Abstract
Microvascular occlusion in sickle cell disease can be initiated by adhesion of sickle red blood cells (RBCs) to the endothelium. Our objective in this study was to verify the relevance in vivo of our discovery that sickle RBCs adhere abnormally to endothelial P-selectin in vitro. We used computer-assisted intravital microscopy to characterize RBC flow velocity (VRBC) in mice. We found faster VRBC of sickle RBCs in P-selectin knock-out and control mice than in sickle cell mice, which have increased endothelial cell P-selectin expression. Agonist peptide for murine protease-activated receptor-1 (PAR-1), which selectively activates mouse endothelial cells but not platelets, was used to assess the effects of endothelial cell P-selectin on microvascular flow. Suffusion of venules with this agonist stopped flow promptly in normal and sickle mice but not in P-selectin knock-out mice or in control mice pretreated with anti-P-selectin monoclonal antibody or unfractionated heparin (UFH). Agonist-induced slowing of flow was reversed rapidly by suffusion with UFH, provided flow had not already stopped. We conclude that endothelial cell P-selectin contributes to the microcirculatory abnormalities in sickle cell disease and that blocking P-selectin may be useful for preventing painful vasoocclusion in sickle cell disease. (Blood. 2004;104:3378-3385)
Introduction
Most of the morbid consequences of sickle cell disease are caused by the impairment of blood flow in the microvasculature.1 Traditional understandings attribute the microvascular occlusion to an increase in blood viscosity caused by intraerythrocytic polymerization of deoxygenated sickle hemoglobin and the consequent sickling and rigidification of red blood cells (RBCs).2 Kinetic considerations predict that, in the absence of preexisting intraerythrocytic polymer, impairment of blood viscosity will occur after the RBC has entered into veins too large to be occluded by rigid sickled RBCs3 and that polymerization will occur within vessels small enough to be occluded by individual sickled RBCs only when their transit through the microcirculation is delayed.
Among the factors that can prolong the transit time of sickle RBCs through the microvasculature are several polymerization-independent processes, including vascular constriction, coagulation, inflammation, and cellular adhesion.4 Experimental evidence supports a 2-step mechanism of vasoocclusion initiated by the binding of an adhesive subset of sickle RBCs to the vascular endothelium and completed by the logjamming of more rigid sickle RBCs behind the adherent nidus.5 This discovery has provided an understanding of the onset of painful vasoocclusion that is lacking from detailed explications of hemoglobin S polymerization and RBC sickling and notions of systemic deoxygenation6 and inspired a profusion of research into mechanisms of adhesion and adhesion-blocking therapies.7,8 The expression of cytoadhesion molecules on endothelial cells isolated from the circulating blood of patients with sickle cell disease9 and on intact endothelial cells in murine models of sickle cell disease10,11 reflects the effects of the several endothelial cell agonists operational in sickle cell disease.4
Adhesion molecules that mediate sickle RBC adherence to the endothelium include many of the same molecules that direct leukocyte adhesion in inflammation,7 a process that is initiated by interactions of selectins with their ligands.12 The increased levels of endothelial cell P-selectin expression in sickle cell disease9-11 and the increased production and activity of the endothelial cell agonist thrombin13,14 suggested to us that P-selectin might have a role in sickle RBC adhesion. Work from our laboratory has demonstrated that sickle RBCs adhere to P-selectin expressed on thrombin-stimulated endothelial cells and to immobilized recombinant P-selectin under both static and flow conditions in vitro,15,16 but the importance of P-selectin-mediated sickle RBC adhesion to the microvascular blood flow in sickle cell disease has yet to be established.
Intravital microscopy has been used to document abnormal microvascular blood flow in patients with sickle cell disease17,18 and to identify the postcapillary venules as the most common sites of sickle RBC adhesion and stoppage of blood flow in sickle cell mouse models.19,20 Other methods of assessing blood flow in vivo also have been used,21-26 and different mechanisms of sickle cell blood flow stoppage have been identified. These include direct adhesion of sickle RBCs to the endothelium24-26 and leukocyte-mediated sickle RBC adhesions.10,27 The experimental conditions used to define those mechanisms in vivo differ from those employed in our in vitro studies in which we employed thrombin as the endothelial cell agonist and depleted leukocytes from the RBC suspensions studied.15,16,28
Our aim in this study was to corroborate in vivo a rheologic effect related to our discovery that sickle RBCs adhere to endothelial cell P-selectin.15,16 We used computer-assisted intravital microscopy (CAIM)20 to establish the microcirculatory flow dynamics of sickle RBCs from transgenic knock-out sickle cell mice (sickle cell mice)29 in the mucosal-intestinal venules of recipient mice. The finding of Coughlin and his associates30 that mice, unlike humans, express protease-activated receptor-1 (PAR-1) activity on endothelial cells but not platelets permitted us to assess in vivo the effects of endothelial cell P-selectin expression independently of activating platelets by using murine PAR-1 agonist peptide. We used the following approaches to test the effect of endothelial cell P-selectin: (1) We compared baseline flow velocities of fluorochrome X-rhodamine-5-[and -6]-isothiocyanate [5[6]-XRITC (XRITC)-labeled RBCs31 in 20- to 40-μm diameter venules of recipient mice having different levels of unstimulated endothelial cell P-selectin expression—P-selectin knock-out, control C57BL/6, and sickle cell mice; (2) we compared the effects of PAR-1 agonist peptide administration in control C57BL/6, sickle cell, and P-selectin knock-out mice; and (3) we pretreated C57BL/6 and sickle cell mice with mouse P-selectin monoclonal antibody (mAb) before administering PAR-1 agonist peptide. We also studied the effects of the P-selectin antagonist, unfractionated heparin (UFH), by pretreating mice with UFH prior to PAR-1 agonist peptide administration and by administering UFH following PAR-1 agonist peptide administration at a time when flow had slowed but not stopped.
Materials and methods
Materials
Antimouse P-selectin mAb RB40.34, antimouse L-selectin mAb MEL-14, antimouse P-selectin glycoprotein ligand-1 (PSGL-1) mAb 2PH1, and antimouse β2 integrin mAb GAME-46 were purchased from BD Pharmingen (San Diego, CA). Thrombin receptor agonist peptide for PAR-1 (PAR-1 agonist peptide; SFLLRN) and scrambled PAR-1 agonist peptide (FSLLRN) synthesized as carboxy-terminal amides were purchased from BioSource (Camarillo, CA). Unfractionated porcine intestine heparin (UFH), rhodamine 6G, and histamine were purchased from Sigma Chemical (St Louis, MO). XRITC dye (X-rhodamine-5-[and -6]-isothiocyanate [5[6]-XRITC) was purchased from Molecular Probes (Eugene, OR). Sodium fluorescein isothiocyanate (FITC) was purchased from IOLABS (25% Funduscein; IOLABS, Claremont, CA).
Mice
Mice weighing from 18 to 35 g were used as sources of RBCs, as recipients of injected fluorochrome (XRITC)-tagged RBCs for studies of RBC flow velocity (VRBC) and flow dynamics, and for studies of flow dynamics of endogenous RBCs. Control mice were C57BL/6 (Charles River Laboratories, Wilmington, MA). Transgenic knock-out sickle cell mice (sickle cell mice) engineered to express only human sickle hemoglobin have hematologic, pathologic, and rheologic features concordant with sickle cell disease of humans.20,29 The sickle cell mice have been backcrossed against a C57BL/6 background with which they are now congenic, as evidenced by the lack of rejection of their bone marrow transplanted into C57BL/6 recipients.27 The P-selectin-deficient mice have been backcrossed against C57BL/6 mice with which they are now congenic.32 The mice used in these studies ranged in age from 6 to 10 weeks. All studies on mice were approved by the committees on animal research of the University of California, San Francisco or the University of California, Davis.
Infusion of XRITC-labeled mouse RBCs, antibodies, and heparin into recipient mice
Control and sickle RBCs for XRITC labeling and infusion into recipient mice were from C57BL/6 or sickle cell mice, respectively. Blood obtained by cardiac puncture was collected into sodium citrate. The buffy coat was removed to deplete leukocytes to a level equivalent to that achieved with an accepted cellulose column method,33 and RBCs were washed and labeled with XRITC according to the method of Sarelius and Duling.31 Recipient mice were anesthetized with intraperitoneal sodium pentobarbital (Veterinary Laboratories, Lenexa, KS), 0.06 to 0.075 mg/g body weight. Labeled RBCs were suspended in phosphate-buffered saline (PBS) to a hematocrit of 0.25 (25%), and 50 μL was infused by tail vein injection into recipient C57BL/6, P-selectin knock-out, or sickle cell mice. These volumes were calculated to obtain a 1:200 in vivo ratio of labeled to unlabeled RBCs and to minimize hemodynamic changes from the infused volumes.
In some experiments mice were preinjected with 50 μL of 600 μg/mL P-selectin mAb RB40.34, 16 U/mL UFH, or the combination of 100 μg/mL L-selectin mAb MEL-14, 400 μg/mL PSGL-1 mAb 2PH1, and 5 mg/mL integrin β2 mAb GAME-46. Each of these reagents or combinations thereof was diluted with PBS to a volume of 50 μL to yield a final effective dosage in 2 mL (approximate total blood volume of an average mouse). A dose of 50 μL rhodamine 6G, 1 mg/mL, was injected into the tail vein to study leukocyte trafficking, as described.34
Computer assisted intravital microscopy (CAIM)
CAIM has been described in detail in our previous study of microvascular blood flow.20 We conducted these studies of blood flow using a prototype intravital microscopy system fabricated from infinity-corrected Olympus optical components (Scientific Instruments, Sunnyvale, CA), a DC 100 W mercury burner (Scientific) light source, and appropriate filters for heat filtering and light intensity calibration. Videomicroscopy was performed using an Olympus MSPLan 10 ∞/f = 180 long-working-distance objective (Olympus, Melville, NY). A computer-aided intravital microscope (CAIM) developed in-house (T.W.C.) was equipped with epi-brightfield and epifluorescence illumination capabilities. The microcirculation was viewed and videotaped at room temperature (20°C) using a high-resolution, low-light level COHU 6415-3000 CCD video camera (COHU Instrument, San Diego, CA). The externalized viscera were moistened continuously with 37°C saline during the procedure. Blood flow was studied and quantified using VASCAN and VASVEL imaging software developed in-house. Two fluorochromes were used in this study: sodium FITC to label the overall microvasculature and XRITC to label RBCs for alternate visualization.20,31
We used reverse epifluorescence illumination20,35 following intramuscular injection of 0.2 mL sodium FITC to identify venules for study and to visualize bulk flow in the microcirculation, and we used the XRITC method described above to track the dynamic flow of RBCs20,31 (Supplemental Movie S1; at the Blood website, see the Supplemental Movies link at the top of the online article). During videotaping of the mucosal-intestinal microcirculation, we interchanged the fluorescein and XRITC filters to monitor overall blood flow and to visualize labeled RBC movement to compute VRBC.
We elected to study the mucosal-intestinal microcirculation because of its high circulatory and metabolic rates, as we described previously.20 We focused specifically on the venules because they are the most common sites of stoppage of sickle cell blood flow19,20 and because uniformity of vessel type provides an internal consistency of vascular reactivity and responses. The branching order of vessels in the mucosal-intestinal circulation is less predictable than in other commonly studied circulations, such as the cremasteric,19,36 but the greater metabolic rate of the mucosal-intestinal circulation provides an important advantage for studies of sickle cell flow. We observed flow dynamics (VRBC and flow stoppage) in venules of 3 size groups according to luminal diameter of the venules: less than 20 μm (small venules); 20 to 40 μm (medium venules); and 40 to 80 μm (large venules). For this study we reported the flow dynamics in medium-size venules, because the consistent VRBC of this size venule provided the most stable background for assessing experimental intervention
VRBC was assessed by computer-assisted tracking of the translocation of XRITC-labeled mouse sickle RBCs in vivo.20,31 To determine whether endothelial cell P-selectin15 is important for sickle RBC adhesion in vivo, we compared the VRBC of XRITC-labeled mouse sickle RBCs injected into mice having different basal levels of P-selectin expression. Recipient mice used were control C57BL/6; P-selectin knock-out, which expresses no P-selectin32 ; and sickle cell mice, which have chronically increased levels of endothelial cell P-selectin.10,11
In heparin pretreatment experiments, 0.8 units UFH in 50 μL PBS or 50 μL PBS alone was injected into a tail vein after surgical externalization of the viscera was complete. This sequence minimized the blood loss experienced when UFH was administered before the surgery.
After the videotaping was completed, we humanely killed the animal by an overdose of sodium pentobarbital. All videotapes were viewed in their entirety, and specific video sequences of interest were coded for subsequent blinded analysis using in-house-developed imaging software (VASCAN and VASVEL programs) to frame-capture, digitize, and objectively measure morphometric characteristics and VRBC.18 Vessel morphometry was measured using VASCAN; vessel diameters and VRBC were measured using VASVEL; and vessel diameters were blindly confirmed later using SCION (a public domain software).
Suffusion of mesenteric endothelium with PAR-1 agonist peptide and heparin (UFH)
Effects of PAR-1 agonist peptide on blood flow dynamics were determined by constant monitoring of RBC movement before and up to 5 minutes after topical administration of 50 μL PAR-1 agonist peptide or scrambled PAR-1 agonist peptide to the mucosal-intestinal vessels by suffusion. The concentration of the agonist peptides varied from 1 to 50 μM. We used the lowest effective concentration as determined by testing each batch of PAR-1 agonist peptide in vitro (by observing cell contraction of human umbilical vein endothelial cells) and in vivo (by observing reduction of VRBC of XRITC-labeled sickle RBCs in C57BL/6 mice). In one experiment 50 μL histamine (10 μg/mL) was suffused as an endothelial call agonist. The ability of UFH to rescue blood flow from complete stoppage was tested by suffusion of 50 μL of 16 U/mL UFH after blood flow had been visibly reduced by PAR-1 agonist peptide suffusion.
Statistical analysis
Statistical analyses were made with the 2-tailed Student t test. P values of less than .05 were accepted as significant.
Results
Baseline microvascular flow velocities in mice having different levels of endothelial P-selectin expression
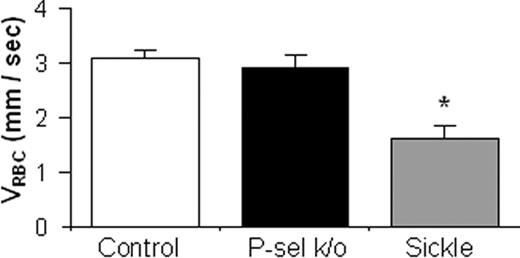
We compared the VRBC of XRITC-labeled sickle RBCs injected into mice having congenic backgrounds but different chronic levels of P-selectin expression—P-selectin knock-out mice, control C57BL/6 mice, and sickle cell mice, which have chronically increased levels of endothelial cell P-selectin.10,11 Measurements of VRBC were made in medium-size venules having very similar diameters (mean diameters ± SEM = 36.6 ± 0.8 μm for C57BL/6, 36.0 ± 0.4 μm for sickle cell, and 36.2 ± 0.6 μm for P-selectin knock-out mice). The mean VRBC in sickle cell mice was 48% lower (P < .001) than in control C57BL/6 mice, but those in P-selectin knock-out and control C57BL/6 mice were not significantly different (Figure 1). The slower VRBC cannot be attributed to the minimal differences in diameters of the venules studied or to inherited differences in vascular endothelial cell gene expression,37 because these mice share a C57BL/6 background. Rather, some microvascular properties of sickle cell mice accounted for the slower VRBC observed. The increased adhesivity of sickle RBCs for P-selectin,15 the similar rapid mean VRBC observed in P-selectin knock-out and C57BL/6 mice in which endothelial cell P-selectin expression had not been activated, and the slower VRBC in sickle cell mice, which have chronically greater levels of endothelial P-selectin expression,10,11 suggest that the level of P-selectin expression may be the vascular property of sickle cell mice responsible for the slower VRBC observed.
Baseline RBC velocity (VRBC) of XRITC-labeled sickle RBCs injected into mouse recipients expected to have different levels of endothelial P-selectin expression. CAIM flow data from visualization of 20 to 40 μm diameter mucosal-intestinal venules are represented as the average VRBC of XRITC-labeled RBCs. Labeled sickle RBCs were injected intravenously into C57BL/6 (control; n = 24), P-selectin knock-out (P-sel k/o; n = 9), and sickle cell mice (n = 10). The error bars indicate the standard error of the mean. * indicates a statistically significant (P < .05) difference from the other groups.
Baseline RBC velocity (VRBC) of XRITC-labeled sickle RBCs injected into mouse recipients expected to have different levels of endothelial P-selectin expression. CAIM flow data from visualization of 20 to 40 μm diameter mucosal-intestinal venules are represented as the average VRBC of XRITC-labeled RBCs. Labeled sickle RBCs were injected intravenously into C57BL/6 (control; n = 24), P-selectin knock-out (P-sel k/o; n = 9), and sickle cell mice (n = 10). The error bars indicate the standard error of the mean. * indicates a statistically significant (P < .05) difference from the other groups.
To explore further the determinants of microvascular hemodynamics we compared the flow rates of sickle and normal mouse RBCs in medium-size venules of C57BL/6 mice. The VRBC of XRITC-labeled normal (n = 18) and sickle (n = 24) RBCs in medium-size venules (20 to 40 μm diameter) were not statistically different (VRBC = 2.71 ± 0.18 mm/s and 3.06 ± 0.13 mm/s, respectively; P = .1). This suggests that in this experimental system the VRBC of normal and sickle RBCs are affected equally by the hemodynamic influences of the vascular walls.
We also compared the microvascular flow velocities of normal RBCs in medium-size venules of C57BL/6, P-selectin knock-out, and sickle cell mice. The VRBC of XRITC-labeled normal RBCs was the same (P = .99) in P-selectin knock-out mice (2.71 ± 0.22 mm/s; n = 14) and in C57BL/6 mice (2.71 ± 0.18 mm/s; n = 18). The VRBC of normal RBCs was significantly slower in sickle cell mice (1.23 ± 0.10 mm/s; n = 3) than in P-selectin knock-out (P = .004) or C57BL/6 mice (P = .001). These results demonstrate the same tendencies for normal RBCs as we found with sickle RBCs, which suggests that in this experimental system the microvascular VRBC of both sickle and normal RBCs is consistently affected by some vascular property of sickle cell mice, perhaps the level of P-selectin expressed on the vascular endothelium.
Microvascular flow velocity changes in mice following activation of endothelium by protease-activated receptor-1 (PAR-1) agonist peptide
To study further the hemodynamic effect of endothelial P-selectin, we utilized the selective activation of murine endothelial cells, but not platelets, by mouse PAR-1 agonist peptide30 to induce higher levels of endothelial P-selectin expression. In these studies we compared in real time the baseline VRBC in medium-size venules (Supplemental Movie S2) with the VRBC after topical suffusion of PAR-1 agonist peptide (Supplemental Movie S4). Inactive scrambled PAR-1 agonist peptide suffused as a control caused no significant changes to blood flow (Supplemental Movie S3). Promptly after endothelial cell activation by PAR-1 agonist peptide administration, XRITC-labeled sickle RBCs were observed to contact, roll, and firmly adhere to the endothelium (Supplemental Movie S4). In a frame-captured photomicrograph, RBCs moving rapidly in the axial stream appear as elongated ellipses, and the RBCs rolling slowly along the vascular wall appear as round dots (Figure 2C).
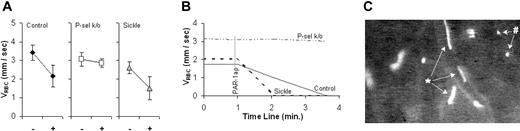
The effect of PAR-1 agonist peptide on RBC velocity (VRBC) of XRITC-labeled sickle RBCs injected into mouse recipients expected to have different levels of endothelial P-selectin expression. Results are described as VRBC in C57BL/6 (control), P-selectin knock-out (P-sel k/o), and sickle cell mice. (A) Average VRBC of labeled sickle RBCs in control (n = 5), P-selectin knock-out (n = 5), and sickle cell mice (n = 4) before (-) and 2 minutes after (+) PAR-1 agonist peptide suffusion. The error bars indicate the standard error of the mean. (B) Real-time course plots of RBC velocity (VRBC) slowing due to PAR-1 agonist peptide (PAR-1ap) suffusion. Each plot represents a single representative experiment. (C) A single frame captured of labeled sickle RBCs in PAR-1 agonist peptide-treated vessels. Sickle RBCs moving rapidly (*) and rolling slowly (#) are indicated.
The effect of PAR-1 agonist peptide on RBC velocity (VRBC) of XRITC-labeled sickle RBCs injected into mouse recipients expected to have different levels of endothelial P-selectin expression. Results are described as VRBC in C57BL/6 (control), P-selectin knock-out (P-sel k/o), and sickle cell mice. (A) Average VRBC of labeled sickle RBCs in control (n = 5), P-selectin knock-out (n = 5), and sickle cell mice (n = 4) before (-) and 2 minutes after (+) PAR-1 agonist peptide suffusion. The error bars indicate the standard error of the mean. (B) Real-time course plots of RBC velocity (VRBC) slowing due to PAR-1 agonist peptide (PAR-1ap) suffusion. Each plot represents a single representative experiment. (C) A single frame captured of labeled sickle RBCs in PAR-1 agonist peptide-treated vessels. Sickle RBCs moving rapidly (*) and rolling slowly (#) are indicated.
Figure 2 shows the VRBC of XRITC-labeled sickle RBCs in venules of sickle cell, control C57BL/6, and P-selectin knock-out mice before and after PAR-1 agonist peptide suffusion. Two minutes after PAR-1 agonist peptide administration, we observed statistically significant reductions in the averaged VRBC of 36% in control C57BL/6 mice (3.4 mm/s to 2.2 mm/s; P = .024) and 42% in sickle cell mice (2.6 mm/s to 1.5 mm/s; P = .031) (Figure 2A). By 4 minutes, blood flow in all venules of PAR-1 agonist peptide-treated control C57BL/6 and sickle cell mice stopped completely, as shown in the representative real-time course plot of VRBC in Figure 2B (see also Supplemental Movie S4). In P-selectin knock-out mice, PAR-1 agonist peptide administration did not result in a significant change in VRBC (7% reduction, P = .194) after 2 minutes. After 4 minutes blood was still flowing in all observed venules, as shown in the representative real-time course plot of VRBC in Figure 2B (Supplemental Movie S6). The slowing and stoppage of microvascular blood flow by PAR-1 agonist peptide in P-selectin-sufficient mice and the absence of these effects in P-selectin-deficient mice are consistent with PAR-1 agonist peptide having an effect on endothelial P-selectin expression and with endothelial cell P-selectin having a role as a determinant of microvascular VRBC.
To confirm that the flow stoppage we observed with PAR-1 agonist peptide suffusion was due to selective endothelial cell activation, rather than activation of both endothelial cells and platelets, we tested the effect of exogenous histamine, which activates endothelial cells but not platelets.38,39 When the mesentery of a C57BL/6 mouse was suffused with 50 μL histamine (10 μg/mL) there was a prompt stoppage of flow of normal blood in the microvasculature detected using FITC-labeled blood flow (data not shown), as had been observed with PAR-1 agonist peptide. This is consistent with histamine-induced expression of P-selectin on endothelial cell surfaces40 causing flow stoppage.
Microvascular flow effects associated with deficient endothelial cell P-selectin activity
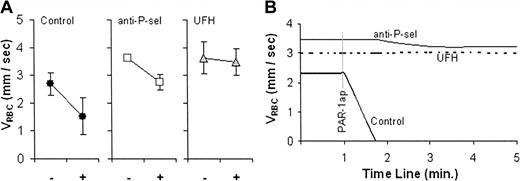
The role of P-selectin as a determinant of VRBC was suggested by the slower flow velocity we observed in sickle cell mice (Figure 1). This was supported by the absence of PAR-1 agonist peptide-induced slowing of microvascular blood flow observed in P-selectin-deficient mice (Figure 2). To test this relationship further, we pretreated control C57BL/6 and sickle cell mice by tail vein injection with P-selectin blocking agents 5 to 15 minutes before PAR-1 agonist peptide administration. Varki et al have defined the P-selectin blocking activity of UFH,41 and we have demonstrated that UFH blocks sickle RBC adhesion to P-selectin.16 Accordingly, we tested the effect of pretreatment with UFH, as well as with anti-P-selectin mAb, on PAR-1 agonist peptide-induced slowing of blood flow. Two minutes after PAR-1 agonist peptide was administered to control C57BL/6 mice that had been pretreated only with PBS there was a 52% reduction in average VRBC from 2.7 mm/s to 1.5 mm/s, which was statistically significant (P = .028) (Figure 3A). In contrast, the reduction of VRBC in mice that had been pretreated with anti-P-selectin mAb or with UFH did not change significantly 2 minutes after suffusion with PAR-1 agonist peptide (23% reduction, P = .071, and 3% reduction, P = .283, respectively) (Figure 3A). By 4 minutes blood flow in all PAR-1 agonist peptide-treated venules in mice pretreated only with PBS stopped completely, which was significantly different from the continued blood flow in mice pretreated with anti-P-selectin mAb (P < .001) or UFH (P < .001), as shown in the representative plots of real-time VRBC changes in Figure 3B.
The effect of pretreatment with anti-P-selectin mAb (anti-P-sel) and unfractionated heparin (UFH) on PAR-1 agonist peptide-reduced RBC velocity (VRBC) of XRITC-labeled sickle RBCs injected into C57BL/6 (control) mice. Results described are VRBC. (A) Average velocities of labeled sickle RBCs (VRBC) in control-(n = 5), anti-P-selectin-(n = 3), or UFH-treated (n = 6) mice before (-) and 2 minutes after (+) PAR-1 agonist peptide suffusion. The error bars indicate the standard error of the mean. (B) Real-time course plots of RBC velocity (VRBC) changes induced by PAR-1 agonist peptide (PAR-1ap) suffusion. Each plot represents a single representative experiment. The representative time course for PAR-1 agonist peptide alone is consistent with the overall findings in which slowing of flow always ensued within 2 minutes and stoppage of flow occurred within 4 minutes.
The effect of pretreatment with anti-P-selectin mAb (anti-P-sel) and unfractionated heparin (UFH) on PAR-1 agonist peptide-reduced RBC velocity (VRBC) of XRITC-labeled sickle RBCs injected into C57BL/6 (control) mice. Results described are VRBC. (A) Average velocities of labeled sickle RBCs (VRBC) in control-(n = 5), anti-P-selectin-(n = 3), or UFH-treated (n = 6) mice before (-) and 2 minutes after (+) PAR-1 agonist peptide suffusion. The error bars indicate the standard error of the mean. (B) Real-time course plots of RBC velocity (VRBC) changes induced by PAR-1 agonist peptide (PAR-1ap) suffusion. Each plot represents a single representative experiment. The representative time course for PAR-1 agonist peptide alone is consistent with the overall findings in which slowing of flow always ensued within 2 minutes and stoppage of flow occurred within 4 minutes.
To determine whether slowing of flow caused by additional P-selectin on the endothelium of sickle cell mice is also amenable to P-selectin blocking, we pretreated these mice with anti-P-selectin mAb or UFH before administering PAR-1 agonist peptide. Using reverse epifluorescence illumination and sodium FITC to measure bulk flow, we found that the time for complete stoppage of flow in sickle cell mice also was significantly prolonged by pretreatment with anti-P-selectin mAb or UFH compared with mice pretreated only with PBS (data not shown). These results provide additional support for endothelial cell P-selectin expression being a determinant of microvascular blood flow dynamics. We detected no changes in vessel diameter related to UFH administration that would have protected against the reduction of flow velocity induced by PAR-1 agonist peptide.
Microvascular flow effects associated with the administration of unfractionated heparin
The evidence for P-selectin as a determinant of microvascular flow dynamics and for UFH as a preventative of sickle RBC adhesion and blood flow stasis (Figure 3) raises the question of whether UFH also might be effective for reversing sickle cell vasoocclusion. To test for this effect, we suffused UFH onto the mucosal-intestinal vasculature of control C57BL/6 mice that had been injected with XRITC-labeled sickle RBCs and treated with PAR-1 agonist peptide suffusion.
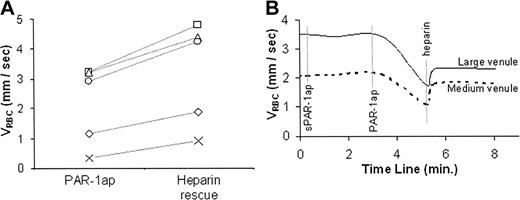
Administration of UFH increased the mean VRBC in PAR-1 agonist peptide-treated venules by 49% (P = .003) (Figure 4; Supplemental Movie S5). In these experiments, when microvascular flow had been slowed but not stopped, UFH treatment prevented complete stoppage. In experiments where blood flow was allowed to stop completely before UFH was administered, UFH did not restore flow (data not shown). These differential effects of UFH on slowed versus stopped flow are consistent with a role of P-selectin in the initial stages of adhesion and flow stoppage and with the potential of UFH for preventing painful vasoocclusion but not for reversing vasoocclusion once it has occurred.
The effect of suffused UFH on PAR-1 agonist peptide-reduced sickle RBC flow velocity (VRBC). Results are described as VRBC of XRITC-labeled sickle RBCs in C57/BL (control) mice. (A) VRBC of sickle RBCs in PAR-1 agonist peptide (PAR-1ap)-treated venules prior to and after UFH suffusion (heparin rescue). Results are of individual VRBC changes in single venules from 5 different experiments. (B) Real-time course plots of RBC velocity (VRBC) in medium (20 to 40 μm) and large (40 μm to 80 μm) venules in C57BL/6 (control) mice treated with inactive scrambled PAR-1 agonist peptide (sPAR-1ap), active PAR-1 agonist peptide (PAR-1ap), and unfractionated heparin (heparin).
The effect of suffused UFH on PAR-1 agonist peptide-reduced sickle RBC flow velocity (VRBC). Results are described as VRBC of XRITC-labeled sickle RBCs in C57/BL (control) mice. (A) VRBC of sickle RBCs in PAR-1 agonist peptide (PAR-1ap)-treated venules prior to and after UFH suffusion (heparin rescue). Results are of individual VRBC changes in single venules from 5 different experiments. (B) Real-time course plots of RBC velocity (VRBC) in medium (20 to 40 μm) and large (40 μm to 80 μm) venules in C57BL/6 (control) mice treated with inactive scrambled PAR-1 agonist peptide (sPAR-1ap), active PAR-1 agonist peptide (PAR-1ap), and unfractionated heparin (heparin).
Microvascular flow effects associated with the blocking leukocyte adhesion
Lutty and Hillery and their associates have reported that sickle RBCs adhere directly to retinal and choroidal capillary endothelium or to cerbrovascular endothelium exposed in vivo to tumor necrosis factor-α (TNF-α) or to reduced nitric oxide levels, respectively.24-26 Others have reported that leukocytes mediate sickle RBCS to cremasteric or cerebral microvascular endothelium activated by large doses of TNF-α or prolonged hypoxia followed by reoxygenation.10,27 We reexamined the role of leukocyte adhesion in vivo using PAR-1 agonist peptide as a selective agonist of the expression of the leukocyte adhesion molecule, P-selectin, on endothelial cells of mucosal intestinal venules. After administration of PAR-1 agonist peptide by suffusion, we detected no slowing of rhodamine 6G-labeled leukocytes preceding the reduction of bulk velocity (data not shown). This suggests that leukocyte adhesion might not have initiated sickle RBC adhesion or flow stoppage. It is possible that unstained leukocytes were involved in the P-selectin-mediated stoppage of blood flow. However, our results seem consistent with direct RBC adhesion to the endothelium as was found in rat retinal, choroidal, and cerebral microvessels in vivo.24-26
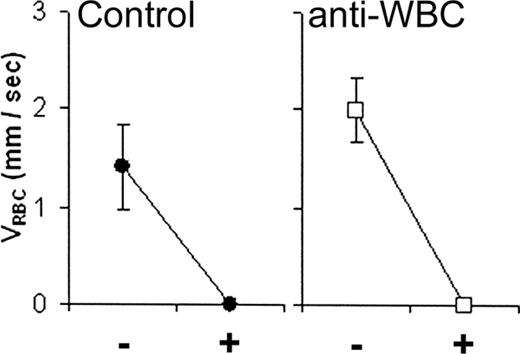
To examine this issue further we pretreated mice with a combination of mAb MEL-14 against mouse L-selectin, mAb GAME-46 against mouse β2 integrins, and mAb 2PH1 against the mouse leukocyte P-selectin ligand PSGL-1 to block leukocyte adhesion using doses that have been shown for each alone to block leukocyte adhesion in vivo or ex vivo.42-44 C57BL/6 mice pretreated with intravenous administration of this combination had hemodynamic responses indistinguishable from those seen in mice not pretreated with the mAb (Figure 5). It is possible that in these studies leukocyte adhesion was not abrogated by the combination of mAb used. While we cannot unequivocally exclude the participation of leukocyte adhesion in the flow stoppage we observed, within the limits of the methodology used our results are consistent with direct adhesion of sickle RBCs to PAR-1 agonist peptide-activated endothelium having initiated vasoocclusion, as was found in rat retinal, choroidal, and cerebral microvessels in vivo.24-26
The effect of pretreatment with a combination of anti-L-selectin, anti-PSGL-1, and anti-β2integrin mAbs (anti-WBC) on PAR-1 agonist peptide-reduced RBC velocity (VRBC) of XRITC-labeled sickle RBCs injected into C57BL/6 (control) mice. Results described are VRBC. Average velocities of labeled sickle RBCs (VRBC) in control (n = 6) or combination antibody-treated (n = 12) mice before (-) and 2 minutes after (+) PAR-1 agonist peptide suffusion. The error bars indicate the standard error of the mean.
The effect of pretreatment with a combination of anti-L-selectin, anti-PSGL-1, and anti-β2integrin mAbs (anti-WBC) on PAR-1 agonist peptide-reduced RBC velocity (VRBC) of XRITC-labeled sickle RBCs injected into C57BL/6 (control) mice. Results described are VRBC. Average velocities of labeled sickle RBCs (VRBC) in control (n = 6) or combination antibody-treated (n = 12) mice before (-) and 2 minutes after (+) PAR-1 agonist peptide suffusion. The error bars indicate the standard error of the mean.
Discussion
The results we describe provide support for the relevance of our prior in vitro findings of elevated static and rolling adhesion of human sickle RBCs to cultured endothelial cells that had been activated with thrombin to express surface P-selectin.15,16 In the current in vivo study, the slower VRBC in venules of sickle cell mice compared with control C57BL/6 or P-selectin knock-out mice is consistent with greater adhesion of sickle RBCs to endothelium having a greater chronic level of P-selectin expression.10,11 Among other potential determinants of VRBC, differences in vessel diameters17 were not responsible for the slower VRBC in sickle cell mice, because the diameters of the venules studied were the same in the 3 groups of mice. Neither can the VRBC difference be attributed to inherited differences in vascular endothelial properties that determine resistance to flow or responses,37 because the 3 strains are congenic. Although the anemia of the sickle cell mice would have increased their flow velocity (and thereby diminished the difference between their flow rates and those of the other 2 strains), the increased blood viscosity caused by the presence of more rigid sickle RBCs would have slowed the flow. Thus, in this analysis the slower VRBC in sickle cell mice is consistent with the hypothesis that endothelial P-selectin could be a determinant of baseline microvascular flow of sickle RBCs in vivo.
We also found that the VRBC of XRITC-labeled sickle mouse RBCs greatly decreased when mucosal-intestinal venules were suffused with PAR-1 agonist peptide, a selective activator of the endothelial thrombin receptor, PAR-1. In addition, both UFH and antibodies that block P-selectin inhibited the rolling adhesion of human sickle RBCs in vitro16 and the PAR-1 agonist peptide-induced flow stoppage of mouse sickle RBCs in vivo.
Further support for the importance of P-selectin to sickle RBC adhesion and blood flow in vivo includes the lack of PAR-1 agonist peptide effect on VRBC in P-selectin-deficient mice and the restoration by UFH of VRBC slowed (but not stopped) by PAR-1 agonist peptide. UFH blocks P-selectin-mediated adhesion, thrombospondin (TSP)-dependent and -independent adhesion of sickle RBCs, and the coagulation processes that are active in sickle cell disease.41,45-48 In our studies we used UFH to block the adhesion of sickle RBCs to P-selectin on the vascular endothelium. We envision an effect on sickle RBCs similar to that in which UFH binds to the walls of heparinase-treated vessels to attenuate the adhesion of circulating leukocytes.49 The specificity of UFH for blocking P-selectin was gained through our use of PAR-1 agonist peptide to activate endothelial cells in vivo. Although thrombin activates the expression of P-selectin by both platelets and endothelial cells,50,51 mice express the PAR-1 thrombin receptor on endothelial cells but not on platelets.30 Taken together with the capacity of UFH to block P-selectin-mediated adhesion16,41 and the selective effect of PAR-1 agonist peptide on mouse endothelial cells, our findings are consistent with the capacity of UFH to prevent flow stoppage by blocking P-selectin.
Induction of the adhesivity of blood and vascular cells by thrombin or thrombin-receptor agonist peptides enhances the adherence of circulating cells to the endothelium and to each other by a variety of mechanisms. We circumvented P-selectin-dependent adhesion of activated platelets52 by using platelet-sparing agonists; thrombin and histamine also induce the expression of von Willebrand factor (VWF) by endothelial cells.40,51 Although the requirement for endothelial cell P-selectin in anchoring VWF to the endothelium53 is consistent with UVF-mediated adhesion having contributed to the differences we observed between P-selectin-deficient and wild-type mice, the antiadhesive effect of VWF on sickle RBC adhesion suggests that VWF was not responsible for these differences.54 Although the adhesion of platelets and leukocytes to activated endothelial P-selectin55-57 should have been blocked by the mAb combination that included anti-PSGL-1 mAb, it is possible that other P-selectin recognition determinants such as sulfatides58 or glycoprotein Ib-IX-V59 may have mediated the adhesion of nonerythroid cells to P-selectin on endothelial cells. Platelet activating factor (PAF) could have mediated the adhesion of sickle RBCs,60-62 but the differences between the responses of P-selectin-deficient and wild-type mice suggest that a PAF effect was not a major one. We cannot exclude the possibility that TSP might have contributed to the changes we observed.46,63,64 Although changes in vascular diameter have a powerful effect on flow resistance,17 we observed no PAR-1-induced changes in vascular diameter that could account for our results. The evidence we provide supports a hemodynamic role of endothelial cell P-selectin on the abnormal microcirculatory flow of sickle RBCs. Much as leukocyte adhesion to the vascular endothelium is initiated by and dependent upon P-selectin-mediated processes,12 our data suggest that endothelial P-selectin is important to the endothelial adhesion of sickle RBCs.
The in vivo findings by Lutty and Hillery's groups of direct adhesion of sickle RBCs to the vascular wall24-26 and by Turhan and Wood and their associates of leukocyte-mediated sickle RBC adhesion10,27 indicate that multiple adhesive mechanisms may contribute to sickle cell vasoocclusion. Our observations appear to resemble those found by Lutty and Hillery's groups. The variance between our findings and those of the Turhan and Wood groups may relate to the different endothelial cell agonists used or to the different types of endothelial cells studied. TNF-α activates neutrophils, causing their margination to the vascular wall and promoting a prothrombotic condition.65-68 Similarly, prolonged hypoxia followed by reoxygenation triggers myriad biologic responses.69-72 The PAR-1 agonist peptide that we used does not activate neutrophils or cause their margination. Different adhesion mechanisms may exist among the vascular endothelia from different genetic and organ-specific sources.37 We studied mucosal-intestinal venular endothelium, while the Turhan and Wood groups studied the cremasteric and cerebrovascular endothelial cells.10,27 In our experimental system we could not confirm that leukocyte adhesion was required for stoppage of microvascular flow. The quantitative relevance of these 2 mechanisms in vivo is difficult to ascertain. Parameters germane to this issue include the thousand-fold greater number of sickle RBCs than neutrophils in the circulation, the increased adhesivity of all sickle RBCs (but some more than others), and the relative capacity of neutrophils and sickle RBCs to participate in heterocellular interactions.
The baseline-increased expression of P-selectin on vascular endothelial cells from human patients9 and in mouse models of sickle cell disease10,11 supports the potential therapeutic relevance of our findings. Because P-selectin acts in the initial phases of vasoocclusion, we predict that blocking P-selectin would be more effective for preventing painful stoppage of blood flow than for treating established pain crises. Numerous approaches to blocking P-selectin are under development for the treatment of inflammatory, malignant, and vascular diseases.73-75 Considering the widespread popularity of low-molecular-weight heparins among clinicians, it is essential to consider that the P-selectin blocking capacity of low-molecular-weight heparin is orders of magnitude less than that of UFH.16,41 In this regard, a small uncontrolled study concluded that minidose UFH could reduce the frequency of sickle cell pain crises.76 While the potential side effects and requirement for injectable administration limit the therapeutic potential of UFH for preventing painful sickle cell vasoocclusion,48 more detailed molecular understandings of the structural determinants the P-selectin blocking activity77 and the technology required to tailor heparin activities according to desired effects78 offer promise for the future use of sulfated anionic polysaccharides for preventing painful vasoocclusion in sickle cell disease. Consideration is now warranted for this therapeutic approach to preventing pain crises in patients with sickle cell disease.
Prepublished online as Blood First Edition Paper, July 22, 2004; DOI 10.1182/blood-2004-02-0713.
Supported in part by grants HL64396 (Steven Embury), HL31579 (N.M.), and HL67432 and HL64396-subcontract (A.T.W.C.), a contract (NO1-CO-12400 [B.A.D.]), and a Sickle Cell Scholars Award (HL20985) (N.M.M.), all from the National Institutes of Health; an American Heart Association Established Investigator Grant (T.N.M.); and a Professional Development Award from the University of California Davis (A.T.W.C.).
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Drs Shaun Coughlin and Gilbert Sambrano for helpful discussions regarding PAR agonist peptide strategies. We acknowledge assistance with and advice regarding mouse breeding provided by Dr Ervin Epstein Jr, Marilyn Parra, Jennifer Hebert, and Yefim Khaimskiy; advice and assistance with the method of XRITC labeling RBCs from Drs Ingrid Sarelius and Jamie Butler; and technical assistance provided by Christi Seto, Christine Baran, Michelle Barbosa, and Colleen Hefner.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal