Magnetic resonance (MR) tracking of magnetically labeled cells is a relatively new technique to noninvasively determine the biodistribution and migration of transplanted or transfused stem cells in vivo.1 The recent paper by Arbab et al2 represents a significant step forward in bringing the prospect of magnetic resonance imaging cell tracking into the clinic. They describe the use of protamine sulfate as a small cationic transfection agent (TA) to shuttle Food and Drug Administration (FDA)-approved ferumoxide (Fe) magnetic nanoparticles (Feridex IV, in an off-label application; Berlex Laboratories, Wayne, NJ) into cells. As protamine sulfate has been approved by the FDA as well (to reverse heparin anticoagulation), and thus also exists as a pharmaceutical preparation, clinical implementation will likely be facilitated.
In this work, the authors have studied the effect that the TA-Fe labeling may have on the differentiation of human mesenchymal stem cells (hMSCs) into adipocytes, osteoblasts, and chondrocytes. Although the viability and proliferation remain unaltered, they reported no adverse effect on cellular differentiation. However, in Figure 2C-D, they failed to demonstrate chondrogenic differentiation, as evidenced by lack of positive (deep red) Safranin-O glycosaminoglycan staining. Furthermore, the immunostaining for collagen II (Figure 2E) lacks significant deposition of brown substrate.
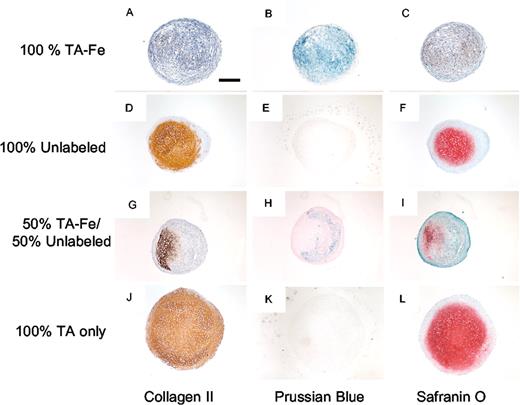
We have extensive experience with MSCs and the process of chondrogenic differentiation,3,4 as well as magnetic labeling of MSCs using TA-Fe.5-7 Using poly-L-lysine (PLL) as a TA, we have labeled hMSCs with Fe at half the dose that was used by Arbab et al2 (25 μg Fe/mL). This results in an intracellular iron incorporation of 13 to 16 pg Fe/cell,6,8 in the same range as obtained by Arbab et al (11 pg Fe/cell).2 Although we found indeed no adverse effect on cell viability, proliferation, adipogenesis, or osteogenesis, we were surprised to observe inhibition of chondrogenesis in the hMSC donors who were studied (Figure 1). The inhibition appeared dose dependent, as halving the Fe concentration induced limited chondrogenesis (data not shown). The inhibition effect was found to be mediated by the Fe itself, and not the TA (ie, PLL) that was used (Figure 1J-L), indicating that the use of other TAs, including protamine sulfate, may not circumvent the observed chondrogenesis inhibition.
Chondrogenic differentiation of MSCs is inhibited after magnetic labeling with ferumoxides. Human MSCs exposed to TA-Fe (A-C) exhibit normal viability, with Prussian Blue staining revealing Fe-containing cells throughout the pellet (B), but fail to demonstrate chondrogenic differentiation (A,C). In contrast, donor MSCs unexposed to TA-Fe exhibit normal chondrogenesis (D-F). To further prove that Fe labeling inhibits chondrogenic differentiation, experiments were performed with 50:50 mixtures of TA-Fe-labeled and unlabeled MSCs (G-I). Only unlabeled, non-Fe-labeled cells (Prussian Blue-negative region in panel H) demonstrate chondrogenesis. Thus, the inhibition of chondrogenesis is mediated by Fe, and not by the TA, as MSCs labeled with only TA (J-L) differentiate normally. All experiments were performed with the same donor cells and passage number. Bar in panel A represents 400 μm. Thin sections of pellets were cleared though xylene and ethanol, then rehydrated. For collagen II indirect immunostaining, sections were reacted against monoclonal antibody C4F6; the brown positive signal was composed of precipitated di-amino-benzidine (DAB). Prussian Blue and safranin O stains were performed as previously described. Sections stained for collagen II and safranin O were also briefly exposed to hematoxylin, to color nuclei blue. Once staining was complete, sections were dehydrated and mounted under No. 1 coverslip glass in Permount (Fisher Scientific, Hampton, NH). The slides were observed at room temperature (22°C) with an Eclipse E400 microscope (Nikon USA, Melville, NY), using a Nikon Plan Fluor 4×/0.13 dry objective lens. Digital images were captured with an attached SPOT RT Slider 2.3.0 digital camera (Digital Instruments, Sterling Heights, MI) using the manufacturer's proprietary capture software (SPOT 4.0.5). Files saved under the JPEG format were then opened in Photoshop 6.0 (Adobe Systems, San Jose, CA), cropped, and combined into a single composite image. Red-Green-Blue colorspace was then adjusted with a single use of the “Levels” command for each set of four similarly-stained pellet sections.
Chondrogenic differentiation of MSCs is inhibited after magnetic labeling with ferumoxides. Human MSCs exposed to TA-Fe (A-C) exhibit normal viability, with Prussian Blue staining revealing Fe-containing cells throughout the pellet (B), but fail to demonstrate chondrogenic differentiation (A,C). In contrast, donor MSCs unexposed to TA-Fe exhibit normal chondrogenesis (D-F). To further prove that Fe labeling inhibits chondrogenic differentiation, experiments were performed with 50:50 mixtures of TA-Fe-labeled and unlabeled MSCs (G-I). Only unlabeled, non-Fe-labeled cells (Prussian Blue-negative region in panel H) demonstrate chondrogenesis. Thus, the inhibition of chondrogenesis is mediated by Fe, and not by the TA, as MSCs labeled with only TA (J-L) differentiate normally. All experiments were performed with the same donor cells and passage number. Bar in panel A represents 400 μm. Thin sections of pellets were cleared though xylene and ethanol, then rehydrated. For collagen II indirect immunostaining, sections were reacted against monoclonal antibody C4F6; the brown positive signal was composed of precipitated di-amino-benzidine (DAB). Prussian Blue and safranin O stains were performed as previously described. Sections stained for collagen II and safranin O were also briefly exposed to hematoxylin, to color nuclei blue. Once staining was complete, sections were dehydrated and mounted under No. 1 coverslip glass in Permount (Fisher Scientific, Hampton, NH). The slides were observed at room temperature (22°C) with an Eclipse E400 microscope (Nikon USA, Melville, NY), using a Nikon Plan Fluor 4×/0.13 dry objective lens. Digital images were captured with an attached SPOT RT Slider 2.3.0 digital camera (Digital Instruments, Sterling Heights, MI) using the manufacturer's proprietary capture software (SPOT 4.0.5). Files saved under the JPEG format were then opened in Photoshop 6.0 (Adobe Systems, San Jose, CA), cropped, and combined into a single composite image. Red-Green-Blue colorspace was then adjusted with a single use of the “Levels” command for each set of four similarly-stained pellet sections.
We believe our inhibition results to be noteworthy. They demonstrate, for the first time, that cellular differentiation into a specific cell type can be affected by Fe labeling while the viability and proliferation remain normal. Future studies will need to implement careful ferumoxide titration and cell differentiation studies, in particular now that clinical trials using magnetically labeled cells are being considered.9
Feride-protamine sulfate labeling does not alter differentiation of mesenchymal stem cells
We read with interest the results of Bulte and colleagues indicating that ferumoxide (Fe)-poly-l-lysine (PLL) complexes inhibited chondrogenic differentiation of mesenchymal stem cells (MSCs) following labeling. They have also raised concerns that the staining characteristics of the cell pellets presented in Figure 2C-E of our report failed to demonstrate chondrogenic differentiation of MSCs labeled with ferumoxide-protamine sulfate (Fe-Pro) complexes.1 Their comments and data provide us with the opportunity to elaborate on our findings and present additional data that clearly demonstrate chondrogenesis from Fe-Pro-labeled MSCs.
We used MSCs and media from the same supplier as the Bulte lab (Cambrex, Baltimore, MD) and procedures designed to result in differentiation of MSCs along different lineages. Chondrogenic differentiation and expression of collagen II depend on incubation time in chemically modified differentiating media and concentrations of factors for differentiation.2 Our original results indicate a similar pattern (ie, morphology and size) and extent of staining for proteoglycans and collagen II in chondrogenic-differentiated cells from both Fe-Pro-labeled and unlabeled MSCs.1
To further demonstrate that Fe-Pro-labeled MSCs undergo chondrogenic differentiation, labeled and unlabeled cell pellets cultured in appropriate differentiation media for 20 days were stained for proteoglycans with Alcian blue 8GX at pH 2.5 (Sigma, St Louis, MO) and for expression of collagen X. Similar to Safranin-O stain, Alcian blue also stains glycosaminoglycans. Figure 1 demonstrates a similar distribution of Alcian blue stain in the Fe-Pro-labeled and unlabeled cells, along with similar cellular morphology and expression of collagen X in both cell pellets,3 indicating that Fe-Pro-labeled MSCs are indeed capable of chondrocytic differentiation. Prussian Blue staining of the section clearly shows iron-labeled cells (Figure 1D-E).
Chondrogenic differentiation is not inhibited by Fe-Pro labeling. Both unlabeled (A) and Fe-Pro-labeled (B) MSC pellets exhibited similar distributions of Alcian blue-stained glycosaminoglycans. Inset shows magnified view (× 100). Staining with collagen X (normally present only in hypertrophic chondrocytes, fetal cartilage, cartilage at growth plates of long bones, and neoplasms arising from chondrocytes) followed by Prussian Blue (PB) shows positive collagen X expression both in unlabeled (C) and Fe-Pro-labeled (D) MSC pellets.4 Magnified view shows collagen X expression in iron-positive cells (E, multiple arrows). Note the lack of Alcian blue stain and expression of collagen X in the cells around extracellular iron oxide complexes (arrow in B, arrow in D). The bars on A-D represent 100 μm and the bar on E represents 20 μm. Photomicrographs were visualized under a Zeiss Axioplan Imaging II microscope (Carl Zeiss, Oberkochen, Germany) equipped with 10×/0.50 (A-D) or 100×/1.30 (A inset, B inset, E) oil immersion objective lenses (Carl Zeiss). Images were acquired with Axiovision 4 software (Carl Zeiss) and processed with Adobe Photoshop 7.0 software (Adobe, San Jose, CA).
Chondrogenic differentiation is not inhibited by Fe-Pro labeling. Both unlabeled (A) and Fe-Pro-labeled (B) MSC pellets exhibited similar distributions of Alcian blue-stained glycosaminoglycans. Inset shows magnified view (× 100). Staining with collagen X (normally present only in hypertrophic chondrocytes, fetal cartilage, cartilage at growth plates of long bones, and neoplasms arising from chondrocytes) followed by Prussian Blue (PB) shows positive collagen X expression both in unlabeled (C) and Fe-Pro-labeled (D) MSC pellets.4 Magnified view shows collagen X expression in iron-positive cells (E, multiple arrows). Note the lack of Alcian blue stain and expression of collagen X in the cells around extracellular iron oxide complexes (arrow in B, arrow in D). The bars on A-D represent 100 μm and the bar on E represents 20 μm. Photomicrographs were visualized under a Zeiss Axioplan Imaging II microscope (Carl Zeiss, Oberkochen, Germany) equipped with 10×/0.50 (A-D) or 100×/1.30 (A inset, B inset, E) oil immersion objective lenses (Carl Zeiss). Images were acquired with Axiovision 4 software (Carl Zeiss) and processed with Adobe Photoshop 7.0 software (Adobe, San Jose, CA).
The differences between the Fe-PLL and Fe-Pro methods may in fact be responsible for the differences in observations between our lab and the Bulte lab. Our group developed and has extensively characterized the approach for magnetic cell labeling combining ferumoxides with transfection agents.4-6 We have shown that labeling stem cells and other cells with Fe-Pro is more efficient and results in a cleaner preparation compared with Fe-PLL, especially if heparin is included in the initial washes.6 We have been able to duplicate Bulte et al's observation by incompletely washing Fe-Pro-labeled MSCs before putting them into differentiation media and observed that extracellular iron complexes could be preventing chondrocytic differentiation (Figure 1D). The dose-dependent effect of Fe-PLL on chondrogenesis from MSCs observed by Bulte's group may be attributable to increasing extracellular complexes with increasing doses of the Fe-PLL label.
In conclusion, our results indicate that chondrocytic differentiation is not inhibited when MSCs are labeled using the Fe-Pro complex. We believe that the observations of Bulte and colleagues using Fe-PLL are interesting but most likely due to differences in labeling techniques and not simply to the presence of ferumoxides in cells. Our results underscore the superiority of the Fe-Pro approach for magnetic cell labeling.
Correspondence: Ali S. Arbab, Experimental Neuroimaging Section, Laboratory of Diagnostic Radiology Research, National Institutes of Health, Bldg 10, Rm no. B1N256, Bethesda, MD 20892; e-mail: saali@cc.nih.gov.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal