Abstract
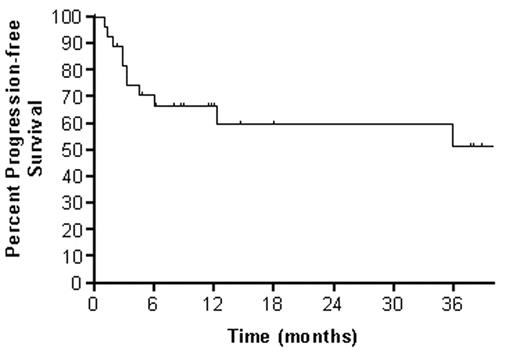
Twenty-eight patients with relapsed or refractory CD20+ NHL have been enrolled in an ongoing phase I trial of dose-escalated 90YZ followed by high-dose BEAM and autotransplant in which the 90YZ dose is patient-specific based on dosimetry. 90YZ doses are calculated to deliver cohort-defined radiation doses (100, 300, 500, ... cGy) to critical organs (liver, lung or kidney), with 3–6 patients per group. On D -22, rituximab (R) 250 mg/m2 is infused followed by the imaging dose of 111In Zevalin® (5 mCi). Imaging is performed immediately post-injection and at 4, 24, 72, and 144 hours; dosimetry is performed on D -15. On D -14, R 250 mg/m2 is administered followed immediately by 90YZ at the dose calculated to deliver the cohort-prescribed absorbed radiation dose to the critical organ. On D -6 through -1, patients receive high-dose BEAM. On D0, a minimum of 2.0 X 106 CD34+ cells/kg is infused and G-CSF 5 μg/kg SQ daily begun. The median age was 54 (range: 25–72) years. NHL histologic subtypes were as follows: mantle cell 5, diffuse aggressive 13, low grade 5, and transformed 5. Most had received 3 or more treatment regimens, including R. The toxicity profile was similar to that associated with high-dose BEAM and included a decrease in DLCO for most patients with one patient at the 500 cGy dose level experiencing a transient decline to below 50% of the predicted value corrected for hemoglobin. The most common grade III/IV toxicities were infection, fever, stomatitis, nausea, vomiting, diarrhea, hemorrhage, and edema. One patient experienced transient veno-occlusive disease at the 700 cGy dose level. Engraftment occurred at a median of 10 days (range:8–18) to granulocytes ≥ 500/μL, and 21 days (range:13–40days) to platelets ≥20,000/μL . With a median follow-up of one year, the 3 year overall and progression-free survivals are 60% and 50%, respectively.
90-Y Zevalin Dosing by Cohort (median; range)
| Cohort (cGy) . | Total Dose (mCi) . | mCi/kg . |
|---|---|---|
| 100 (n=3) | 5 (2–14) | .06(.05–.12) |
| 300 (n=7) | 22(14–57) | .25(.18–.63) |
| 500 (n=6) | 31(16–48) | .40(.14–.63) |
| 700 (n=6) | 37(26–55) | .38(.27–.73) |
| 900 (n=3) | 28(27–37) | .32(.27–.44) |
| 1100 (n=3) | 48(29–65) | .57(.50–.75) |
| Cohort (cGy) . | Total Dose (mCi) . | mCi/kg . |
|---|---|---|
| 100 (n=3) | 5 (2–14) | .06(.05–.12) |
| 300 (n=7) | 22(14–57) | .25(.18–.63) |
| 500 (n=6) | 31(16–48) | .40(.14–.63) |
| 700 (n=6) | 37(26–55) | .38(.27–.73) |
| 900 (n=3) | 28(27–37) | .32(.27–.44) |
| 1100 (n=3) | 48(29–65) | .57(.50–.75) |
The liver was the critical organ in nearly all cases. Patient-specific doses calculated to deliver a cohort-prescribed absorbed radiation dose to the critical organ were highly variable suggesting that dosing based on weight and not dosimetry is likely to result in a wide range of absorbed dose to critical organs. In the context of this study, 90YZ has been administered to eight patients at doses of .5 mCi/kg or greater. We conclude that with careful dosimetry, 90YZ doses higher than the conventional .4 mCi/kg may be safely combined with BEAM and autotransplant. Accrual continues at the 1300 cGy dose level.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal