Abstract
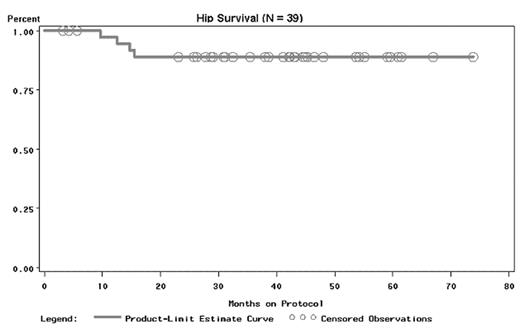
Avascular necrosis (AVN) of the hips affects half of sickle cell anemia (SCA) patients and can rapidly lead to total collapse of the hip. Treatment with supportive therapy alone is unsuccessful and results in half of all patients requiring hip surgery within 2 years of diagnosis. Prognosis in advanced cases is dismal, with surgery eventually required in all patients. Alternative therapies including aggressive physical therapy (PT) and hip coring decompression (HCD) may improve the natural history of AVN but neither have been evaluated prospectively in SCD. The goal of the prospective randomized National AVN Trial in SCA is to evaluate the safety of HCD and PT for AVN. 46 patients were randomized to have either HCD of their study hip followed by 6 weeks of an aggressive PT protocol, or to undergo only the PT protocol. Both treatment arms are balanced for Ficat stage and patient age. Outcome measures include clinical improvement as measured by the Children’s Hospital Oakland Hip Evaluation Scale (CHOHES), a 100 point validated scale that assesses hip function and pain associated with AVN in SCA. Additional measures include X-ray progression and the need for surgical intervention. This report presents hip survival rates in 39 study patients: 17 patients underwent HCD and 22 were treated in the PT arm. Average patient age was 26 (range 10 to 50). 67% had bilateral disease. The average follow-up is currently 40 months. In the survival graph, treatment failure was defined as additional hip surgery. 4 of the 39 study hips (10%) were treatment failures: 1/9 (11%) of Stage I, 3/19 (16%) of Stage II, and 0/11 (0%) of Stage III hips.
In addition to a low treatment failure rate, hips in each Ficat stage show evidence of clinical improvement. CHOHES scores improved 17 points at 1 year and 22 points at 2 years; even Stage III shows a 20 point improvement at 1 year and 21 points at 2 years. Our data indicates that both HCD and aggressive PT are beneficial in the treatment of AVN in SCA. PT may be effective in the treatment of even fairly advanced Stage III AVN. The AVN Trial in SCA is ongoing; patients will be followed for 3 to 5 years, and a comparison of HCD to aggressive PT will be made upon completion.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal