Abstract
Background: Dengue Fever (DF) illness caused by any of the four serotypes of dengue virus (flavivirus) is currently the most important mosquito borne viral disease in the tropical areas of the world. Dengue hemorrhagic fever (DHF) is a potentially lethal complication of DF.
An estimated 500,000 cases of DHF require hospitalisation each year, of which a very large proportion are children. The case fatality rate varies from 1 to 5% and can exceed 20% without proper treatment.
Thrombocytopenia in DHF: Thrombocytopenia is a constant finding in DHF/Dengue Shock Syndrome (DSS). The platelet count usually decreases to below 100,000 /mm3, 1–2 days before defervescence and remains low for 3–5 days in most cases. The cause of thrombocytopenia is not clearly understood and may be due to decreased platelet production, increased peripheral destruction, or both.
Method: We report a case series in 19 patients from different hospitals around Manila. Pediatric patients who fulfilled the WHO criteria of DHF: high fever, bleeding, positive tourniquet test, moderate to severe thrombocytopenia (<100,000 /mm3) and hemoconcentration (Hematocrit increase> 20%) were included in the case study after voluntary, written, informed consent was provided by parent or proxy.
All subjects meeting the eligibility criteria received a single dose of WinRho® SDF which was administered at a dose of 20 μg/kg over 15 minutes. WinRho® SDF was administered when the platelet count dropped below 100,000 /mm3. Platelet counts were measured every 24 hours.
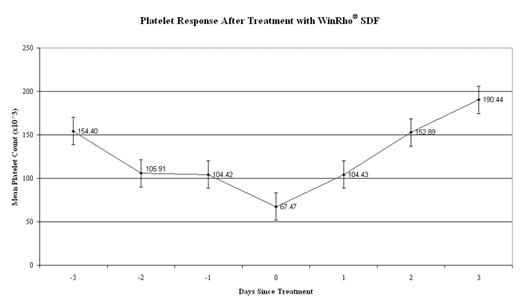
Findings: All 19 patients showed an increase in the platelet count after administration of WinRho® SDF. Figure 1 summarizes the average platelet count for the 19 patients before and after treatment with WinRho® SDF. Day 0 indicates the date of administration of WinRho® SDF. 48 hours after administration of WinRho® SDF, the platelet count returned to normal (> 150,000) in all patients. All 19 patients also showed clinical improvement and were discharged from hospital within 96 hours. There were no serious adverse events observed in any patient.
Change in average platelet count before and after treatment with WinRho® SDF.
Conclusion:
All 19 patients showed a rapid increase in the platelet count after administration of WinRho® SDF. 48 hours after administration of WinRho® SDF, the platelet count returned to normal in all patients. All the patients showed clinical improvement and were discharged from hospital within 96 hours.
WinRho® SDF is currently licensed to treat immune thrombocytopenia purpura (ITP) in pediatric or adult patients. Unlike other disease states which may produce ITP, the thrombocytopenia associated with DF has an acute onset and a high mortality rate if untreated. The response observed in these patients demonstrates that the thrombocytopenia associated with DF responds rapidly to treatment with WinRho® SDF.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal