Abstract
The mechanism by which ATO induces hematologic responses in patients with MDS is not well defined, and a better understanding might help to optimize the efficacy of ATO. Among other effects ATO induces apoptosis via inhibition of NFkB by interfering with IkB kinases. TNFa, a potent NFkB stimulus, is upregulated in MDS. ATO has been shown to prevent TNFa-induced NFkB activation, and to induce apoptosis in various neoplastic cells.
Methods: 1) We used eletrophoretic mobility shift assays (EMSA) to determine NFkB activation in marrow mononuclear cells (MMC) from MDS and normal marrows. 2) Apoptosis was determined by Annexin V and propidium iodide following treatments with ATO (2-200uM), Enbrel, a soluble TNF-receptor (5ug/ml), and TNFa (20ng/ml), alone or in combination, in marrows from healthy donors, patients with MDS, and in ML1 cells. 3) The effect of ATO and Enbrel on NFkB inhibition was evaluated by EMSA, and phosphorylation of IKBα was determined using western blots. 4) To define the role of TNFa receptor (R) 1 and 2 signaling for ATO activity, apoptosis and NFkB activation were determined while blocking TNFR 1 and 2 respectively. 5) Levels of FLIP, an NFkB-dependent gene that modulates death signaling, were determined in response to treatment using real-time PCR. 6) Apoptosis in response to ATO/TNF/Enbrel was also determined in ML1 cells transduced to overexpress FLIP long.
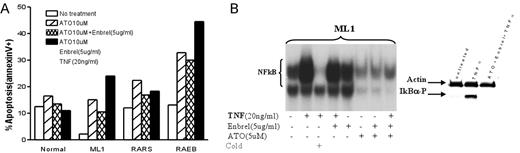
Results: MMC from 7 of 8 patients, and CD34+ cells from all patients with RAEB tested showed high constitutive NFkB activation (compared to RA/RARS marrows, which showed low activity comparable to normal marrows). Treatment with ATO induced similar patterns of NFκB inhibition in normal, ML1 and primary MDS (RAEB) cells, even in the presence of exogenous TNFa. Interestingly, Enbrel failed to prevent TNF-induced NFkB activation, and did not enhance ATO-induced NFkB inhibition (Figure B: EMSA;Western blot for P-IKB). However, there were distinct patterns of apoptosis: ATO induced apoptosis in ML1 and in primary MDS cells to a greater extent than in normal controls. While addition of (exogenous) TNF to ATO treated cells enhanced apoptosis in ML1 and RAEB cells (less in RA marrow), apoptosis was decreased in normal cells (Figure A). TNF-a induced NFkB activation in ML1 and primary MDS cells was mediated through TNFR 1, and blocking of TNFR 1 (but not TNFR2) resulted in a significant reduction of apoptosis in ML1 cells in response to ATO + TNFa, suggesting a role of TNF-α/TNFR1 in ATO-induced apoptosis. FLIP levels were down regulated in ML1 and primary MDS cells treated with ATO (or ATO plus Enbrel) in the presence of exogenous TNF; overexpression of FLIPlong in ML1 cells failed to significantly reduce ATO induced apoptosis.
Conclusions: These studies show complex interactions of TNFa and ATO in MDS cells. ATO and TNF-a (TNFR1) signals may interact at the level of IkB, but the exact mechanism remains to be determined. The data obtained with Enbrel suggest that additional pathways are involved. The data also suggest that combinations of ATO and Enbrel may be exploited therapeutically in patients with MDS (showing up-regulation TNFa) by eliminating primarily clonal cells while protecting normal hemopoiesis.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal