Abstract
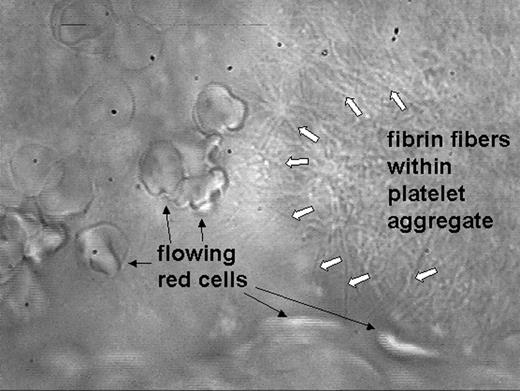
Lipid-rich atherosclerotic plaques are vulnerable, and upon disruption trigger intraarterial thrombus formation. Tissue factor activating blood coagulation is viewed as the major prothrombotic stimulus within the plaque. We isolated lipid-rich atheromatous plaques from 50 patients with carotid artery stenosis and identified morphologically diverse collagenous structures within in the plaques. They stimulated platelet adhesion, dense granule secretion and aggregation, and triggered thrombus formation in hirudin-anticoagulated blood under arterial flow conditions. Even in fully anticoagulated flowing blood, i.e. in the absence of tissue factor-mediated coagulation, plaques were able to activate platelets. Thrombus formation was more rapid and stable when blood was anticoagulated with a low concentration of heparin, but, although fibrin was detectable within the thrombus, the initial step was always single platelet adhesion and not fibrin formation. In contrast, absence or inhibition of the platelet collagen receptor glycoprotein VI prevented platelet adhesion to atheromatous plaques and thrombus formation. We thus identified platelet glycoprotein VI as being essential and sufficient to mediate plaque-induced thrombus formation. Our study suggests a novel anti-thrombotic strategy to prevent and treat atherothrombosis in patients with vulnerable atherosclerotic plaques.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal