Abstract
In 2001, the GIMEMA Group started a phase II study to evaluate Imatinib both in adult (study A, 18–60 years) and elderly (Study B, >61 years) patients with Philadelphia positive (Ph+) acute lymphoblastic leukemia (ALL).
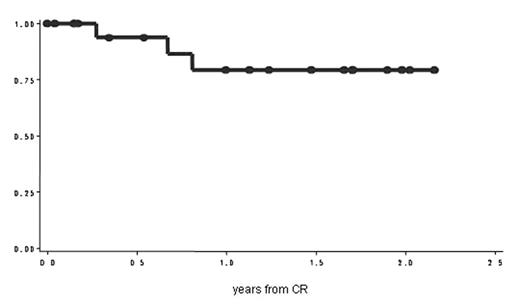
In study A, adult patients with Ph+ ALL received the standard GIMEMA induction (VCR, DNR, PDN, L-ASP) and consolidation (MTZ and high-dose Ara-C) treatments, without imatinib. All patients in hematological complete remission (HCR) after the consolidation, either with or withouth a donor, received imatinib p.o. at the dosage of 800 mg/day for 6 months. After completing the 6-months therapy, in the absence of safety concerns, if the investigator considered the patient eligible for treatment, the therapy with imatinib was continued. A total of 22 patients have been enrolled [7 in molecular complete remission (MCR) and 15 still BCR/ABL+]: 18 are still in HCR after a median follow up of 20 months (2 – 26). In particular, all the 7 patients PCR- when started imatinib are still in HCR (6 PCR- and 1 becoming PCR+), after a median follow-up of 19 months (7.6 – 24) and eleven of the 15 PCR+ patients are in CHR after a median follow-up of 19 months (1.2 – 26). Of the remaining 4 PCR+ patients, 1 died in CR after an alloSCT and 3 patients relapsed at 4, 9 and 11 months, respectively. The probability of disease-free survival at 2 years is 79% (C.I. 95%: 58,4 – 100,3).
In study B, planned for elderly patients usually not eligible for intensive treatments, the induction therapy consisted of imatinib 800 mg/day p.o. associated to prednisone 40 mg/sqm/day for 30 days, withouth any other chemotherapy. Up to date only 12 patients have been enrolled. Median age was 67.5 yrs (61–78), 9 were females. Eleven (92%) obtained a CR with the prednisone-Imatinib association induction treatment, 1 patient discontinued the treatment for toxicity. The post-remissional treatment mainly consisted of imatinib alone: 8 patients are still in CR, after a median follow up of 7 months (4 – 15), 2 relapsed at 4 and 4 months and 1 died in CR due to a 2nd neoplasia. In conclusion, these studies, planned 3 years ago to verify the feasibility and toxicity of imatinib in adult and elderly Ph+ ALL patients, clearly suggest a strong activity of Imatinib also in this disease, not only giving the possibility of inducing a CR as monotherapy, but even of maintaining long-lasting hematological and molecular CR withouth allogeneic stem cell transplantation. Further studies are mandatory to finally identify the best way to integrate imatinib in the entire treatment strategy of this disease.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal