Abstract
BACKGROUND: The Clot Formation and Lysis (CloFAL) assay, modified from prior global assay methods by He et al., 1999, and Smith et al., 2003, involves activation of coagulation and fibrinolysis in platelet-poor plasma via addition of a physiologic reactant solution in a multi-well microassay plate. Continuous spectropohotometric data analysis allows measurement of kinetic absorbance changes of the plasma sample over three hours, which yields a unique clot formation and lysis curve. Using parameters of the curve, coagulation and fibrinolytic indices (CI and FI) are calculated relative to a simultaneously run pooled normal plasma standard.
METHODS: Platelet-poor plasma obtained from pregnant women at term (n=24), neonatal cord blood (n=29), and healthy children (n=22) were analyzed using the CloFAL assay. Healthy adult (n=22) plasma samples, as well as those of individuals with factor deficiencies, were obtained commercially. Fibrinolytic alterations in vitro were also investigated. Intra-assay coefficients of variation (CVs) for normal controls ranged from 3–12% for all assay parameters, with inter-assay CVs of 5–15%.
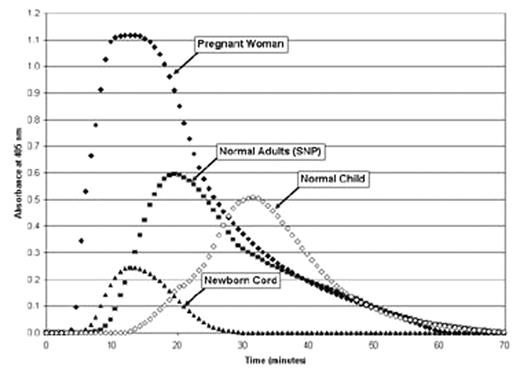
RESULTS: Representative CloFAL curves for healthy adults and children, pregnant women, and newborn infants are shown in Figure 1. Coagulation potential (measured by median CI) was significantly increased, while fibrinolytic capacity (measured by median FI) was markedly decreased, in pregnant women as compared to healthy adults (CI: 239% vs. 115%, FI: 59% vs. 95%; P<0.001 for each). By contrast, CI was decreased, and FI notably increased, in neonatal cords versus children, although the former comparison did not achieve statistical significance (CI: 58% vs. 69%, P=0.09; FI: 210% vs. 142%, P<0.001). The influence of deficiencies of coagulation factors and fibrinolytic regulators upon CloFAL parameters was also investigated. The greatest impact upon CI occurred with severe deficiency of fibrinogen or factors II, V, VII, VIII, IX, or X. Furthermore, CI was sensitive to deficiencies of factor VIII and fibrinogen in a concentration-dependent manner. In addition, FI was increased by PAI-1 deficiency and by inhibition of thrombin-activatable fibrinolysis inhibitor (TAFI) activation, and was zero in the setting of aminocaproic acid treatment. Further studies revealed that the influence of heparin concentrations of up to 2 U/mL in plasma was completely reversible by heparinase treatment of samples prior to assay.
CONCLUSION: These results indicate that the CLoFAL assay is reproducible and analytically sensitive to known physiologic and pathologic alterations in coagulation and fibrinolysis. The application of this global assay to patients with a variety of disorders of thrombosis and hemostasis is currently ongoing.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal