Abstract
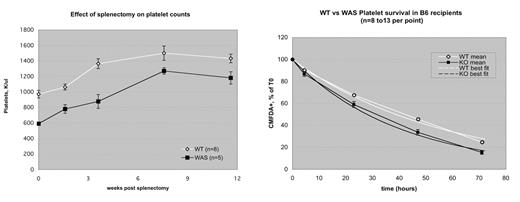
The Wiskott-Aldrich Syndrome (WAS) is an X-linked recessive condition manifesting as variable degrees of immunodeficiency and thrombocytopenia. The strong clinical improvement in platelet count seen in WAS patients after splenectomy suggests that increased platelet consumption is a major contributor to the thrombocytopenia. Here we show that splenectomy improves but does not normalize platelet count in a murine (C57Bl/6) model of WAS, suggesting (a) that murine WAS parallels clinical WAS in this regard, and (b) that either increased extrasplenic consumption or diminished platelet production (or both) contributes to the thrombocytopenia. We quantified platelet turnover by labeling WAS and WT platelets with CMFDA and following their disappearance after injection into WT recipients. Platelet loss rates in this model fit best to a simple exponential decay curve. We found that WAS platelets turn over significantly faster than WT (survival times: 56 hr (WT) vs 40 hr (WAS), p <.01). By comparison, we have observed a 42% reduction in platelet count in WAS mice (508K/ul, vs 881K/ul for age matched WT littermates, p<.01). WAS mice also show increased splenic extramedullary hematopoiesis, and increased megakaryocyte counts in bone marrow as assayed by flow cytometry. These results suggest that both increased platelet consumption and reduced thrombopoiesis contribute to the thrombocytopenia of WAS in this system, while megakaryopoiesis may not be inhibited. To determine the site of increased consumption of WAS platelets, we repeated the turnover studies in splenectomized C57Bl/6 mice. The results fit a two-hit gamma function, with estimated lifespans of 65 hr (WT) vs 53 hr (WAS)(p <. 01), indicating that splenectomy improved but did not normalize turnover of WAS platelets. The data suggests that the spleen is not the site of increased platelet consumption in WAS mice. To further delineate the role played by splenic platelet consumption in the thrombocytopenia of WAS, we crossed C57Bl/6 WAS mice for three generations onto the reportedly hypersplenic mouse strain C3H. Suprisingly, WAS (C3H) males showed no significant thrombocytopenia. Nonetheless, we found that WAS (C3H) platelets turn over more rapidly than WT platelets in C3H mice (estimated lifespans 61 hr (WT) vs 42 hr (WAS), p <.01), despite the fact that in comparison to C57Bl/6J (but not C57Bl/6Nhsd) mice, C3H mice do not in fact have significantly larger spleens. These results suggest that WAS (C3H) mice are able to compensate for increased platelet turnover rates by increasing platelet production appropriately, and imply that WAS (C57Bl/6) mice are not. Taken together, these studies suggest that the thrombocytopenia of murine WAS (C57Bl/6) is due to both increased extrasplenic platelet consumption and deficient platelet production. The inter-strain variation in WAS phenotype we report here reproduces the marked inter- and intra-kindred phenotypic variation seen in clinical WAS.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal