Abstract
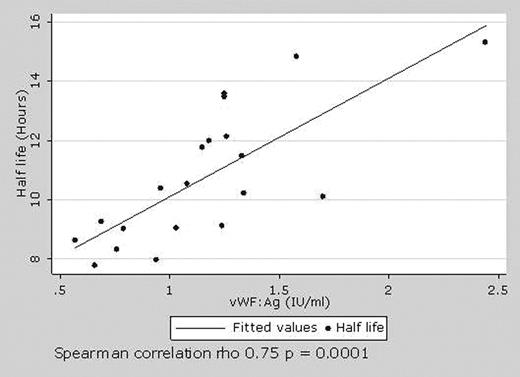
Understanding the pharmacokinetics of factor VIII (FVIII) is critical to maximise the clinical benefit of clotting factor replacement therapy. Little is known about the pharmacokinetics of recombinant FVIII (rFVIII) in children and current dosing regimes, largely based on adult pharmacokinetic data, may be inappropriate. Further, there is limited information about factors determing the pharmacokinetic variability of FVIII between children. We performed a pharmacokinetic study in 20 children with severe haemophilia A (mean age 12.8 years) following a bolus of 50 U/kg of rFVIII (Kogenate-FS, Bayer Health Care). FVIII:C levels were assessed at 10 time points to determine the effect of patient size, age and pre infusion vWF:Ag level on FVIII pharmacokinetics. The patients had no history of inhibitors to FVIII (>0.5 BU). The mean incremental FVIII recovery was 1.87 (U/ml)/(U/kg) (range 1.25– 2.76) which is significantly lower than adult pharmacokinetic studies. (Table 1) FVIII recovery showed a greater correlation with body surface area (BSA, Spearman correlation p = 0.037) than to weight (p = 0.048) suggesting dosing based on BSA may lead to more predictable FVIII recovery in children. The mean half-life of FVIII was 10.7 hours (range 7.8 to 15.3) and is reduced in comparison to adult studies. FVIII half-life showed a positive correlation with levels of vWF:Ag (p = 0.0001) (Figure 1) and half life was reduced in six patients with FVIII inhibitor titres between 0.05–0.15 Nijmegen modified BU (p = 0.06). The correlation of rFVIII half-life with vWF:Ag levels may provide a basis for pharmacological augmentation of vWF:Ag levels to prolong rFVIII half life and may be of major benefit in countries with a limited supply rFVIII. The finding that FVIII half-life is reduced in patients with inhibitor titres that have previously been regarded as clinically insignificant suggests that standards and methodologies for the measurement of inhibitors should be re-evaluated. This study shows that the pharmacokinetics of rFVIII in children are unique and that BSA, pre infusion levels of vWF and the presence of low, previously considered non-clinically significant inhibitors may affect the pharmacokinetics of Kogenate-FS in children. These relationships, and the potential to improve dosing schedules for FVIII in children, need further study.
Comparison of results from our centre with other published pharmacokinetic studies of rFVIII.
| Reference . | Number of patients . | Mean age (range) (Years) . | Mean incremental recovery (U/ml)/(U/kg) . | Mean half life (hours) . | Mean clearance (ml/hr/kg) . | Mean volume of distribution steady state (ml/kg) . |
|---|---|---|---|---|---|---|
| ¥ Results represent pooled data of one rFVIII product prepared at two different sites assessed by one stage assay. Only week 1 of serial pharmacokinetic studies is presented. | ||||||
| Barnes (2004) | 20 | 12.8 (4.4 – 18.1) | 1.9 | 10.7 | 4.1 | 59.2 |
| Lee (1999)¥ | 30 | N/A | 2.5 | 12.7 | 2.0 | 62.6 |
| Fijnvandraat (1997) | 12 | 34.0 (17 – 64) | 2.4 | 11.3 | 3.2 | 44.8 |
| Harrison (1991) | 14 | 34.1 (23 – 72) | 2.8 | 16.5 | 2.4 | 51.2 |
| Schwartz (1990) | 17 | N/A | 2.7 | 15.8 | 2.5 | 50.5 |
| Reference . | Number of patients . | Mean age (range) (Years) . | Mean incremental recovery (U/ml)/(U/kg) . | Mean half life (hours) . | Mean clearance (ml/hr/kg) . | Mean volume of distribution steady state (ml/kg) . |
|---|---|---|---|---|---|---|
| ¥ Results represent pooled data of one rFVIII product prepared at two different sites assessed by one stage assay. Only week 1 of serial pharmacokinetic studies is presented. | ||||||
| Barnes (2004) | 20 | 12.8 (4.4 – 18.1) | 1.9 | 10.7 | 4.1 | 59.2 |
| Lee (1999)¥ | 30 | N/A | 2.5 | 12.7 | 2.0 | 62.6 |
| Fijnvandraat (1997) | 12 | 34.0 (17 – 64) | 2.4 | 11.3 | 3.2 | 44.8 |
| Harrison (1991) | 14 | 34.1 (23 – 72) | 2.8 | 16.5 | 2.4 | 51.2 |
| Schwartz (1990) | 17 | N/A | 2.7 | 15.8 | 2.5 | 50.5 |
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal