Abstract
Introduction: Stability of a FVIII preparation in mini-pumps is an important consideration for its use in a continuous infusion manner. Our goal was to evaluate the stability of a specific recombinant FVIII (Kogenate®FS; KOGENATE®Bayer) in two commonly used infusion mini-pumps, with and without anticoagulant additives that may be used in clinical practice to prevent local thrombophlebitis.
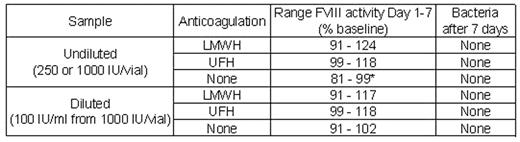
Methods: Kogenate® FS (250 IU/vial and 1000 IU/vial) was reconstituted using aseptic technique according to the manufacturer’s instructions with normal water for injection (100 IU/ml and 400 IU/ml, respectively); an additional dilution of 100 IU/ml was made from the 1000 IU/vial size. Reconstituted material was spiked with unfractionated heparin (UFH) or low molecular weight heparin (LMWH; Enoxaparin/Clexane) to a final concentration ~5 U/ml, or left untreated and then transferred to reservoirs of 2 mini-pumps (Walkmed, Medfusion Inc., Duluth, GA and CADD, Deltec, Inc., St. Paul, MN). All bags were stored in a light-protected environment at room temperature (22°C) for 7 days. Samples were drawn at baseline (immediately post reconstitution), 3h, 6h, 12h, 24h, and days 2–7, frozen at −30°C, and assayed for FVIII activity by one-stage assay. On Day 7, the residual volume of the mini-pump containers was cultured for bacteria.
Results: All samples except one* had FVIII activity >90% of baseline.
Conclusions: Kogenate® FS appears to retain excellent stability at room temperature during 7 days in both CADD and Walkmed infusion pumps with and without addition of UFH or LMWFH. No bacterial growth was observed. These results indicate that Kogenate® FS may be useful in continuous infusion therapy.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal