Abstract
We report the use of the in vitro cobblestone area-forming cell (CAFC) assay to investigate the hematopoietic progenitor cell (HPC) profile of pre-selection, post-selection positive (CD133+) and post-selection negative (CD133−) fractions of cord blood (CB). The CAFC assay provides a qualitative measure of the HPC contained within a given cell population at a given time-point. Briefly, CAFC give rise to cobblestone areas (CA) beneath a stromal cell layer that can be visualized by light microscopy at weekly intervals and their frequencies determined by the use of a limiting dilution strategy. CA that persist in culture for a relatively long period of time are considered to be derived from more primitive, potentially higher quality HPC than those that persist in culture for a shorter period of time. Using this approach, changes within the HPC compartment within a specific cell population can be quantified.
Methods: Three frozen CB units were thawed, washed and pre-selection samples removed for CAFC and flow cytometric (CD133+) analysis. Samples were then subjected to CD133+ selection using the Miltenyi MidiMACS device and positive and negative fractions collected for CAFC and CD133+ analysis. Limiting dilution within the CAFC assay was performed in flat-bottomed 96-well plates, over 12, 2.5-fold serial dilutions with 10 wells per dilution. The murine FBMD-1 cell line provided the stromal component of the assay. Culture was performed at 33°C in Iscove’s Modified Dulbecco’s Medium containing 10% each of fetal bovine and horse serum and supplemented with 10ng/ml recombinant human interleukin 3 (R & D Systems) and 20ng/ml recombinant human granulocyte colony-stimulating factor (Neupogen, Amgen). Cultures were assayed and medium refreshed at weekly intervals.
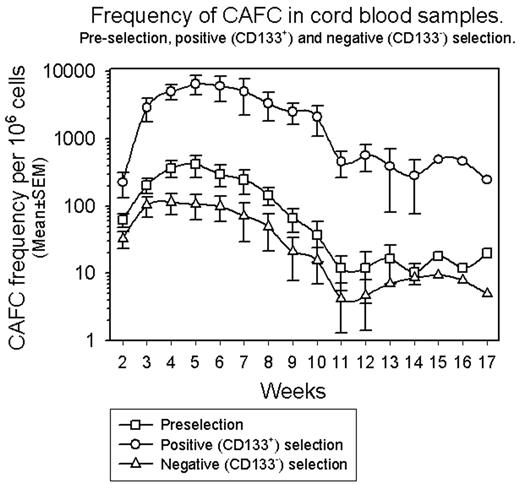
Results: Purity of CD133+ cells in pre-selection CB samples ranged from 1-3% and reached a maximum of 98% following positive selection. Analysis of negative fractions revealed a CD133+ cell purity of 1–2%. As shown in the figure, the frequency of CAFC in the pre-selection fraction reached a maximum at week 5 (~400/106 cells) and by week 10 of culture was reducing (~40/106 cells). The frequency of CAFC in the positive fraction also reached a maximum at week 5 (~6500/106 cells) however, by week 10 of culture the frequency of CAFC was still ~2000/106 cells. The pattern of CAFC in the negative fraction closely mirrored that observed in the pre-selection sample although with reduced frequencies of CAFC at each time-point.
Conclusion: CAFC activity present in CB is concentrated in the CD133+ fraction rather than the CD133− fraction and, in particular, CAFC that persist longer in culture (potentially higher quality HPC) are also concentrated in the CD133+ fraction. The CAFC assay is currently being used to track changes that occur within the HPC compartment with ex vivo expansion of isolated CD133+ CB cells to determine whether any one population is preferentially expanded at the expense of any other. This will address concerns that ex vivo expansion techniques expand more mature HPC at the expense of more primitive HPC thereby potentially compromising stem cell reserve.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal