Abstract
BACKGROUND: When neutropenia without infection is encountered while treating Hodgkin’s Lymphoma (HL) with ABVD chemotherapy clinicians may choose to delay or reduce the chemotherapy dose or maintain the dose intensity and schedule with or without neutrophil growth factor support out of concern for neutropenic complications. We sought to determine the frequency of neutropenia and febrile neutropenia (FN) during ABVD chemotherapy.
METHODS: We examined the medical records of all patients seen at Univ. of Iowa diagnosed with HL between 1/1/1990 and 12/31/2002 treated with ABVD chemotherapy. We reviewed the charts with complete chemotherapy dosing records to determine the incidence of neutropenia, dose reduction, dose delay and chart record of febrile neutropenia (FN).
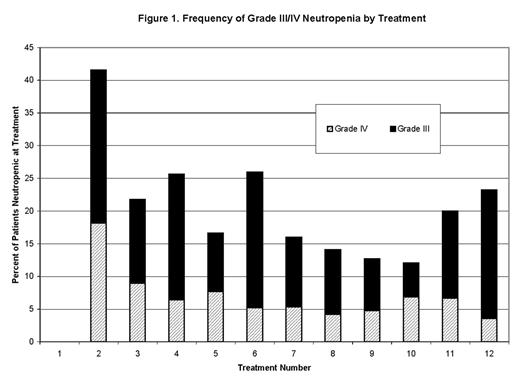
RESULTS: 218 patients were seen at UIHC with the diagnosis of HL. 104 patients were treated with ABVD chemotherapy. Adequate dosing data was available for 82 of these patients (894 treatments). On scheduled day of treatment (Day 1 and 15 of each cycle) at least Grade III neutropenia (ANC<1000) was present in 51 pts and 165 (18.45%) treatments. Grade IV (ANC<500) neutropenia was present in 28 pts and 56 (6.26%) treatments. Grade III/IV neutropenia was most frequent at treatment 2 (C1, D15) (n=32). Figure 1 illustrates the frequency of Grade III/IV neutropenia on day 1 of treatment. Dose modification (DM) defined as dose delay of > 4 days and/or dose reduction to < 80% of original doxorubicin dose following neutropenia occurred at only 10 of 165 treatments. No episodes of FN developed in this cohort. 155 treatments at the time of neutropenia were administered without dose reduction or dose delay. Growth factor support was co-administered in 55 (36.77%) of these treatments and the remaining treatments at the time of neutropenia (73.23%, n=100) were administered without growth factor support. One episode of FN developed in each of these subsets. No FN was observed following the 56 treatments administered with an ANC < 500, including 54 managed without DM (32 with GCSF support and 22 without). 672 treatments were administered with ANC ≥ 1000 resulting in 6 episodes of FN. DM was observed in 78 of these 672 treatments with 3 subsequent episodes of FN. 57 treatments (4 with DM, 23 with GCSF, 20 without GCSF, 14 unknown GCSF) had no available ANC data and were associated with one episode of FN. FN developed a total of 9 times in 8 of 82 patients over 894 treatments.
CONCLUSION: Among HL patients treated with ABVD chemotherapy we found a high frequency of neutropenia - most commonly following treatment 1. We found a very low incidence of FN that was independent of neutropenia at the time of chemotherapy administration. Dose modification for neutropenia without infection at the time of ABVD administration is not required to reduce risk of FN, and should be avoided in settings where maintaining dose intensity is considered valuable.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal