Abstract
SLP-76(SH2 domain-containing leukocyte protein of 76 kD) is a hematopoietic adapter protein that is expressed in myeloid and T cells. SLP-76 is a substrate for tyrosine kinase in the src and syk family activation pathway required for T-cell receptor-mediated signaling. Cross-linking of the human FcgammaRIIa1 (CD32) in myeloid cells which contains an immune receptor tyrosine-based activation motif (ITAM) causes phosphorylation of SLP-76. Mice deficient in SLP76 develop fetal hemorrhage along with failure of T cell development and perinatal mortality. We have found that K562 cells express SLP-76. We hypothesized that SLP-76 or associated proteins may be substrates of Bcr-Abl in the K562 cell line and thus promote survival signals.
Materials and Methods: The K-562 cell line was obtained from the American collection of Cell Cultures. SLP deficient (−/−) KO mice were obtained from Dr. James Clements (Roswell Park Cancer Institute). Antibodies used include Sheep polyclonal IgG Anti-Human SLP 76, Peroxidase-conjugated Affinipure Donkey Anti-Sheep IgG at 0.8mg/ml, Mouse monoclonal IgG Anti-Phosphotyrosine, and Polyclonal goat Anti-mouse.
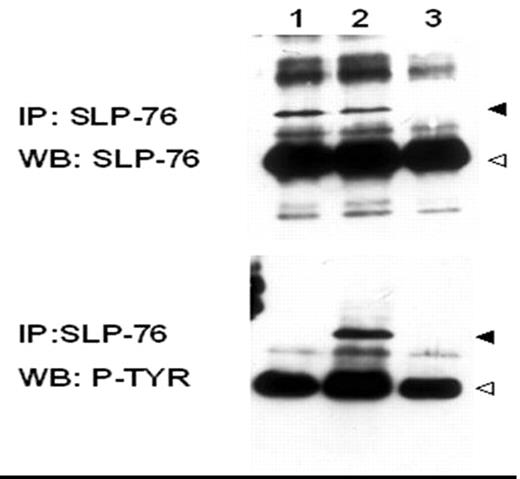
Immunoprecipitation and Immunoblot: Cells were either untreated or stimulated with purvanidate (phosphatase inhibitors). Purvanidate treated cells were used as a positive control. Spleenocytes isolated from a SLP-76 deficient mouse (KO) were used as negative control. Lysates were then subject to standard immunoprecipitation for SLP-76 followed by immunoblotting with a SLP-76 specific antibody. The denatured samples were then resolved by SDS-PAGE.
Results: SLP-76 is expressed in untreated and treated K562 with purvanidate and not detectable from lysates derived from SLP-76 KO spleenocytes. To assess the phosphorylation status of SLP-76 and any co-associated proteins in K562 cells, the SLP-76 blot was stripped and then immunoblotted for total phosphotyrosine content. SLP-76 does not appear to be constitutively phosphorylated in the K562 cells. However, significant tyrosine phosphorylation of SLP-76 was readily detectable in the purvanidate treated K562 cells.
Conclusion: The current studies reveal that although SLP-76 is indeed found in the K562 cell line expressing the Bcr-Abl oncogene. Somewhat surprisingly, despite the constitutive activation of the Bcr-Abl tyrosine kinase in K562 cells, we detected no obvious phosphoproteins co-precipitating with SLP-76 in the absence of purvanidate stimulation. Together, the lack of SLP-76 tyrosine phosphorylation and the lack of co-associated proteins in K562 cells suggest that SLP-76 is not a major player in the signal transduction pathways emanating from Bcr-Abl. However, a more stringent confirmation of this conclusion would require inhibiting SLP-76 expression in K562 cells and than assessing the growth and survival characteristics.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal