Abstract
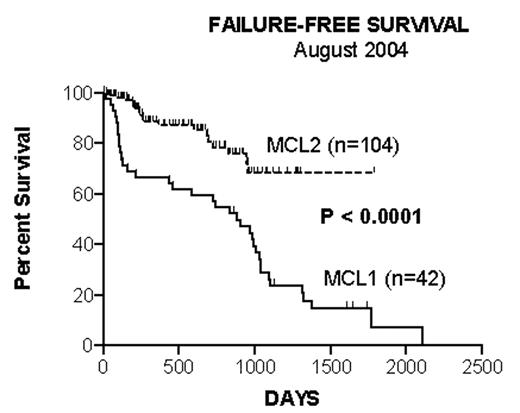
Methods: In the Nordic Lymphoma Group MCL Project newly diagnosed stage II-IV MCL patients < 66 years receive induction treatment and peripheral-blood stem-cell (PBSC) harvest, followed by BEAM/BEAC with PBSC support. In the MCL1 Protocol, the induction was maxi-CHOP x 3 (CTX 1200 mg/m2, doxorubicin 75 mg/m2, VCR 2mg D1, prednisolone 100 mg D1-5). Stem-cells were mobilised by maxi-CHOP + G-CSF. Because of the high failure rate in MCL1 (Figure 1) the MCL2 protocol was activated adding 2 series of high-dose Ara-C (3g/m2 BID D1-2) and 2 standard doses of rituximab (R) (375 mg/m2) to the induction program. Stem cells were mobilised by Ara-C + G-CSF + rituximab D1 + D9 for in-vivo purging. In both protocols patient-specific molecular markers were sought established before treatment start, and stem-cells and follow-up blood and bone-marrow samples assessed for MCL by PCR or flow cytometry.
Results: Table 1
Compared Results of the Nordic Lymphoma Group MCL1 and MCL2 Protocols
| NORDIC MCL PROTOCOL # . | MCL1 (1997–2000) . | MCL2 (2000-) . | P value . |
|---|---|---|---|
| Supported by the Nordic Cancer Union | |||
| No. of Pts included/eval. for response | 42/42 | 120/88 | |
| Response pretransplant | 31/42 | 86/88 | 0.002 |
| CR/resp pretransplant | 11/31 | 51/86 | 0.04 |
| No. transplanted | 27 | 86 | |
| Eval. for response posttransplant | 27 | 82 | |
| CR/Response posttransplant | 25/27 | 76/82 | NS |
| Molecular response posttransplant | 5/13 | 38/42 | 0.0003 |
| PCR-neg. stem-cell products (of tested) | 2/16 | 22/25 | 0.00005 |
| 3-year failure-free survival (104 pts eval.) | 24% | 68% | 0.0001 |
| 3-year relapse-free survival (RFS) | 45% | 76% | 0.04 |
| 3-year molecular RFS survival | ND | 67% | |
| 3-year overall survival | 60% | 85% | 0.02 |
| NORDIC MCL PROTOCOL # . | MCL1 (1997–2000) . | MCL2 (2000-) . | P value . |
|---|---|---|---|
| Supported by the Nordic Cancer Union | |||
| No. of Pts included/eval. for response | 42/42 | 120/88 | |
| Response pretransplant | 31/42 | 86/88 | 0.002 |
| CR/resp pretransplant | 11/31 | 51/86 | 0.04 |
| No. transplanted | 27 | 86 | |
| Eval. for response posttransplant | 27 | 82 | |
| CR/Response posttransplant | 25/27 | 76/82 | NS |
| Molecular response posttransplant | 5/13 | 38/42 | 0.0003 |
| PCR-neg. stem-cell products (of tested) | 2/16 | 22/25 | 0.00005 |
| 3-year failure-free survival (104 pts eval.) | 24% | 68% | 0.0001 |
| 3-year relapse-free survival (RFS) | 45% | 76% | 0.04 |
| 3-year molecular RFS survival | ND | 67% | |
| 3-year overall survival | 60% | 85% | 0.02 |
Posttransplant maintenance treatment: In MCL2, patients in clinical stable response but molecular relapse are offered R 4 std. doses. So far, isolated molecular relapse occurred in 11 pts. of whom 10 received R: Four became PCR neg., one did not respond and later relapsed, five are not yet evaluable. Conclusion: By comparing MCL1 results with preliminary results of the still ongoing MCL2 study we conclude that the addition of HD Ara-C and R to the induction treatment significantly increases the
• clinical response rate pretransplant
• molecular response rate
• No. of tumor-cell free grafts
• failure-free, relapse-free and overall survival
Rituximab maintenance treatment can induce 2nd molecular remission, the clinical significance hereof still unknown.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal