Comment on Zennadi et al, page 3774
Epinephrine activates erythroid ICAM-4 and enhances sickle cell adhesion to endothelium via ICAM-4–αVβ3 interactions, supporting the hypothesis that physiologic stress may promote vaso-occlusion in sickle cell disease.
In addition to sickling behavior, sickle erythrocytes (SS RBCs) exhibit an increased propensity of adhesion to vascular endothelium. SS RBC adhesion has been considered an important factor in the initiation of vasoocclusive episodes in sickle cell disease. In microcirculatory networks, SS RBC–endothelial interaction can prolong red cell residence times, induce hypoxia, and initiate sickling. Several adhesive mechanisms involving multiple adhesion molecules (RBC receptor–adhesive protein–endothelial receptor) have been defined. Among the putative endothelial receptors, αVβ3 integrin can interact with several adhesive bridging proteins (ie, thrombospondin [TSP], von Willebrand factor, and possibly plasma laminin), and antibodies directed against this integrin can dramatically inhibit SS RBC adhesion in an ex vivo microvascular preparation.1 Because of its ability to bind to several adhesive proteins, αVβ3 is considered a potential target in designing antiadhesive therapy.2 FIG1
Epinephrine modulates SS RBC adhesion to ECs. See the complete figure in the article beginning on page 3774.
Epinephrine modulates SS RBC adhesion to ECs. See the complete figure in the article beginning on page 3774.
Recent studies have shown that signaling mechanisms may play an important role in SS RBC adhesion via activation of RBC adhesion receptors. For example, integrin-associated protein (IAP; CD47) on SS RBCs can induce signal transduction to activate RBC adhesion receptors for TSP.3 In this issue, Zennadi and colleagues have identified an adhesion receptor, intercellular adhesion molecule-4 (ICAM-4; [Landsteiner-Weiner (LW) protein or CD242]), on SS RBCs, which can mediate adhesion to endothelium through at least one direct ligand, αVβ3 integrin. Importantly, activation of ICAM-4 by physiologic stress mediator epinephrine significantly enhances SS RBC adhesion. The epinephrine-induced activation of ICAM-4 involves a cyclic adenosine monophosphate (cAMP)–dependent signaling pathway, probably via stimulation of β-adrenergic receptors. Previously, this group of investigators has shown a role of cAMP-dependent signaling pathways in SS RBC adhesion wherein epinephrine-induced activation of basal cell adhesion molecule/Lutheran (B-CAM/Lu) on SS RBCs caused increased adhesion to immobilized laminin.4
Although epinephrine can activate both B-CAM/Lu and ICAM-4 in SS RBCs, the latter is specifically involved in adhesion to endothelial cells, as antibodies to B-CAM/Lu and laminin are ineffective in the ICAM-4–mediated interaction. Nevertheless, the fact that epinephrine enhances sickle cell adhesion toboththeextracellularmatrix(via B-CAM/LU and laminin) and endothelium (via ICAM-4 and αVβ3) supports the association between physiologic stress and vaso-occlusion in sickle cell disease.
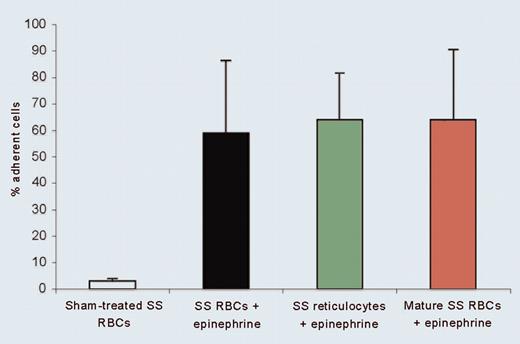
ICAM-4 can be activated by epinephrine, at least partially via a cAMP–protein kinase A (PKA)–dependent signaling pathway, resulting in increased adhesion of SS RBCs. The authors show that raising intracellular cAMP results in greatly increased adhesion of SS RBCs but not of normal RBCs. Furthermore, ICAM-4 on SS RBCs, but not on normal RBCs, undergoes PKA-dependent serine phosphorylation upon activation. Accordingly, epinephrine causes increased adhesion of SS cells but not of normal RBCs. It is important to note that both SS reticulocytes and mature SS RBCs, expressing similar levels of ICAM-4, can adhere to endothelium with almost the same avidity when stimulated with epinephrine. These are important observations in that they present a novel mechanistic basis for adhesion of both SS reticulocytes and mature SS RBCs, a phenomenon that has been repeatedly observed in many previous studies.
Among the ICAM family of adhesive proteins, ICAM-4 is unique in its expression on erythroid cells. ICAM-4 binds a diverse array of integrins including several αV integrins, β2 integrins, and α4β1 integrin, suggesting a multiple function for this adhesion molecule.5 Using a panel of monoclonal antibodies and specific inhibitors, the authors have identified endothelial αVβ3 integrin as the major counterreceptor for ICAM-4. These studies enhance our understanding of the mechanism of SS RBC adhesion to endothelium and provide a new approach to abrogate SS RBC–endothelial interaction by identifying new receptor ligand targets.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal