Platelet aggregation is initiated by the release of mediators as adenosine diphosphate (ADP) stored in platelet granules. Possible candidates for transport proteins mediating accumulation of these mediators in granules include multidrug resistance protein 4 (MRP4, ABCC4), a transport pump for cyclic nucleotides and nucleotide analogs. We investigated the expression of MRP4 in human platelets by immunoblotting, detecting a strong signal at 170 kDa. Immunofluorescence microscopy using 2 MRP4-specific antibodies revealed staining mainly in intracellular structures, which largely colocalized with the accumulation of mepacrine as marker for delta-granules and to a lower extent at the plasma membrane. Furthermore, an altered distribution of MRP4 was observed in platelets from a patient with Hermansky-Pudlak syndrome with defective delta-granules. Adenosine triphosphate (ATP)–dependent cyclic guanosine monophosphate (cGMP) transport codistributed with MRP4 detection in subcellular fractions, with highest activities in the dense granule and plasma membrane fractions. This transport was inhibited by dipyramidole, indomethacin, and MK571 with median inhibitory concentration (IC50) values of 12, 22, and 43 μM, and by ibuprofen. Transport studies with [3H]ADP indicated the presence of an orthovanadate-sensitive ADP transporting system, inhibited by dipyramidole, MK571, and cyclic nucleotides. The results indicate a function of MRP4 in platelet mediator storage and inhibition of MRP4 may represent a novel mechanism for inhibition of platelet function by some anti-inflammatory drugs.
Introduction
The critical role played by platelets in hemostasis and thrombosis is related to their function as exocytotic cells that secrete effector molecules at the side of vascular injury. Platelets contain at least 3 types of intracellular granules, in which these mediators are stored and concentrated, known as alpha, dense, and lysosomal granules.1 While alpha granules contain mainly polypeptides, as fibrinogen, von Willebrand factor, growth factors, and protease inhibitors, dense granules contain small molecules, specifically adenosine diphosphate (ADP), adenosine triphosphate (ATP), serotonin, and calcium.2 Humans with defective dense granule exocytosis suffer from delta storage pool disease associated with a moderate bleeding tendency. The most severe delta storage pool disease is observed in Hermansky-Pudlak syndrome (HPS), a rare autosomal recessive disorder in which oculocutaneous albinism, bleeding, and lysosomal ceroid storage result from defects of melanosomes, platelet-dense granules, and lysosomes.3-5 Little is known, however, about transport proteins mediating accumulation of the effector molecules in granules or their transport across the plasma membrane. Possible candidates include the multidrug resistance protein 4 (MRP4/ABCC4) and MRP5 (ABCC5). These belong to the C-branch of the human ATP-binding cassette (ABC) transporter superfamily, which consists of 12 members, 9 of which comprise the group of multidrug resistance proteins (MRP1-9; ABCC1-6 and ABCC10-12).6,7 MRPs are integral membrane glycoproteins that mediate the primary active unidirectional export of organic anions from cells. Conjugates of lipophilic compounds with glutathione, glucuronate, and sulfate are preferred substrates of MRP1-3,8-10 while cyclic purine nucleotides and nucleotide analogs have been identified as substrates for MRP4 and MRP5.11-15 MRP5 mRNA has been detected in many tissues,16,17 and the MRP5 protein could be localized in erythrocytes,12 in smooth muscle cells of the genitourinary tract,18 and in human heart cardiomyocytes, vascular endothelial, and smooth muscle cells.19 MRP4 mRNA was detected in prostate, liver, testis, ovary, brain, kidney, and adrenal gland.16,20-22 Studies in membrane vesicles containing recombinant MRP4 indicate that it represents a transporter with a relatively broad substrate spectrum, including cyclic nucleotides as well as bile acids in cotransport with reduced glutathione, and prostaglandins.14,15,22-25 It also confers resistance to antiviral nucleoside analogs and cytotoxic thiopurine nucleosides, probably by the cellular export of the intracellularly formed respective nucleotide.11,14,26-28
Proceeding from the initial finding that MRP4 is highly expressed in human platelets, we investigated the subcellular localization and function of MRP4 in these blood cells. The results indicate a function of MRP4 in platelet-dense granule storage and shed light on a novel molecular component of the action of dipyramidole and some other nonsteroidal antiinflammatory drugs (NSAIDs).
Patients, materials, and methods
Materials
[8-3H] Cyclic guanosine monophosphate (cGMP, 326 GBq/mmol) and [2,8-3H] 3′,5′ cyclic adenosine monophosphate (cAMP, 480 GBq/mmol) were obtained from Hartmann Analytic (Braunschweig, Germany) and [2,8-3H]ADP (1.1 TBq/mmol), from American Radiolabeled Chemicals (St Louis, MO). Unlabeled nucleotides, dipyridamole, serotonin, mepacrine (quinacrine), ibuprofen, indomethacin, and sodium orthovanadate were from Sigma (Munich, Germany). MK571 (3-([{3-(2-[7-chloro-2-quinolinyl]ethenyl)phenyl}-{[3-dimethylamino-3-oxopropyl)-thio}-methyl]thio) propanoic acid) was from Alexis (San Diego, CA).
Antibodies
The peptides corresponding to the 15 amino-terminal and carboxy-terminal amino acids of the human MRP4 sequence (MLPVYQEVKPNPLQD and SNGQPSTLTIFETAL, respectively; National Center for Biotechnology Information [NCB] accession no. O15439) were synthesized and coupled to keyhole limpet hemocyanin (Peptide Specialty Laboratories, Heidelberg, Germany). Rabbits were immunized with these conjugates to raise the polyclonal MLP and SNG antisera at the Deutsches Krebsforschungszentrum, Heidelberg. Both antibodies were characterized for their specificity before.23 The AMF antibody was generated against the deduced carboxy-terminal sequence AMFAAAENKVAVKG specific for human MRP5 at the Deutsches Krebsforschungszentrum, Heidelberg, as described.12,18 The mouse monoclonal CD62P antibody against P-selectin was obtained from Beckman Coulter (Krefeld, Germany), and the mouse monoclonal antibody against LAMP2 (lysosome-associated membrane glycoprotein 2, H4B4) as well as the goat polyclonal antibodies to glycoprotein Ib (GPIb, CD42Ib) and P-selectin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Alexa Fluor 488–conjugated goat anti–rabbit immunoglobulin G (IgG) and Alexa Fluor 568–conjugated anti–mouse IgG were from Molecular Probes (Eugene, OR).
HPS patient
The patient is a member of a large kindred with the Hermansky-Pudlak syndrome, extensively studied as described before.3,4 He is an albino with white skin, numerous freckles, marked nystagmus, severely impaired vision, and a bleeding tendency characterized with easy bruising, gingival bleeding, and bleeding after tooth extraction. He has a prolonged bleeding time and a severe deficiency of granule-bound ATP and ADP in the platelets.29 A mutation in the HPS4 gene has been found in this patient.30
Isolation of human platelets and subfractions
Preparation of human platelets for membrane studies. Platelets were obtained from a healthy donor, who did not take any medication during the previous 2 weeks by double-arm cytapheresis using a Fresenius AS104 (Fresenius, Bad Homburg, Germany) separator using the C4F Plt 5d set, adenine-citrate dextrose (Fresenius) as an anticoagulant, and saline 0.9% (Braun, Melsungen, Germany). The platelet concentrate was then filtered via a leukocyte depletion filter (> 99.999% reduction of leukocytes). By cell counting using the Nagotte chamber and flow cytometry, leukocyte contamination of 1/μL or more was excluded. For immunohistochemical studies, platelet-rich plasma (PRP) was isolated from citrated blood by differential centrifugation (180g, 20 minutes).
Washing of platelets. PRP was centrifuged (7 minutes, at 650g) after addition of 111 μL/mL azide-citrate-dextrose-anticoagulant (ACD-A) and 5 μL/mL apyrase PRP. The supernatant was discarded and the platelets were washed with buffer containing 5 U/mL apyrase (Sigma, Taufkirchen, Germany) and 2 U/mL hirudin (Pharmion, Hamburg, Germany). Finally the platelets were resuspended in Tyrode buffer (150 mM NaCl, 12 mM NaHCO3, 2.5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 5.5 mM d-glucose, and 1 mg/mL bovine serum albumin [BSA], pH 7.4).
Preparation of platelet membrane vesicles. The washed platelets were pelleted and resuspended in homogenization buffer (100 mM KCl, 25 mM NaCl, 2 mM MgSO4, 12 mM Na3 citrate, 10 mM d-glucose, 25 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 5 mM ATP, 0.35% BSA, pH 7.0, 340 milliosmolar)31 supplemented with protease inhibitors (0.1 mM phenylmethylsulfonyl fluoride, 0.3 μM leupeptin, and 1 μM aprotinin). The platelets were lysed by repeated cycles of freezing and thawing (4 times) and centrifuged at 100 000g (30 minutes, 4°C) to obtain pellets of crude membranes or subjected to subcellular fractionation on a linear 30% to 60% sucrose density gradient, performed essentially as described by Broekman.32 Following ultracentrifugation (200 000g for one hour at 4°C) in a swing-out rotor (Sorvall TH-641; Kendro, Hanau, Germany), visible whitish bands of different density zones were harvested separately. Each of them, as well as the crude membranes, were suspended in incubation buffer (250 mM sucrose and 10 mM Tris [tris(hydroxymethyl) aminomethane]/HCl [pH 7.4]), homogenized by 30 passes with a tight-fitting Dounce B homogenizer, and centrifuged at 100 000g (30 minutes, 4°C). The resulting pellets were suspended in a small volume of incubation buffer and vesicles were formed by 20 passes through a 27-gauge needle. The membrane vesicles were frozen and stored in liquid N2.
To follow the granule purification and enrichment, part of the platelets was incubated for 30 minutes at 37°C with mepacrine (100 μM) and washed twice before lysis. Mepacrine accumulation in the obtained subcellular fractions was analyzed by fluorescence detection (excitation at 485 nm, emission at 535 nm). Activity of β-glucuronidase was measured as described previously.33
Immunoblot analysis
Membrane fractions were loaded onto a 7.5% sodium dodecylsulfate–polyacrylamid gel after incubation in sample buffer at 37°C for 30 minutes. Immunoblotting was performed using a tank blotting system (Bio-Rad, Hercules, CA) and an enhanced chemiluminescence detection system (Amersham Pharmacia Biotech, Piscataway, NJ). Primary antibodies were diluted in Tris-buffered saline containing 0.05% Tween 20 and 1% BSA to the following final concentrations: AMF and SNG sera, 1:1000; MLP serum, anti–LAMP-2, and polyclonal anti–P-selectin, 1:500; and anti-GPIb, 1:50. Secondary horseradish peroxidase–conjugated goat anti–rabbit and goat anti–mouse IgG antibodies (Bio-Rad) or horse anti–goat IgG antibody (Vector Laboratories, Burlingame, CA) was used at 1:2000 or 1:1000 dilutions.
Vesicle transport studies
Transport of cyclic nucleotides. The ATP-dependent transport of [3H]cGMP or [3H]cAMP into membrane vesicles was measured by rapid filtration through nitrocellulose filters essentially as described.12 Vesicles were incubated in the presence of 4 mM ATP, 10 mM MgCl2, 10 mM creatine phosphate, 100 μg/mL creatine kinase, and [3H]cGMP or [3H]cAMP in the concentrations indicated in incubation buffer containing 250 mM sucrose and 10 mM Tris/HCl (pH 7.4). The final incubation volume was 75 μL. For inhibition studies, compounds were added from a stock solution in an appropriate solvent (incubation buffer, dimethyl sulfoxide, or ethanol at a final concentration of the solvent below 0.5% vol/vol); identical concentrations of the solvent were used in control samples. Aliquots (20 μL) of the incubations were taken at the times indicated, diluted in 1 mL ice-cold incubation buffer and filtered immediately through nitrocellulose filters (0.2-μm pore size, presoaked in incubation buffer). Filters were rinsed with 5 mL incubation buffer, dissolved in liquid scintillation fluid, and counted for radioactivity. In control experiments, ATP was replaced by an equal concentration of 5′-AMP. Rates of net ATP-dependent transport were calculated by subtracting values obtained in the presence of 5′-AMP as a blank from those in the presence of ATP and are given in pmol [3H]cGMP or [3H]cAMP × mg protein–1 (1 pmol × mg protein–1 = 528 atomic disintegrations per minute [DPM] or 780 DPM, respectively).
Transport of [3H]ADP. ATP-dependent transport of [3H]ADP (1 μM) into membrane vesicles was measured by rapid filtration as described for cyclic nucleotides, except that vesicles were incubated in incubation buffer supplemented with 0.4 mM ATP and 10 mM MgCl2 in the presence or absence of sodium orthovanadate (1 mM). For studying the effect of an increased osmolarity of the extravesicular medium, the vesicles were preincubated for 45 minutes at 4°C in buffer containing 1 M sucrose or in standard incubation buffer containing 250 mM sucrose.
Immunofluorescence microscopy
Coverslips cleaned in acetone (5 minutes) and washed with water were covered with 40 μL human collagen type I (1 mg/mL; Sigma, Munich, Germany), incubated (1.5 hours, 37°C), washed twice with 1 mL phosphate-buffered saline (PBS), blocked by BSA (2 mg/mL, one hour, 37°C), washed twice with 1 mL PBS, incubated with 20 μL platelet suspension (100 × 109/L, 30 minutes, room temperature [RT]), washed with 1 mL PBS, pH 7.3, thrice, and fixed by formaldehyde in PBS (1%, 30 minutes, RT). After 3 washes with PBS, platelets were permeabilized with 1% saponin in PBS (30 minutes RT) and blocked by 20% human serum in PBS (15 minutes). Antibody staining was carried out using the primary antibodies at the following dilutions: SNG (or preimmune serum), 1:125; MLP (or preimmune serum), 1:50; anti-LAMP2, 1:10; and anti–P-selectin (CD62P), 1:5. The respective secondary antibodies, either conjugated to Alexa Fluor488 or Alexa Fluor568, were used at a dilution of 1:250 or 1:50, respectively. Fluorescence micrographs were taken with a confocal laser scanning microscope (Chromaphor Analysen Technik, Duisburg, Germany). Samples were observed with a Nikon inverted microscope and a 100 × oil-immersion objective. A CCD camera and VoxCell scan software from VisiTech International (Sunderland, United Kingdom) were used for analysis. For peptide competition experiments, the SNG serum was preincubated for 60 minutes at room temperature with 90 μM of the synthetic peptide used to generate this antibody. For staining of dense granules with mepacrine, platelets were incubated with 100 μM mepacrine for 30 minutes at 37°C and washed twice with calcium-free Tyrode buffer before they were placed on coverslips.
For comparison of normal platelets and Hermansky-Pudlak syndrome platelets, staining intensities were assessed by immunofluorescence microscopy using MetaMorph Imaging series 4.6 software (Visitron Systems, Puchheim, Germany).
Results
Detection of MRP4 in human platelets
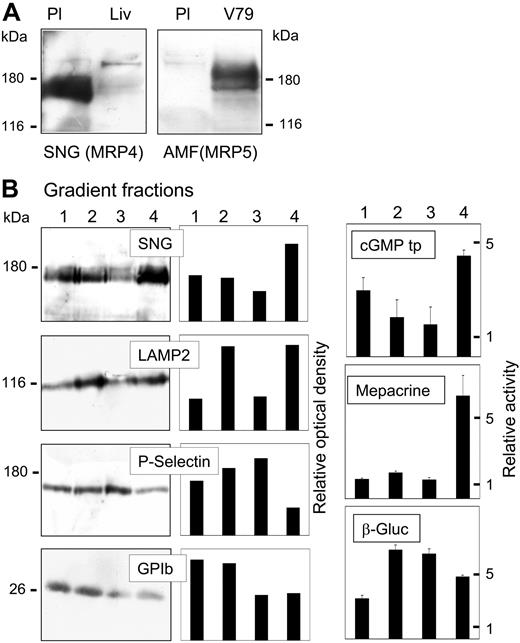
Expression of MRP4 and MRP5 was analyzed by immunoblot analysis as shown in Figure 1A. The SNG antibody directed against the carboxyl terminus of human MRP4 detected a strong broad double-band at 170 to 180 kDa in crude membranes (100 000g pellets) from human platelets (Figure 1A, left panel). The double-band is probably due to different glycosylation, since both bands were shifted to one major band with a lower apparent molecular mass after treatment of the membrane proteins with peptide N-glycosidase F prior to immunoblot analysis. Both bands disappeared when the SNG antiserum was preincubated with the synthetic SNG peptide, while an additional band at about 130 kDa representing probable crossreactivity of the antiserum remained unchanged (not shown). Crude membranes from human liver, shown to express MRP4 before,23 were analyzed for comparison, indicating the relatively high expression of MRP4 in platelets. In contrast, there was only a very weak signal obtained with the AMF anti-MRP5 antibody (Figure 1A, right panel).
Detection of MRP4 and MRP5 in platelet membranous fractions. (A) Immunoblot analysis of MRP4 (left panel) and MRP5 (right panel) in platelet crude membranes (Pl; 40 μg protein) detected by the SNG and AMF antisera directed against the carboxyl terminus of human MRP4 and MRP5, respectively. Crude membranes of human liver (Liv) or MRP5-transfected V79 cells (V79) were used as positive controls. (B) Platelet subcellular fractions were separated on a linear 30% to 60% sucrose gradient and visible bands were collected. According to Broekman,32 4 fractions of increasing density (1-4: 30%, 35%-40%; 50%-55%, 60% sucrose) enriched in (1) plasma membrane, (2) lysosomes, (3) α-granules, or (4) dense granules were further analyzed. MRP4 was detected by immunoblotting using the antiserum SNG (20 μg protein/lane). Blots were further incubated with specific antibodies against LAMP2 (lysosome-associated membrane glycoprotein 2), P-selectin, and the surface antigen GPIb (CD42b), as marker for lysosomes/dense granules, α-granules, and plasma membrane, respectively (left panels). The results were quantified by densitometric analysis and the relative optical density of the specific bands was plotted (middle panels). Right panels: ATP-dependent cGMP transport (cGMP tp) as well as mepacrine accumulation and β-glucuronidase (β-Gluc) activity were measured in the fractions as described in “Patients, materials, and methods” and plotted as relative specific activities (mean values ± SD, n = 3).
Detection of MRP4 and MRP5 in platelet membranous fractions. (A) Immunoblot analysis of MRP4 (left panel) and MRP5 (right panel) in platelet crude membranes (Pl; 40 μg protein) detected by the SNG and AMF antisera directed against the carboxyl terminus of human MRP4 and MRP5, respectively. Crude membranes of human liver (Liv) or MRP5-transfected V79 cells (V79) were used as positive controls. (B) Platelet subcellular fractions were separated on a linear 30% to 60% sucrose gradient and visible bands were collected. According to Broekman,32 4 fractions of increasing density (1-4: 30%, 35%-40%; 50%-55%, 60% sucrose) enriched in (1) plasma membrane, (2) lysosomes, (3) α-granules, or (4) dense granules were further analyzed. MRP4 was detected by immunoblotting using the antiserum SNG (20 μg protein/lane). Blots were further incubated with specific antibodies against LAMP2 (lysosome-associated membrane glycoprotein 2), P-selectin, and the surface antigen GPIb (CD42b), as marker for lysosomes/dense granules, α-granules, and plasma membrane, respectively (left panels). The results were quantified by densitometric analysis and the relative optical density of the specific bands was plotted (middle panels). Right panels: ATP-dependent cGMP transport (cGMP tp) as well as mepacrine accumulation and β-glucuronidase (β-Gluc) activity were measured in the fractions as described in “Patients, materials, and methods” and plotted as relative specific activities (mean values ± SD, n = 3).
The platelet crude membranes were further separated on a linear sucrose density gradient according to Broekman.32 After ultracentrifugation, visible bands were obtained in basically the same density zones and with similar marker enzyme distribution as described in the article. Further analyzed were 4 density zones enriched in (1) plasma membrane, (2) lysosomes, (3) α-granules, or (4) dense granules32 (Figure 1B). MRP4 was detected by immunoblotting and ATP-dependent cGMP transport, as indication to MRP4 transport activity (see “[3H]ADP transport in vesicular fractions from platelets”) was measured. As marker of the different subcellular compartments, antibodies against GPIb, LAMP2, and P-selectin as well as detection of β-glucuronidase activity and mepacrine accumulation were used. Fraction 4 exhibited the highest MRP4 antibody detection and cGMP transport activity, followed by fraction 1. Similar results were obtained using the MLP antiserum directed against the amino terminus of MRP4 (not shown). The α-granule marker, P-selectin, was highest in fraction 3, where MRP4 detection was lowest. The major platelet surface antigen GPIb (CD42b) was most abundant in fractions 1 and 2, but traces were also present in fraction 4. LAMP2 (lysosome-associated membrane glycoprotein 2), a marker for dense granules and lysosomes,34 peaked in fraction 4 and in fraction 2, the fraction of major β-glucuronidase activity. This fraction seems to be a mixed fraction containing lysosomes but also a high proportion of plasma membrane, as indicated by GPIb. Accumulation of the fluorescent mepacrine was used as an additional dense granule marker. Mepacrine has been shown to be rapidly and specifically concentrated in dense granules.35 Labeling appeared only in the densest fraction. However, note that here the intact platelets were loaded with mepacrine prior to homogenization, and, thus, transport into membrane vesicles, formed during the preparation, could not occur, in contrast to the cGMP transport measured in the isolated fractions.
Immunolocalization of MRP4 in human platelets
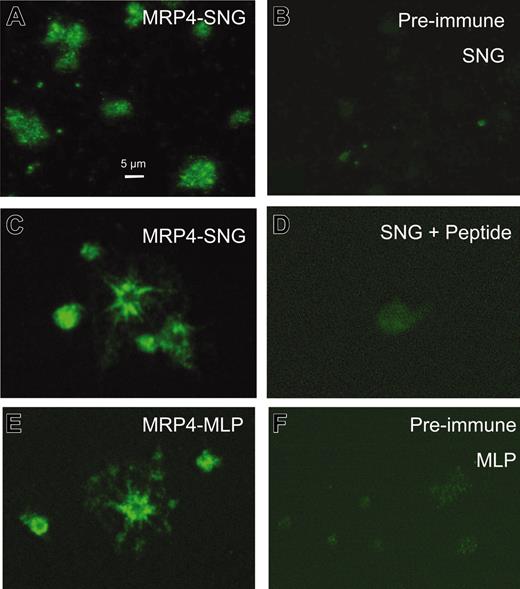
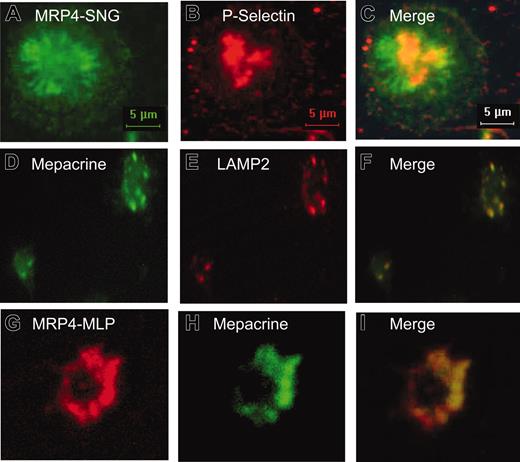
The subcellular localization of MRP4 in human platelets was further investigated using immunofluorescence microscopy (Figures 2, 3). As shown in Figure 2, both anti-MRP4 antibodies SNG, directed against the carboxyl terminus, and MLP, directed against the amino terminus of MRP4, revealed a similar positive staining, mainly in intracellular structures and to a lower extent at the plasma membrane depending on the activation of the platelets indicated by the different shape of platelets (Figure 2A, C, E). Control staining with the preimmune sera or after preincubation with the recognition peptide indicates the specificity of the signal (Figure 2 B, D, F). To identify the intracellular structures, where MRP4 is localized, double-label experiments were performed using costaining with P-selectin as marker for α-granules and mepacrine, as well as the anti-LAMP2 antibody, as markers for dense granules (Figure 3). The overlay images in Figure 3 (C, F, I) indicate no colocalization with the α-granule marker but a partial colocalization with mepacrine-storing and LAMP2-positive structures, most likely to be dense granules (Figure 3D-I).
Immunofluorescence microscopy analysis of MRP4 localization in human platelets. Platelets were adhered on collagen, fixed, permeabilized, and incubated with the primary anti-MRP4 antibodies SNG (A, C) and MLP (E) or, as control, with the respective preimmune sera (B, F). (D) Staining with SNG after preincubation with the synthetic peptide used to generate the SNG antiserum. Images were obtained by confocal laser scanning microscopy using an Alexa488-conjugated antirabbit secondary antibody and identical parameters for each set.
Immunofluorescence microscopy analysis of MRP4 localization in human platelets. Platelets were adhered on collagen, fixed, permeabilized, and incubated with the primary anti-MRP4 antibodies SNG (A, C) and MLP (E) or, as control, with the respective preimmune sera (B, F). (D) Staining with SNG after preincubation with the synthetic peptide used to generate the SNG antiserum. Images were obtained by confocal laser scanning microscopy using an Alexa488-conjugated antirabbit secondary antibody and identical parameters for each set.
Immunolocalization of MRP4 in human platelets. (A-C) Double-label immunofluorescence microscopy of platelets stained with the anti-MRP4 antibody SNG (green fluorescence, Alexa488-conjugated antirabbit secondary antibody) and a monoclonal antibody against P-selectin, as marker for α-granules (red fluorescence, Alexa568-conjugated antimouse secondary antibody). (D-I) Platelets were preincubated with the fluorescent dye mepacrine (green fluorescence), known to be concentrated in dense granules, and stained with an anti-LAMP2 antibody (E, red fluorescence, Alexa568-conjugated antimouse secondary antibody) or MRP4-MLP (G, red fluorescence, Alexa568-conjugated antirabbit secondary antibody). (C,F,I) Superimposed images.
Immunolocalization of MRP4 in human platelets. (A-C) Double-label immunofluorescence microscopy of platelets stained with the anti-MRP4 antibody SNG (green fluorescence, Alexa488-conjugated antirabbit secondary antibody) and a monoclonal antibody against P-selectin, as marker for α-granules (red fluorescence, Alexa568-conjugated antimouse secondary antibody). (D-I) Platelets were preincubated with the fluorescent dye mepacrine (green fluorescence), known to be concentrated in dense granules, and stained with an anti-LAMP2 antibody (E, red fluorescence, Alexa568-conjugated antimouse secondary antibody) or MRP4-MLP (G, red fluorescence, Alexa568-conjugated antirabbit secondary antibody). (C,F,I) Superimposed images.
Altered localization of MRP4 in dense granule–deficient platelets (HPS)
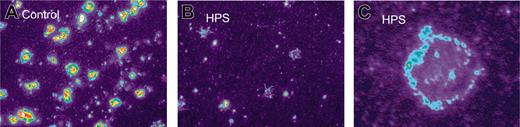
Furthermore, the distribution of MRP4 in platelets from a patient with HPS was studied. It has been established before that the patient had a severe deficiency of granule-bound ATP (0.08 μmol/1011 platelets; controls, 2.02 ± 0.20) and granule-bound ADP (0.06 μmol/1011 platelets; controls, 1.74 ± 0.21).29 Immunofluorescence microscopy of permeabilized and nonpermeabilized platelets from this patient revealed surface expression of MRP4 with decreased intracellular staining compared with controls (Figure 4). By immunoblot analysis of whole platelet lysates, MRP4 was also found in HPS platelets, most likely due to the plasma membrane–expressed MRP4 (not shown).
Altered localization of MRP4 in platelets from HPS patients. Platelets adhered to collagen were stained by the anti-MRP4 antibody SNG and a tetramethylrhodamine isothiocyanate–labeled secondary antibody (1:200) as described in “Patients, materials, and methods.” Differences in fluorescence were enhanced using pseudocolor (× 100 magnification, MetaMorph Imaging series 4.6 software, Visitron Systems, Puchheim, Germany). Control platelets (A) show membrane staining and 1 to 3 strong staining clusters, most likely resembling dense granules, whereas HPS platelets mostly showed membrane staining only (B). Higher magnification of HPS platelet (C).
Altered localization of MRP4 in platelets from HPS patients. Platelets adhered to collagen were stained by the anti-MRP4 antibody SNG and a tetramethylrhodamine isothiocyanate–labeled secondary antibody (1:200) as described in “Patients, materials, and methods.” Differences in fluorescence were enhanced using pseudocolor (× 100 magnification, MetaMorph Imaging series 4.6 software, Visitron Systems, Puchheim, Germany). Control platelets (A) show membrane staining and 1 to 3 strong staining clusters, most likely resembling dense granules, whereas HPS platelets mostly showed membrane staining only (B). Higher magnification of HPS platelet (C).
MRP4 transport activity in vesicular fractions from platelets
ATP-dependent transport of [3H]cGMP and [3H]cAMP, established substrates of MRP4,14 was studied as index of MRP4 transport activity (Figure 5). ATP-dependent transport can proceed into the fraction of inside-out–oriented membrane vesicles or into granules representing preformed inside-out vesicles. [3H]cGMP transport was observed in crude membranes as well as in all membranous fractions of the density gradient analyzed. The relative transport activities correlated with the detection of MRP4 (Figure 1B). The absolute rates of net ATP-dependent [3H]cGMP transport amounted to 1.50 ± 0.29 pmol × mg protein–1 × minute–1 and 2.28 ± 0.13 pmol × mg protein–1 × minute–1 (mean values ± SD, n = 3) in the plasma membrane and dense granule fraction, respectively, at a cGMP concentration of 2 μM and 75 μg membrane protein/75 μL incubation volume. ATP-dependent [3H]cAMP transport could also be detected with a transport rate of 0.45 pmol × mg protein–1 × minute–1 at a substrate concentration of 4 μM (light membrane fraction; Figure 5, right panel). The [3H]cGMP transport was further characterized by inhibition studies with several compounds previously shown to affect recombinant MRP4,24,25 including dipyridamole and the leukotriene analog MK571 as well as the nonsteroidal anti-inflammatory drug indomethacin (Figure 6). The median inhibitory concentration (IC50) values calculated from the concentration dependency curves for dipyridamole, indomethacin, and MK571 were 12, 22, and 43 μM, respectively. Dipyridamole also inhibited the ATP-dependent cAMP transport by 42% at 10 μM (Figure 5, right panel). The [3H]cGMP transport was further inhibited by ibuprofen to 64.5 ± 15.4% of the control at 50 μM. In concentrations up to 100 μM, no significant inhibition by serotonin was observed, suggesting that serotonin is not a high-affinity substrate for MRP4. Also mepacrine only slightly affected cGMP transport (69.7 ± 5.6% of control at 100 μM).
Transport of cyclic nucleotides in platelet membranes. Platelet membrane vesicles (100 μg protein) were incubated with [3H]cGMP (left panel) or [3H]cAMP (right panel) (4 μM) in the presence of 4 mM ATP (▴) or 4 mM 5′-AMP (♦), and the vesicle-associated radioactivity was determined as described in “Patients, materials, and methods” (mean values ± SD, n = 3). The rate of net ATP-dependent transport (▪) was calculated by subtracting transport in the presence of 5′-AMP as a blank from transport in the presence of ATP. Right panel: ATP-dependent transport of cAMP in the presence (♦) or absence (▪) of 10 μM dipyridamole.
Transport of cyclic nucleotides in platelet membranes. Platelet membrane vesicles (100 μg protein) were incubated with [3H]cGMP (left panel) or [3H]cAMP (right panel) (4 μM) in the presence of 4 mM ATP (▴) or 4 mM 5′-AMP (♦), and the vesicle-associated radioactivity was determined as described in “Patients, materials, and methods” (mean values ± SD, n = 3). The rate of net ATP-dependent transport (▪) was calculated by subtracting transport in the presence of 5′-AMP as a blank from transport in the presence of ATP. Right panel: ATP-dependent transport of cAMP in the presence (♦) or absence (▪) of 10 μM dipyridamole.
Inhibition of cGMP transport into platelet membranes. Membrane vesicles were incubated with [3H]cGMP (2 μM) for 10 minutes at 37°C in the presence of dipyridamole (▪), indomethacin (♦), or MK571 (▴) at the concentrations indicated. Rates of ATP-dependent [3H]cGMP transport were determined as described in “Patients, materials, and methods” and calculated as percent inhibition of control [3H]cGMP transport in the presence of the identical concentrations of the solvent used. Data represent mean values ± SD from 3 determinations.
Inhibition of cGMP transport into platelet membranes. Membrane vesicles were incubated with [3H]cGMP (2 μM) for 10 minutes at 37°C in the presence of dipyridamole (▪), indomethacin (♦), or MK571 (▴) at the concentrations indicated. Rates of ATP-dependent [3H]cGMP transport were determined as described in “Patients, materials, and methods” and calculated as percent inhibition of control [3H]cGMP transport in the presence of the identical concentrations of the solvent used. Data represent mean values ± SD from 3 determinations.
[3H]ADP transport in vesicular fractions from platelets
To assess whether ADP could be a substrate for MRP4-mediated ATP-dependent transport, the uptake of [3H]ADP (1 μM) into platelet membrane vesicles (crude membranes) was measured in the presence of 0.4 mM ATP during a time period of 2 minutes. As shown in Figure 7, a time-dependent increase of the vesicle-associated [3H]ADP was observed at a rate of 6.74 ± 1.9 pmol × mg protein–1 × minute–1 (mean value ± SD of 3 different experiments with triplicate determinations). When ATP was replaced by 5′-AMP or the nonhydrolyzable ATP analog AMPPNP, conventionally used in the control incubations to calculate the ATP-dependent component of transport, we observed about a 3- to 4-fold higher background binding of the [3H]ADP to the membranes, which was only slightly increasing with time (not shown). This lower unspecific binding of the [3H]ADP in the presence of ATP compared with 5′-AMP is probably due to the presence of unlabeled ADP, competing with and diluting the 3H-labeled ADP. Source of this unlabeled ADP could be ADP contamination in the commercially available ATP and the formation of ADP through ATP hydrolysis. To demonstrate ATP dependency and ensure at the same time equal initial ADP concentrations, we measured transport in the presence of ATP with or without 1 mM orthovanadate, an inhibitor of ATP hydrolysis. We observed a time-dependent increase of the vesicle-associated radioactivity in the presence of ATP, despite the fact that the [3H]ADP was diluted by the simultaneous formation of unlabeled ADP. This indicates that there is an active incorporation counteracting the dilution effect. The vesicle-associated radioactivity was significantly reduced in the presence of ATP plus orthovanadate, despite the fact that inhibition of ADP formation should enhance binding of the labeled compound, thus indicating that the observed decrease represents inhibition of the active transport process. The dilution effect on the [3H]ADP by formation of unlabeled ADP was also the reason for reducing the ATP concentration from the standard concentration of 4 mM to 0.4 mM.
Transport of ADP in platelet membranes. (A-D) Platelet membrane vesicles (100 μg protein; crude membranes) were incubated with [3H]ADP (1 μM) in the presence of 0.4 mM ATP (▪) or 0.4 mM ATP + 1 mM orthovanadate (♦), and the vesicle-associated radioactivity was determined as described in “Patients, materials, and methods” (mean values ± SD, n = 3; 1 pmol × mg protein–1 = 1724 DPM). (A-B) Vesicles were preincubated for 45 minutes at 4°C in standard incubation buffer containing 250 mM sucrose (A) or in buffer containing 1 M sucrose (B). (C-D) ADP transport in the absence (C) or presence (D) of 100 μM dipyridamole.
Transport of ADP in platelet membranes. (A-D) Platelet membrane vesicles (100 μg protein; crude membranes) were incubated with [3H]ADP (1 μM) in the presence of 0.4 mM ATP (▪) or 0.4 mM ATP + 1 mM orthovanadate (♦), and the vesicle-associated radioactivity was determined as described in “Patients, materials, and methods” (mean values ± SD, n = 3; 1 pmol × mg protein–1 = 1724 DPM). (A-B) Vesicles were preincubated for 45 minutes at 4°C in standard incubation buffer containing 250 mM sucrose (A) or in buffer containing 1 M sucrose (B). (C-D) ADP transport in the absence (C) or presence (D) of 100 μM dipyridamole.
To determine whether the observed difference in [3H]ADP uptake by the vesicles in the presence and absence of orthovanadate reflects transmembrane movement rather than binding to the membrane surface, the influence of high osmolarity was studied. At a concentration of 1 M sucrose outside the vesicles, the rate of [3H]ADP transport in the absence of orthovanadate was markedly reduced, indicating active transport (Figure 7B). In the presence of orthovanadate, however, 1 M sucrose only slightly affected [3H]ADP association to the vesicles, indicating that the amount of radioactivity measured represents the proportion of [3H]ADP binding to the vesicle membrane independent of transmembranal transport. Furthermore, the vanadate-sensitive ADP accumulation could also be detected in the dense granule fraction of the sucrose gradient and was inhibited by dipyridamole, MK571, and cGMP (Table 1).
Inhibition of [3H]ADP transport into platelet dense vesicles
Compound . | Concentration, μM . | [3H]ADP transport, % of control . |
|---|---|---|
| None (control) | NA | 100 |
| Dipyridamole | 10 | 52.7 ± 5.2 |
| 100 | 8.8 ± 0.4 | |
| MK571 | 100 | 40.7 ± 3.9 |
| cGMP | 100 | 28.7 ± 2.3 |
Compound . | Concentration, μM . | [3H]ADP transport, % of control . |
|---|---|---|
| None (control) | NA | 100 |
| Dipyridamole | 10 | 52.7 ± 5.2 |
| 100 | 8.8 ± 0.4 | |
| MK571 | 100 | 40.7 ± 3.9 |
| cGMP | 100 | 28.7 ± 2.3 |
Platelet-dense membrane vesicles (100 μg protein) were incubated with [3H]ADP (1 μM) in the presence of 0.4 mM ATP or 0.4 mM ATP + 1 mM orthovanadate for 2 minutes, and the difference in the vesicle-associated radioactivity was calculated. The compounds listed were added in the given concentrations to incubations with and without orthovanadate. The difference is given as percent of control (mean values ± SD from 3 determinations). The control vanadate-sensitive [3H]ADP transport at 2 minutes in these experiments was 3.0 ± 0.4 pmol/mg protein-1.
Discussion
The release of platelet-dense body constituents such as ADP has a fundamental role in hemostasis. The content in platelet-dense bodies is probably established in megakaryocytes, with ADP concentrations exceeding 0.6 M and indicating an active transport. However, very little is known about this process. The presence of plasma membrane proteins such as GPIb in the dense granule membrane suggests that dense granules arise from both endogenous syntheses in the megakaryocyte as well as from heterotypic fusion with endocytic vesicles budding from the plasma membrane.36 The present study identifies MRP4 as a major candidate for the active transport of organic anions, especially nucleotides, into these granules. This is based on the following observations: Immunoblotting (Figure 1) and immunofluorescence microscopy (Figures 2, 3, 4) showed the high abundance of MRP4 in platelets and its predominate localization in dense granules as indicated by colocalization with mepacrine, which is specifically concentrated in dense granules35 (Figure 3), as well as by the altered distribution in platelets from a patient with Hermansky-Pudlak syndrome (HPS) (Figure 4). Mutations in different genetic loci have been identified in patients with HPS5,37 and in strains of animals demonstrating HPS-like storage pool defects such as pale-ear mice.38 Although the relationships between the genetic defects and the molecular pathogenesis are not yet fully defined, the normal development of platelet-dense granules, which is related to lysosomal vesicle trafficking, is diminished, leading to reduced or absent platelet-dense granules. Our immunodetection of MRP4 in these granules (besides the plasma membrane and possibly other notidentified intracellular membranes) is supported by functional studies demonstrating ATP-dependent transport of 3H-labeled cGMP, which paralleled MRP4 detection in subcellular fractionation (Figure 1B). This transport proceeds into the fraction of inside-out membrane vesicles formed from the plasma membrane during the vesicle preparation as well as into the granules representing “preformed” inside-out vesicles (Figure 8). Despite an unavoidable activation of the platelets during the homogenization procedure leading to fusion of granules with the plasma membrane and the incomplete organelle separation, the relative distribution of MRP4 and cGMP transport in comparison with that of the other marker proteins indicates an enrichment in dense granules besides the plasma membrane fraction. Furthermore, the inhibition profile of the observed transport (Figure 6) points at MRP4 as a major candidate for mediating this process. The determined IC50 values of 12 μM, 22 μM, and 43 μM for dipyridamole, indomethacin, and MK571, respectively, were slightly higher but in the same order of magnitude than those that have been found to inhibit MRP4-mediated transport in other systems. In membranes from MRP4-containing Sf9 cells, 5 μM indomethacin inhibited transport to about 50%.25 MRP4-mediated PMEA (9-(2-phosphonylmethoxyethyl)adenine) efflux from intact cells was inhibited by dipyridamole and MK571 with IC50 values of 2 μM and 10 μM, respectively.24 Amongst the MRPs, MRP4 has a unique broad substrate and inhibitor specificity. The leukotriene antagonist MK571 also effectively inhibits MRP1,9 which, however, does not transport cyclic nucleotides. MRP5, a cyclic nucleotide transporter like MRP4,12 is inhibited by dipyridamole but is not or only slightly affected by MK571.12,24 Thus, the combined inhibition by dipyridamole, MK571, and indomethacin strongly argues for a major contribution of MRP4. In addition, we detected MRP5 only at a low level in platelets (Figure 1A). However, we cannot fully exclude a contribution of other not-yet-identified proteins to the observed transport.
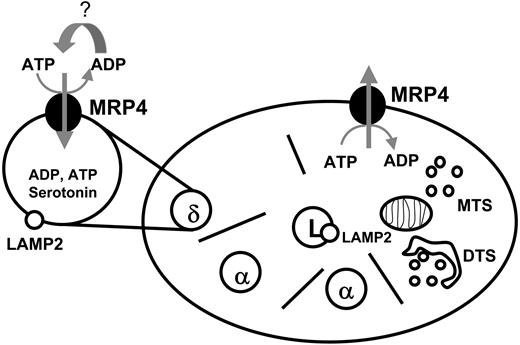
Possible involvement of MRP4 in platelet transmitter storage and release. Platelets contain the ATP-dependent export pump MRP4 in the membrane of dense (δ)-granules and in the plasma membrane depending on platelet activation. MRP4 may be involved in the active transport of ADP into the dense granules and in the ADP release from the platelets. MTS indicates microtubular system; DTS, dense tubular system.
Possible involvement of MRP4 in platelet transmitter storage and release. Platelets contain the ATP-dependent export pump MRP4 in the membrane of dense (δ)-granules and in the plasma membrane depending on platelet activation. MRP4 may be involved in the active transport of ADP into the dense granules and in the ADP release from the platelets. MTS indicates microtubular system; DTS, dense tubular system.
Dipyridamole and, in particular, dipyridamole in combination with low-dose aspirin are very effective in preventing recurrent stroke.39 However, the mechanisms underlying this dipyridamole effect have not been fully elucidated. Dipyridamole has been shown to enhance nitric oxide (NO)/cGMP–mediated effects in intact human platelets40 as well as to interfere with the ADP-dependent platelet activation.41 Both effects could be related to MRP4, assuming a possible effect of this transporter on the cytosolic cGMP concentration28 as well as a role in adenosine nucleotide transport. Similarly, inhibition of MRP4-mediated transmitter storage and release may represent a novel molecular mechanism for the action of indomethacin and ibuprofen, besides the known inhibition of cyclooxygenases (COX1 and COX2).
Beside ADP and ATP, serotonin is also a well-established compound of platelet-dense granules. Platelets exhibit a sodium- and chloride-coupled serotonin uptake transporter (5-hydroxytryptamine transporter (5-HTT); SLC6A4) in their plasma membrane.42 In addition, a pH gradient–dependent serotonin transport system has been described in platelet-dense granules,43 however, the molecular identity of this transporter has not been elucidated so far. The weak inhibition of the MRP4-mediated cGMP transport by serotonin suggests that serotonin is not a high-affinity substrate for MRP4.
Since ADP is the major signaling molecule concentrated in platelet-dense granules, the question of whether MRP4 represents an ADP transporter is of central interest. The fact that MRP4 mediates the transport of cyclic nucleotides and nucleotide analogs and exhibits a remarkably broad substrate specificity among the MRPs14,15,22-25 supports this speculation. However, ADP translocation by ABC transporter is experimentally difficult to demonstrate due to the complex coupling of substrate transport to hydrolysis of ATP at the 2 nucleotide binding domains resulting in ADP formation. However, we could observe a time-dependent increase of the vesicle-associated [3H]ADP in the presence of ATP, which was significantly reduced by the addition of orthovanadate, which inhibits the ATP hydrolysis (Figure 7). This vanadate-sensitive uptake of [3H]ADP was markedly reduced by an increase in osmolarity of the extravesicular medium, which is expected to decrease the intravesicular volume without affecting the surface, suggesting that this process reflects transport into an osmotically sensitive space rather than binding to the membrane surface. Furthermore, this uptake was significantly affected by dipyridamole, MK571, and cGMP (Table 1). These data indicate the presence of an ATP-driven ADP transporting system in platelet membrane vesicles and granules and pinpoint MRP4 as a major candidate protein involved in this process.
The novel concept of the involvement of an ABC transporter in platelet transmitter storage and release (Figure 7) remains to be speculative but is strongly supported by our findings. This may have a major impact on understanding the role of dipyramidole and other NSAIDs in primary and secondary prevention of arterial occlusions. Further evidence for the role of MRP4 as a platelet ADP transporter may be provided by ADP transport assays with recombinant MRP4 or by the identification of mutations in the MRP4 gene affecting platelet function.
Prepublished online as Blood First Edition Paper, August 5, 2004; DOI 10.1182/blood-2003-12-4330.
Supported by the German Federal Ministry for Education and Research (NBL3 program, reference 01 ZZ 0103).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The antisera AMF, SNG, and MLP were generated at the Deutsches Krebsforschungszentrum, Heidelberg, Germany, in the division of Tumor Biochemistry headed by Dr Dietrich Keppler. We acknowledge the excellent technical assistance of Carmen Blumentritt, Department of Immunology and Transfusion Medicine, Greifswald, and of Tina Brüggmann, Department of Pharmacology, Greifswald.

![Figure 5. Transport of cyclic nucleotides in platelet membranes. Platelet membrane vesicles (100 μg protein) were incubated with [3H]cGMP (left panel) or [3H]cAMP (right panel) (4 μM) in the presence of 4 mM ATP (▴) or 4 mM 5′-AMP (♦), and the vesicle-associated radioactivity was determined as described in “Patients, materials, and methods” (mean values ± SD, n = 3). The rate of net ATP-dependent transport (▪) was calculated by subtracting transport in the presence of 5′-AMP as a blank from transport in the presence of ATP. Right panel: ATP-dependent transport of cAMP in the presence (♦) or absence (▪) of 10 μM dipyridamole.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/12/10.1182_blood-2003-12-4330/5/m_zh80230470130005.jpeg?Expires=1763531140&Signature=4IG4rI8aSSeAR20pcYdL7fyyPByM1NCnwMzQf21e~en-mo6FeNz~TXvPV1VvIHHerWHY4wdfpoRgEIKGxVirBcK3oVlp~HkQu05TwUQCgrR7AD5r0piJox1KOP83Be5~T0bdL-h~2Z7OdfSLcYTsLHir2Dwi2Xb1QM327rpKJGo0vzlzL0qJboeCLQkJAxfyE8txlLVmQr~QhS6SqvEnCs2yfLpr7u5TQDm4JDw~W5lKPGfoBiTLRsmttnkqsNm207cqHwlfcyYfqCSRotUoDI8MNUT1mjuPckQ8LT7m5JFYI8sLAJe0dfMkKnQWQaKBa8WupPVG3dnkKKiGThbXfQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Inhibition of cGMP transport into platelet membranes. Membrane vesicles were incubated with [3H]cGMP (2 μM) for 10 minutes at 37°C in the presence of dipyridamole (▪), indomethacin (♦), or MK571 (▴) at the concentrations indicated. Rates of ATP-dependent [3H]cGMP transport were determined as described in “Patients, materials, and methods” and calculated as percent inhibition of control [3H]cGMP transport in the presence of the identical concentrations of the solvent used. Data represent mean values ± SD from 3 determinations.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/12/10.1182_blood-2003-12-4330/5/m_zh80230470130006.jpeg?Expires=1763531140&Signature=Tx1jNt05oeo1Fh16e8Srty3V4jjqg3L26PpqBTbLu5EEmqIdqwDmukiwaJ3jsg0s6~msWat7tLthpGOw2n1PNusYRzsrqOobXkpF-n-t4CU1uM1isdeFukwfHeOEFJ-xsA4UDUPWrsrk3kWWLzFPyoFcwMLmQ-p41thfWozPY-5fAPRHzKAFMOphCBEKLl38KazXoIw9Jw40plrKzioA5HNwbn27MOBW06y2OzdpPnyRDmZuEum0frs791-gbCwbL-AWppAygZ4V2wxgZ3Aw6A9cBwUg-u4NsNgktRwNqecvBTF8yc08rFQO8YRGhufbUpO7WkSh-zuvkQ0aEGJACg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Transport of ADP in platelet membranes. (A-D) Platelet membrane vesicles (100 μg protein; crude membranes) were incubated with [3H]ADP (1 μM) in the presence of 0.4 mM ATP (▪) or 0.4 mM ATP + 1 mM orthovanadate (♦), and the vesicle-associated radioactivity was determined as described in “Patients, materials, and methods” (mean values ± SD, n = 3; 1 pmol × mg protein–1 = 1724 DPM). (A-B) Vesicles were preincubated for 45 minutes at 4°C in standard incubation buffer containing 250 mM sucrose (A) or in buffer containing 1 M sucrose (B). (C-D) ADP transport in the absence (C) or presence (D) of 100 μM dipyridamole.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/12/10.1182_blood-2003-12-4330/5/m_zh80230470130007.jpeg?Expires=1763531140&Signature=WsAVaBApkwxL89SNPRmRdfOs5b2Gq23pwR7EE6C-sxyHrxyXfl5t~aMEDGdjju5MGRbMlOO-jZthfMM4eYGFQQPV1RloROmY~s4DTmEIXIO4K3-QQ8OwzNeWt2aXKGvbFsI0Te6s-ONcJIbZ5TAWxAVB~4EAjXibw0RKEOHlvzQdZgpOJhZy5FCrIqendFftYen8MdjWvl9ZJ-nn~8LeWM3S89Ga-85ukLXJsUMFcFgwR8oDKzlvJvJ5E2PnDtzziSCUErXz~xSrSujbLMR7Hi~QhFMlhhJSH8JCf9plAANhDqb9j2yup6sXZXU~IyVYWYIY5BLRp5QaSqvcNO0HlQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal