P-selectin glycoprotein-1 (PSGL-1) supports P-selectin–dependent rolling in vivo and in vitro. However, controversy exists regarding the importance of PSGL-1–dependent and –independent E-selectin rolling. Using antibodies against PSGL-1 and PSGL-1-/- mice, we demonstrated abolition of P-selectin–dependent rolling but only partial inhibition of E-selectin–mediated rolling in the cremaster microcirculation following local administration of tumor necrosis factor α (TNF-α). In vitro studies demonstrated that binding of recombinant mouse E-selectin chimera to PSGL-1-/- neutrophils was dramatically decreased in mice treated systemically but not locally with TNF-α. Further, PSGL-1 blockade abolished E-selectin–dependent rolling in wild-type mice following systemic TNF-α administration but not local TNF-α administration. Together, these data support an E-selectin ligand present on PSGL-1-/- neutrophils that is down-regulatable upon systemic but not local activation. To determine whether the PSGL-1–independent E-selectin ligand was physiologically important, we used a P- and E-selectin–dependent cutaneous contact hypersensitivity model. Binding studies showed no E-selectin ligand down-regulation in this model. The few cells that rolled on E-selectin ligand following PSGL-1 antibody administration or in PSGL-1 deficiency were sufficient to induce profound contact hypersensitivity. In conclusion, E-selectin mediates PSGL-1–dependent and independent rolling and the latter can be down-regulated by systemic activation and can replace PSGL-1 to support the development of inflammation.
Introduction
Leukocyte accumulation into sites of injury or infection requires a multistep process that involves rolling, adhesion, and emigration. The selectin family of adhesion molecules is responsible for the initial contact of leukocytes with the vascular endothelium. The selectin family consists of 3 closely related cell-surface molecules termed L-selectin, E-selectin, and P-selectin.1 L-selectin is constitutively expressed on leukocytes and binds to ligands on other leukocytes and on activated endothelial cells. E-selectin, expressed on activated endothelial cells, and P-selectin, expressed on activated platelets and endothelial cells, bind to ligands on leukocytes. Recent studies using double- or triple-selectin knockout mice revealed that selectins have overlapping and distinct functions.2,3 Indeed, single-selectin knockout mice revealed only minor deficiencies in leukocyte recruitment in response to tumor necrosis factor α (TNF-α) or thioglycollate4,5 ; more profound deficiencies in double-knockout mice3 ; and the greatest degree of leukocyte recruitment impairment in E-, L-, and P-selectin triple-knockout mice.2,3,6 Therefore, inhibition of a common ligand for the 3 selectins is a very attractive mode of therapeutic intervention in leukocyte recruitment.
P-selectin glycoprotein ligand-1 (PSGL-1) was first identified in 1992 by Western blotting of membrane extracts of neutrophils and the myeloid cell line HL-60.7 PSGL-1 is a high-affinity ligand for P-selectin and it is preferentially localized to the tips of the microvilli on resting leukocytes.8 In vivo studies have shown that PSGL-1 interaction with P-selectin is required for rolling of human leukocytes or myeloid cells in mesenteric venules of the rat9 as well as in the recruitment of mouse neutrophils into the cremaster muscle.10 However, PSGL-1 is also a ligand for L-selectin11 and for E-selectin.12 Some debate exists over the importance of E-selectin–PSGL-1 interactions. An in vivo study demonstrated that microspheres coated with human PSGL-1–immunoglobulin G (IgG) chimera attached and rolled on E-selectin in TNF-α–stimulated mouse mesenteric venules.13 This study contradicts a study using PSGL-1-/- mice by Yang et al,14 which showed no defect of E-selectin–mediated rolling in TNF-α–stimulated cremasteric venules. Yet another study using PSGL-1-/- mice showed attenuated E-selectin–dependent leukocyte rolling under flow conditions.15
In this study we systematically examined the importance of PSGL-1–dependent and –independent rolling using anti–PSGL-1 antibodies and PSGL-1-/- mice. We report that PSGL-1 can bind both P-selectin and E-selectin. More importantly, we report an E-selectin ligand distinct from PSGL-1 that is rapidly down-regulated from circulating leukocytes during systemic inflammation making the rolling on P-selectin and E-selectin entirely PSGL-1–dependent.
Materials and methods
Animals
Animals used in this study were male and female C57Bl/6, P-selectin-/-, and PSGL-1-/- mice weighing between 20 and 30 g and older than 8 weeks of age. All mice were on a C57Bl/6 background. C57Bl/6 and P-selectin-/- mice were purchased from The Jackson Laboratories (Bar Harbor, ME) and PSGL-1-/- mice were supplied by Dr Daniel Bullard (University of Alabama) and originally made by Dr B. Furie (Center for Haemostasis and Thrombosis Research, Harvard Medical School, Boston, MA). A minimum of 3 to 6 animals was used in each experimental group.
Intravital microscopy
Animals were anesthetized by intraperitoneal injection of a mixture of 10 mg/kg xylazine (MTC Pharmaceutical, Cambridge, ON, Canada) and 200 mg/kg ketamine hydrochloride (Rogar/STB, London, ON, Canada). All mice were kept at 36°C to 37°C. The right jugular vein was cannulated to administer anesthetic, fluorescent dyes, and antibodies. Animals were then prepared as follows to view either the skeletal muscle (cremaster) microcirculation or dermal (ear preparation) microcirculation.
Cremaster muscle preparation. An incision was made in the scrotal skin to expose the left cremaster muscle, which was then carefully removed from the associated fascia. A lengthwise incision was made on the ventral surface of the cremaster muscle. The testicle and epididymis were separated from the underlying muscle and reintroduced into the abdominal cavity. The muscle was then spread out over an optically clear viewing pedestal and secured along the edges with 3-0 suture. The exposed tissue was superfused with warm bicarbonate-buffered saline (pH 7.4). The cremaster microcirculation was observed through an intravital microscope (Axioskop; Carl Zeiss, Don Mills, ON, Canada) with a × 10 eyepiece and a × 25 objective lens. Single unbranched venules (20-40 μm in diameter) were selected for study and images of the microcirculation were recorded using a video camera (Panasonic-Digital 5100; Panasonic, Secaucus, NJ) and videocassette recorder (VCR).
Ear skin preparation. The hair on the left ear was removed using hair removal lotion. The left ear was covered with physiologic saline and gently positioned between a microscope slide and a coverslip on a stage of an intravital microscope as previously described.16 Due to the thickness of the ear, leukocyte–endothelial cell interactions were not visible by transillumination. Therefore, for this protocol, animals were injected with the fluorescent dyes fluorescein isothiocyanate (FITC)–bovine albumin (10 mg/kg intravenously; Sigma Chemical, St Louis, MO) and rhodamine 6G (0.3 mg/kg intravenously; Sigma Chemical) immediately before microscopic visualization. FITC–bovine albumin allowed the visualization of the microvasculature. At the dose used, rhodamine 6G labels leukocytes and has been shown to allow detection of the same number of rolling leukocytes as transmitted light. It has no effect on leukocyte kinetics per se.17 Rhodamine 6G–associated fluorescence was visualized by epi-illumination at 510- to 560-nm emission filter.17 The ear microcirculation was observed through the same intravital microscope as described for cremaster muscle preparation but with a × 40 water immersion objective lens. A fluorescent camera (model C-2400-08; Hammamatsu Photonics, Hammamatsu City, Japan) was used to project the images onto a monitor, and the images were recorded for playback analysis using a VCR. Single unbranched venules (20-40 μm in diameter) were selected and, to minimize variability, the same section of the venule was observed throughout the experiment. The number of rolling leukocytes and leukocyte velocity were determined off-line during video playback analysis.
Measurements. For both skin and muscle preparations, rolling leukocytes were defined as leukocytes that rolled at a velocity slower than that of red blood cells. Leukocyte rolling velocity was measured for the first 10 to 20 leukocytes entering the field of view at the time of recording and was determined as the time required for a leukocyte to traverse a 100-μm venule length. Leukocytes were considered adherent to the venular endothelium if they remained stationary for 30 seconds or longer. Red blood cell velocity (VRBC) was measured online using an optical Doppler velocimeter (Microcirculation Research Institute, Texas A&M University, College Station, TX) and was only measured for the skeletal muscle preparation, as determination of VRBC using fluorescence was not possible. Venular blood flow in the skeletal muscle preparation was calculated from the product of cross-sectional area and mean red blood cell velocity (Vmean = VRBC/1.6), assuming cylindrical geometry. Venular wall shear rate (γ) was calculated based on the Newtonian definition [α = 8 (Vmean/DV)] and venular wall shear stress was γ × blood viscosity, where blood viscosity was assumed to be 0.0025 poise.18 None of the hemodynamic and microvascular parameters measured (vessel diameters, centerline red blood cell velocity, and wall shear rate) were statistically different among the groups studied (data not shown). Images of the dermal (ear skin preparation) and skeletal muscle (cremaster) microcirculation were recorded before and after administration of various antibodies in mice 3 to 4 hours after injection of TNF-α (R&D Systems, Minneapolis, MN).
Experimental protocol. TNF-α (0.5 μg/mouse) was injected locally 3 hours before surgical exposure of the muscle. In a separate series of experiments, TNF-α (0.5 μg/mouse) was injected intraperitoneally 3 hours before preparation of either the muscle or skin. This protocol induced a systemic inflammation causing notably altered leukocyte traffic in all vasculatures examined.19,20 The recordings for the intravital microscopy experiments were made between 3 and 4 hours after TNF-α injection. The following monoclonal antibodies were used intravenously: RB40.34 against mouse P-selectin21 (20 μg/mouse), 2PH1 against mouse PSGL-110 (50 μg/mouse), 4RA10 against mouse PSGL-122 (50 μg/mouse), and RME-1 against mouse E-selectin23 (100 μg/mouse; kindly supplied by Dr A. C. Issekutz). These concentrations of antibodies have been shown to be optimal in our preliminary work and in published results.10,15 TNF-α–induced leukocyte rolling was also studied in P-selectin-/- and PSGL-1-/- mice.
Oxazolone-induced contact hypersensitivity (CHS). Mice were sensitized for CHS response by topical application of 50 μL of 5% oxazolone (Sigma Chemical) in acetone–olive oil vehicle (4:1) to the shaved flank. One week later, mice received a 10-μL challenge of 1% oxazolone solution on the ventral aspect of the left ear. Just prior to this antigen challenge, blocking antibodies against adhesion molecules (anti–E- and anti–P-selectin or anti–PSGL-1) or saline were administered intraperitoneally. At 2 hours and 24 hours after antigen challenge, ear skin venules were visualized via intravital microscopy as previously described.16
Flow cytometry
In all groups of experiments described above, blood was withdrawn by cardiac puncture using heparin (10 U/mL). To detect E-selectin ligands, 100 μL of blood was first incubated for 30 minutes with 2.5 μg of recombinant mouse E-selectin/Fc chimera (R&D Systems). Subsequently, red blood cell lysis was performed with OptiLyse B solution (Immunotech, Marseille, France). Binding of neutrophils to E-selectin chimera was detected by incubation for 30 minutes with goat antihuman IgM conjugated to biotin (1:100 antibody dilution; Sigma Chemical) followed by 30 minutes incubation with streptavidin-phycoerythrin (PE) conjugate (Sav-PE; BD Pharmingen, San Diego, CA; 1:50 antibody dilution). Cells stained with secondary and tertiary antibodies alone were used as negative controls. Neutrophil binding to E-selectin chimera was then analyzed on a FACscan machine using CellQuest software (Becton Dickinson Immunocytometry Systems, San Jose, CA). Neutrophils, lymphocytes, and monocytes were identified using FSC (forward scatter) and SSC (side scatter) profiles, which identify size and granularity, respectively.16
Statistical analysis
All data are displayed as mean ± SEM. All data were analyzed using Student t test and a Bonferroni correction was applied where multiple comparisons were necessary. A value of P less than .05 was deemed significant.
Results
TNF-α–induced leukocyte rolling in cremaster microcirculation is dependent on both endothelial selectins
In Figure 1A, approximately 50 to 60 cells/minute rolled in control C57Bl/6 (first column), whereas approximately 30 cells/minute rolled after 3 to 4 hours of local TNF-α injection (second column). Treatment with anti–P-selectin antibody reduced rolling by 70% and subsequent addition of anti–E-selectin antibody eliminated all remaining rolling (Figure 1A). This is consistent with previous work, suggesting that leukocyte rolling in the cremaster microcirculation is entirely dependent on P- and E-selectin following local TNF-α administration.24,25 Rolling velocity was approximately 5 μm/second and was not affected by anti–P-selectin antibody. As no leukocytes rolled after treatment with anti–P-selectin antibody and anti–E-selectin antibody, leukocyte rolling velocity could not be measured (Figure 1B). Previous reports have shown that E-selectin mediates slow rolling (∼5 μm/s) in TNF-α–treated C57Bl/6 mice.26 This differs from rolling on P-selectin at approximately 50 μm/second in untreated mice.27
Leukocyte rolling flux and velocity in cremaster microcirculation of C57Bl/6 mice treated with TNF-α. Leukocyte rolling flux (A) and leukocyte rolling velocity (B) in cremaster microcirculation of C57Bl/6 mice treated 3 hours before with TNF-α applied locally (0.5 μg/mouse). The effects of anti–P-selectin, anti–E-selectin, or anti–PSGL-1 alone or in combination were tested. ▪ represents the values for leukocyte rolling and leukocyte rolling velocity under control conditions. Data are presented as mean ± SEM. *P < .05 relative to C57Bl/6 mice treated with TNF-α (second column). N/D indicates not determined.
Leukocyte rolling flux and velocity in cremaster microcirculation of C57Bl/6 mice treated with TNF-α. Leukocyte rolling flux (A) and leukocyte rolling velocity (B) in cremaster microcirculation of C57Bl/6 mice treated 3 hours before with TNF-α applied locally (0.5 μg/mouse). The effects of anti–P-selectin, anti–E-selectin, or anti–PSGL-1 alone or in combination were tested. ▪ represents the values for leukocyte rolling and leukocyte rolling velocity under control conditions. Data are presented as mean ± SEM. *P < .05 relative to C57Bl/6 mice treated with TNF-α (second column). N/D indicates not determined.
P-selectin–independent rolling in TNF-α–treated cremaster microcirculation is inhibited by anti–PSGL-1 antibody
In another set of experiments we assessed whether the E-selectin component of leukocyte rolling in TNF-α–stimulated cremasteric venules could be inhibited by a PSGL-1 monoclonal antibody. C57BL/6 mice received the PSGL-1 antibody 4RA10 intravenously after treatment with P-selectin antibody. Figure 1A demonstrates that the P-selectin–independent rolling that was inhibited by anti–E-selectin antibody (fourth column) was almost completely inhibited by anti–PSGL-1 antibody (fifth column). However, 2 to 3 cells/minute consistently rolled in these preparations, whereas no cells rolled in P-selectin and E-selectin antibody–treated mice. Rolling velocity was approximately 5 μm/second and again was not affected by anti–P-selectin antibody or by subsequent addition of 4RA10 antibody (Figure 1B). Clearly, the E-selectin component of slow rolling remained in the absence of PSGL-1. In another set of experiments, addition of only PSGL-1 antibody to C57BL/6 mice treated with TNF-α was as effective as addition of P-selectin and PSGL-1 antibody in tandem (Figure 1A, sixth column). Again, 2 or 3 cells could be seen rolling per minute and the leukocyte rolling velocity was not significantly altered after treatment with anti–PSGL-1 antibody (Figure 1B). These few cells rolling after PSGL-1 blockade were abolished with anti–E-selectin antibody (seventh column). This suggests that PSGL-1 mediates a large component of the E-selectin–dependent rolling but that a small amount of E-selectin–dependent rolling is PSGL-1 independent. These few cells also exhibited very slow rolling velocity.
PSGL-1 antibody also inhibits E-selectin–mediated leukocyte rolling in TNF-α–treated cremaster microcirculation of P-selectin-/- mice
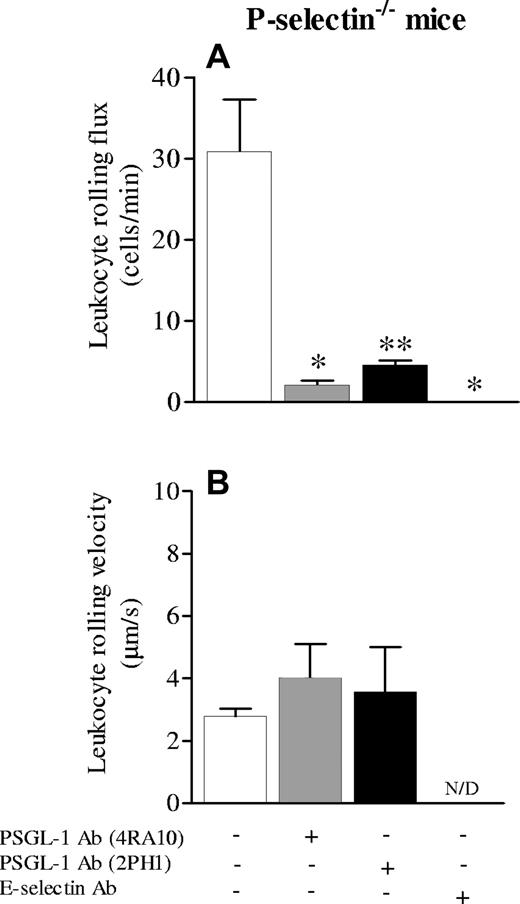
A limitation of the P-selectin antibody (Ab) is potentially incomplete P-selectin inhibition. Therefore, we also studied P-selectin-/- mice. In control conditions, P-selectin-/- mice have absolutely no rolling leukocytes.28 As reported previously, following TNF-α stimulation, P-selectin-/- mice have significant E-selectin–dependent rolling29 and we have observed additional E-selectin synthesis in the P-selectin-/- mice relative to wild-type mice in response to some stimuli.30 Indeed, leukocyte rolling in P-selectin-/- mice after 3 to 4 hours of local TNF-α was about 30 cells/minute, a value not dissimilar to wild-type mice. All of this rolling is E-selectin dependent.25 To confirm this, P-selectin-/- mice were treated with anti–E-selectin antibody. No rolling cells were observed after addition of anti–E-selectin antibody (Figure 2A, fourth column). Addition of PSGL-1 antibody (4RA10) inhibited leukocyte rolling by more than 95% (Figure 2A), demonstrating that anti–PSGL-1 antibody is able to inhibit almost all E-selectin–dependent rolling. However, some E-selectin–dependent and PSGL-1–independent rolling persisted. Leukocyte rolling velocity was very slow and was not altered after treatment with anti–PSGL-1 antibody (Figure 2B). We also tested a second antibody, 2PH1, thought to be less effective as a PSGL-1 inhibitor.10 After addition of 2PH1, a 70% reduction in leukocyte rolling was noted in P-selectin-/- mice. Although this is not as effective as the 4RA10 antibody, there does appear to be some E-selectin–dependent inhibition (Figure 2A). Leukocyte rolling velocity was not altered after addition of 2PH1 (Figure 2B).
Leukocyte rolling flux and velocity in cremaster microcirculation of P-selectin-/- mice treated with TNF-α. Leukocyte rolling flux (A) and leukocyte rolling velocity (B) in cremaster microcirculation of P-selectin-/- mice treated 3 hours before with TNF-α applied locally (0.5 μg/mouse). The effects of anti–PSGL-1 antibody (4RA10 or 2PH1) and anti–E-selectin antibody were tested. Data are presented as mean ± SEM. *P < .05 relative to untreated value; **P < .05 relative to 4RA10 treatment.
Leukocyte rolling flux and velocity in cremaster microcirculation of P-selectin-/- mice treated with TNF-α. Leukocyte rolling flux (A) and leukocyte rolling velocity (B) in cremaster microcirculation of P-selectin-/- mice treated 3 hours before with TNF-α applied locally (0.5 μg/mouse). The effects of anti–PSGL-1 antibody (4RA10 or 2PH1) and anti–E-selectin antibody were tested. Data are presented as mean ± SEM. *P < .05 relative to untreated value; **P < .05 relative to 4RA10 treatment.
TNF-α–induced leukocyte rolling in cremaster venules of PSGL-1-/- mice is inhibited by anti–E-selectin antibody
In this series of experiments we used mice deficient in PSGL-1 after 3 to 4 hours of local TNF-α injection. Compared with wild-type mice (Figure 1A), PSGL-1-/- mice had reduced numbers of rolling leukocytes (Figure 3A, second column) as already described.14 Addition of anti–E-selectin antibody essentially eliminated the number of rolling leukocytes in PSGL-1-/- mice (Figure 3A, third column). This result strongly suggests that E-selectin ligands other than PSGL-1 also exist. The leukocyte rolling velocity increased dramatically following E-selectin antibody administration, consistent with the view that E-selectin mediates slow rolling26 (Figure 3B). Note that in PSGL-1-/- mice, no leukocyte was seen rolling in control conditions (Figure 3A, first column). It is noteworthy that the PSGL-1-/- mouse has much more E-selectin–dependent rolling (approximately 10 cells/min; Figure 3A) versus wild-type mice treated with the 4RA10 anti–PSGL-1 antibody (Figure 1A), suggesting that these other E-selectin ligands are up-regulated in this knockout mouse. It is worth noting that the anti–PSGL-1 antibody (4RA10) had absolutely no effect in the PSGL-1-/- mouse (data not shown), suggesting that the greater reduction in rolling with the antibody in the wild-type mice was not due to effects beyond PSGL-1.
Leukocyte rolling flux and velocity in cremaster microcirculation of PSGL-1-/- mice treated with TNF-α. Leukocyte rolling flux (A) and leukocyte rolling velocity (B) in cremaster microcirculation of PSGL-1-/- mice treated 3 hours before with TNF-α applied locally (0.5 μg/mouse). The effect of anti–E-selectin antibody was tested. The first bar at left represents the values for leukocyte rolling and leukocyte rolling velocity under control conditions. Data are presented as mean ± SEM. *P < .05 relative to PSGL-1-/- mice treated with TNF-α (second column).
Leukocyte rolling flux and velocity in cremaster microcirculation of PSGL-1-/- mice treated with TNF-α. Leukocyte rolling flux (A) and leukocyte rolling velocity (B) in cremaster microcirculation of PSGL-1-/- mice treated 3 hours before with TNF-α applied locally (0.5 μg/mouse). The effect of anti–E-selectin antibody was tested. The first bar at left represents the values for leukocyte rolling and leukocyte rolling velocity under control conditions. Data are presented as mean ± SEM. *P < .05 relative to PSGL-1-/- mice treated with TNF-α (second column).
An E-selectin ligand other than PSGL-1 is down-regulated upon activation
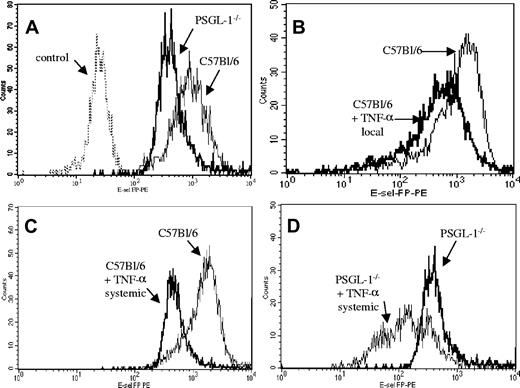
Figure 4A demonstrates that neutrophils from wild-type mice bound E-selectin chimera with great avidity (mean fluorescent intensity [MFI] = 2000). There was an 8-fold reduction of E-selectin binding in PSGL-1-/- mice (MFI = 300); however, some binding remained. This binding was not nonspecific, as it was elevated above control values obtained by staining with the secondary and tertiary antibodies alone (data not shown). Interestingly, the local injection of TNF-α into C57Bl/6 mice caused a small but consistent decrease in E-selectin binding (Figure 4B). We were intrigued by this observation and rationalized that the local injection of TNF-α may cause some minor systemic activation leading to down-regulation of either PSGL-1 or some other E-selectin ligand. To see if this down-regulation could be enhanced, we injected mice systemically with TNF-α (intraperitoneal injection causes systemic inflammation in all tissues examined to date). Indeed, systemic TNF-α significantly reduced E-selectin binding from MFI = 2000 to MFI = 500 (Figure 4C). Interestingly, when systemic TNF-α was given to PSGL-1-/- mice, E-selectin binding was significantly reduced to near-control values (Figure 4D). Notably, this down-regulation of E-selectin ligand(s) was observed for only the neutrophils. Neither lymphocytes nor monocytes exhibited a significant reduction in expression of E-selectin ligand following TNF-α treatment (data not shown).
Flow cytometric analysis showing the binding in vitro of recombinant mouse E-selectin chimera to neutrophils from C57Bl/6 and PSGL-1-/- mice. (A) Binding to neutrophils of untreated C57Bl/6 and PSGL-1-/- mice along with cells alone as controls. (B) Binding to C57Bl/6 neutrophils and C57Bl/6 neutrophils after 3 hours of TNF-α local injection (intrascrotal). (C) Binding to C57Bl/6 neutrophils and C57Bl/6 neutrophils after 3 hours of TNF-α injection (intraperitoneal). (D) Binding to PSGL-1-/- neutrophils and PSGL-1-/- neutrophils after 3 hours of TNF-α systemic injection (intraperitoneal). Binding was detected with PE. The data are representative of 4 experiments.
Flow cytometric analysis showing the binding in vitro of recombinant mouse E-selectin chimera to neutrophils from C57Bl/6 and PSGL-1-/- mice. (A) Binding to neutrophils of untreated C57Bl/6 and PSGL-1-/- mice along with cells alone as controls. (B) Binding to C57Bl/6 neutrophils and C57Bl/6 neutrophils after 3 hours of TNF-α local injection (intrascrotal). (C) Binding to C57Bl/6 neutrophils and C57Bl/6 neutrophils after 3 hours of TNF-α injection (intraperitoneal). (D) Binding to PSGL-1-/- neutrophils and PSGL-1-/- neutrophils after 3 hours of TNF-α systemic injection (intraperitoneal). Binding was detected with PE. The data are representative of 4 experiments.
The enhanced E-selectin ligand down-regulation with systemic TNF-α prompted us to examine leukocyte rolling in the cremaster muscle following systemic (intraperitoneal) TNF-α administration. In PSGL-1-/- mice that received local TNF-α, approximately 10 cells rolled per minute (Figure 5A). When TNF-α was given systemically into PSGL-1-/- mice, the number of rolling leukocytes decreased to near-zero values (Figure 5A). TNF-α administered systemically to C57Bl/6 mice resulted in approximately 30 cells/minute rolling in the cremaster microcirculation (Figure 5A). Addition of PSGL-1 antibody (4RA10) to C57Bl/6 mice treated systemically with TNF-α eliminated all rolling (Figure 5B). This is in contrast to local TNF-α administration, wherein a few rolling cells were always evident. Clearly, the few cells rolling independent of PSGL-1 (following local TNF-α administration; Figure 1A) did so via a down-regulatable E-selectin ligand.
Leukocyte rolling flux in cremaster microcirculation of C57Bl/6 and PSGL-1-/- mice treated 3 hours before with TNF-α intraperitoneally or locally (0.5 μg/mouse). The effect of systemic or local injection of TNF-α is shown in C57Bl/6 and PSGL-1-/- mice (A). Also, the effect of PSGL-1 antibody (4RA10) was tested in C57Bl/6 mice injected systemically with TNF-α (B). Data are presented as mean ± SEM. *P < .05 relative to PSGL-1-/- mice that received local injection of TNF-α.
Leukocyte rolling flux in cremaster microcirculation of C57Bl/6 and PSGL-1-/- mice treated 3 hours before with TNF-α intraperitoneally or locally (0.5 μg/mouse). The effect of systemic or local injection of TNF-α is shown in C57Bl/6 and PSGL-1-/- mice (A). Also, the effect of PSGL-1 antibody (4RA10) was tested in C57Bl/6 mice injected systemically with TNF-α (B). Data are presented as mean ± SEM. *P < .05 relative to PSGL-1-/- mice that received local injection of TNF-α.
Systemic TNF-α induces exclusively PSGL-1–dependent rolling in dermal skin microcirculation
Next, we performed experiments in skin, a vasculature wherein E-selectin is the dominant selectin.31 Figure 6A demonstrates that approximately 8 to 10 cells/minute rolled in the C57Bl/6 mouse ear following 3 to 4 hours of systemic TNF-α administration (second column) as opposed to 35 to 40 cells/minute in control conditions (first column). No leukocyte rolling could be detected in the ear skin preparation in PSGL-1-/- mice after systemic TNF-α (Figure 6A). Next, the 2 antibodies against PSGL-1 (4RA10 and 2PH1) were tested in the skin of C57Bl/6 mice. Just like in the PSGL-1-/- mice, 4RA10 abolished all leukocyte rolling flux after systemic TNF-α. One cell was seen to roll in the entire series of experiments. Interestingly, 2PH1 again did not abolish all rolling (Figure 6A), consistent with this being a less effective PSGL-1 inhibitor. The 2PH1 antibody did not affect leukocyte rolling velocity (Figure 6B).
Leukocyte rolling flux and velocity in ear skin microcirculation of C57Bl/6 and PSGL-1-/- mice. Leukocyte rolling flux (A) and leukocyte rolling velocity (B) in ear skin microcirculation of C57Bl/6 and PSGL-1-/- mice. ▪ represent the values for leukocyte rolling flux (A) and leukocyte rolling velocity (B) in C57Bl/6 under control conditions. The remaining bars represent the experiments done in C57Bl/6 and PSGL-1-/- mice treated 3 hours before with TNF-α intraperitoneally (0.5μg/mouse). The effects of PSGL-1 antibodies (4RA10 or 2PH1) were tested in C57Bl/6 mice. Data are presented as mean ± SEM. *P < .05 relative to C57Bl/6 mice treated with TNF-α.
Leukocyte rolling flux and velocity in ear skin microcirculation of C57Bl/6 and PSGL-1-/- mice. Leukocyte rolling flux (A) and leukocyte rolling velocity (B) in ear skin microcirculation of C57Bl/6 and PSGL-1-/- mice. ▪ represent the values for leukocyte rolling flux (A) and leukocyte rolling velocity (B) in C57Bl/6 under control conditions. The remaining bars represent the experiments done in C57Bl/6 and PSGL-1-/- mice treated 3 hours before with TNF-α intraperitoneally (0.5μg/mouse). The effects of PSGL-1 antibodies (4RA10 or 2PH1) were tested in C57Bl/6 mice. Data are presented as mean ± SEM. *P < .05 relative to C57Bl/6 mice treated with TNF-α.
PSGL-1–independent E-selectin ligand has an important physiologic role in a CHS
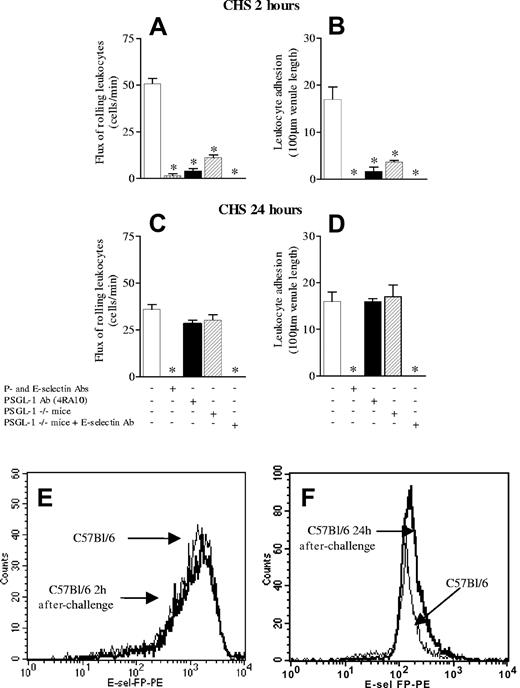
Since the PSGL-1–independent E-selectin ligand only mediates rolling of a few cells, we wished to determine whether the E-selectin ligand is important pathologically. We have previously shown that early leukocyte recruitment (within the first 2 hours of antigen challenge) is entirely dependent on P- and E-selectin,16 an observation confirmed in Figure 7A-B. Further, leukocyte recruitment observed at 24 hours of contact sensitivity is dependent upon this early P- and E-selectin–dependent leukocyte recruitment.16 In fact, the administration of anti–P- and E-selectin antibodies at the time of antigen challenge completely abrogated leukocyte recruitment at 24 hours of CHS (Figure 7C-D) in C57Bl/6 mice. This lack of leukocyte recruitment paralleled a lack of inflammation or edema as assessed by histology and ear thickness measurements, respectively (Table 1). We observed that treatment of C57Bl/6 mice with anti–PSGL-1 antibody, 4RA10, greatly attenuated leukocyte rolling and adhesion at 2 hours of CHS (Figure 7A-B). However, this did not translate into an attenuated CHS response at 24 hours; indeed, the inflammatory response was similar to that seen in untreated mice as assessed by intravital microscopy (Figure 7C-D) and ear thickness measurement (Table 1). PSGL-1-/- mice yielded similar results as with the application of 4RA10 in wild-type mice (Figure 7; Table 1). However, when an anti–E-selectin antibody was administered at the time of challenge in a PSGL-1-/- mouse, no leukocyte recruitment was seen at either 2 or 24 hours of CHS. Clearly, the small number of cells recruited early in CHS by PSGL-1–independent, E-selectin–mediated rolling were sufficient to reconstitute a full inflammatory response. Figures 7E and 7F are flow cytometry profiles showing that in this model of CHS there is absolutely no down-regulation of E-selectin ligand in either neutrophils or any other cell type at 2 or 24 hours, respectively.
Leukocyte rolling flux and leukocyte adhesion in contact hypersensitivity (CHS) model 2 and 24 hours after challenge. (A,C) Leukocyte rolling flux at 2 and 24 hours after challenge, respectively. (B,D) Leukocyte adhesion at 2 and 24 hours after challenge, respectively. The effects of anti–P- and E-selectin antibodies in combination are shown as well as the anti–PSGL-1 antibody (4RA10) and in PSGL-1-/- mice. Finally, the effect of anti–E-selectin antibody in PSGL-1-/- is shown. (E,F) Flow cytometric analysis showing the binding in vitro of recombinant mouse E-selectin chimera to neutrophils from C57Bl/6 at 2 and 24 hours of CHS, respectively. Data are presented as mean ± SEM. *P < .05 relative to untreated value.
Leukocyte rolling flux and leukocyte adhesion in contact hypersensitivity (CHS) model 2 and 24 hours after challenge. (A,C) Leukocyte rolling flux at 2 and 24 hours after challenge, respectively. (B,D) Leukocyte adhesion at 2 and 24 hours after challenge, respectively. The effects of anti–P- and E-selectin antibodies in combination are shown as well as the anti–PSGL-1 antibody (4RA10) and in PSGL-1-/- mice. Finally, the effect of anti–E-selectin antibody in PSGL-1-/- is shown. (E,F) Flow cytometric analysis showing the binding in vitro of recombinant mouse E-selectin chimera to neutrophils from C57Bl/6 at 2 and 24 hours of CHS, respectively. Data are presented as mean ± SEM. *P < .05 relative to untreated value.
Comparison of leukocyte adhesion and change in ear thickness at 24 hours of CHS
Mouse . | Antibody treatment at antigen challenge . | Adhesion, cells/100 μm . | Change in ear thickness, cm-3 . |
|---|---|---|---|
| Wild-type | No treatment | 16 ± 2 | 15 ± 1.5 |
| Wild-type | Anti-P- and anti-E-selectin antibody | 0* | 0* |
| Wild-type | Anti-PSGL-1 antibody (4RA10) | 16 ± 0.6 | 13.3 ± 1.6 |
| PSGL-1-/- | No antibody | 17 ± 2.5 | 13 ± 1.3 |
| PSGL-1-/- | Anti-E-selectin antibody | 0* | 0* |
Mouse . | Antibody treatment at antigen challenge . | Adhesion, cells/100 μm . | Change in ear thickness, cm-3 . |
|---|---|---|---|
| Wild-type | No treatment | 16 ± 2 | 15 ± 1.5 |
| Wild-type | Anti-P- and anti-E-selectin antibody | 0* | 0* |
| Wild-type | Anti-PSGL-1 antibody (4RA10) | 16 ± 0.6 | 13.3 ± 1.6 |
| PSGL-1-/- | No antibody | 17 ± 2.5 | 13 ± 1.3 |
| PSGL-1-/- | Anti-E-selectin antibody | 0* | 0* |
Data are presented as mean ± SEM.
P < .05 relative to untreated value
Discussion
In the present study we report that PSGL-1 inhibition completely blocks P-selectin–dependent rolling, and a large amount of E-selectin–dependent rolling. In fact, more than 98% of all rolling was eliminated using the PSGL-1 strategy. However, our data also reveal that there is a small yet consistent number of rolling cells on E-selectin that appear to be resistant to PSGL-1 inhibition. This particular rolling was sensitive to activation inasmuch as TNF-α administered systemically caused down-regulation of the PSGL-1–independent E-selectin ligand (as assessed by flow cytometry) and eliminated these few cells from interacting with the microvessels. Finally, our data reveal that this very small amount of PSGL-1–independent leukocyte rolling was functionally important. In a localized inflammatory response like CHS, those cells that rolled via E-selectin but not PSGL-1 were entirely sufficient to reconstitute a full dermatitis at 24 hours. Clearly, our data would strongly advocate the tandem use of P-selectin and E-selectin antibodies or E-selectin and PSGL-1 antibodies for therapeutic use in localized models of inflammation but would caution against PSGL-1 inhibition therapy alone.
It appears that upon activation, potentially all of the selectin-dependent rolling ligands are down-regulatable molecules, suggesting that down-regulation may be a universally important mechanism for selectin de-adhesion or some other as yet unknown process. Indeed, L-selectin is rapidly shed following leukocyte activation. PSGL-1 has also been reported to be shed following activation with chemoattractants such as platelet-activating factor (PAF), phorbol myristate acetate (PMA),32 as well as proteases and pharmacologic molecules.33 Our data also showed down-regulation of PSGL-1 in vivo following systemic TNF-α administration (Figure 4C). In fact, even local administration of TNF-α caused some down-regulation of PSGL-1. Clearly, PSGL-1 is more amenable to down-regulation in vivo than the weak shedding reported in vitro with inflammatory molecules.33 Our data suggest that the PSGL-1–independent E-selectin ligand was also down-regulated in vivo following systemic TNF-α administration. However, at this time it is unclear whether the ligand is internalized or shed. Nevertheless, our data also suggest that this down-regulation translated into an important decrease in function, as PSGL-1–independent rolling was completely lost following systemic TNF-α administration.
The PSGL-1–independent, E-selectin–dependent rolling has been described before by Yang et al.14 They observed ample rolling of cells following TNF-α stimulation in PSGL-1-/- mice. Xia et al15 observed PSGL-1–independent, E-selectin–dependent rolling in vitro. These investigators reported that there were fewer PSGL-1-/- than wild-type cells rolling on E-selectin but the rolling velocity was not different, entirely consistent with our in vivo observations. It was tempting to conclude that the PSGL-1-/- rolling was negligible since in vivo only a few cells rolled independent of this selectin ligand. However, when we examined the importance of this ligand in a model of CHS, it was clear that these few cells allowed for complete reconstitution of the inflammatory response. We have previously reported that in this particular model, P-selectin and E-selectin are absolutely required in the first 2 hours for the inflammation to occur at 24 hours.16 However, it is important to note that if the selectins were inhibited after 2 hours, the inflammation progressed without any limitations. Clearly, PSGL-1 was important only within the first phase of CHS. It is clear that despite the fact that PSGL-1 is the dominant ligand for P-selectin and E-selectin, the residual E-selectin–dependent rolling observed over the first 2 hours was sufficient to allow for the 24-hour inflammatory response to progress.
Clearly, our data demonstrate that the “other” E-selectin ligand(s) can be an important molecule for leukocyte recruitment. Numerous investigators have spent much time trying to identify E-selectin ligands other than PSGL-1 with limited success. Our data would suggest that PSGL-1 is not the only E-selectin ligand, since PSGL-1-/- mice still had E-selectin–dependent rolling. Although cutaneous lymphocyte-associated antigen (CLA) is another ligand for E-selectin, PSGL-1 is the major glycoprotein carrier of this carbohydrate modification. Therefore, the PSGL-1-/- mice would also be deficient in CLA. L-selectin fulfills some of the characteristics of our unknown E-selectin ligand inasmuch as L-selectin is shed from leukocytes during activation. Moreover, L-selectin was shown to be an E-selectin ligand in humans.34,36 However, mouse L-selectin has been shown not to bind mouse E-selectin, making this an unlikely ligand in our study.35 E-selectin ligand-1 (ESL-1),37,38 CD66-nonspecific cross-reacting antigen,39 CD43,40 and β2 integrin41,42 have all been proposed as potential ligands on leukocytes for E-selectin, although CD43 does not appear to support rolling and to our knowledge β2 integrin and CD66 are not down-regulated. Recently, a family of glycolipids, namely α2,3-sLex glycosphingolipids, have also been shown to support E-selectin–dependent rolling.43 That study demonstrated that α2,3-sLex glycosphingolipids were effective at tethering to E-selectin and mediating stable slow rolling in vitro, consistent with the slow rolling we observed in vivo.
Using PSGL-1 antibodies and the PSGL-1-/- mice has helped us to demonstrate that there exists a PSGL-1–independent ligand that is easily down-regulated during systemic inflammation but can play a very important role in leukocyte recruitment to local sites of inflammation. The loss of this ligand in models like systemic TNF-α administration may explain the complete loss of leukocyte rolling in PSGL-1-/- mice, whereas the retention of the E-selectin ligand in local inflammation would explain its importance in local models of inflammation.
Prepublished online as Blood First Edition Paper, August 10, 2004; DOI 10.1182/blood-2004-02-0578.
Supported by a group grant from the Canadian Institutes of Health Research (CIHR; P.K.). P.K. is a Canada Research Chair and an Alberta Heritage Foundation for Medical Research (AHFMR) scientist. G.A. and C.S.B. are fellows of the Canadian Association for Gastroenterology and J.M.H. is an AHFMR and CIHR training program student. R.C.O.Z. is a Multiple Sclerosis Society fellow.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal