Abstract
APEX2/APE2 is a secondary mammalian apurinic/apyrimidinic endonuclease that associates with proliferating cell nuclear antigen (PCNA), and the progression of S phase of the cell cycle is accompanied by its expression. To determine the biologic significance of APEX2, we established APEX2-null mice. These mice were about 80% the size of their wild-type littermates and exhibited a moderate dyshematopoiesis and a relatively severe defect in lymphopoiesis. A significant accumulation of both thymocytes and mitogen-stimulated splenocytes in G2/M phase was seen in APEX2-null mice compared with the wild type, indicating that APEX2 is required for proper cell cycle progression of proliferating lymphocytes. Although APEX2-null mice exhibited an attenuated immune response against ovalbumin in comparison with wild-type mice, they produced both antiovalbumin immunoglobulin M (IgM) and IgG, indicating that class switch recombination can occur even in the absence of APEX2. (Blood. 2004;104: 4097-4103)
Introduction
Oxidation or other chemical modification of nucleotides and genomic DNA is a major threat to living organisms because the damage inflicted may cause alterations in base pairs or may block progression of DNA replication and transcription. Base excision repair (BER) is one of the major cellular defense mechanisms for eliminating damaged bases in genomic DNA.1,2 DNA glycosylases catalyze the first step of BER by excising the damaged bases, and leave apurinic/apyrimidinic (AP) sites. Among the various mammalian DNA glycosylases, uracil DNA glycosylase (UNG) and MutY homolog (MUTYH) excise misincorporated bases during DNA replication, and both enzymes associate with proliferating cell nuclear antigen (PCNA), a scaffold protein for DNA replication machinery3,4 ; thus, both can initiate replication-associated BER.4-7 APEX2/APE2,8,9 a secondary mammalian AP endonuclease, is known to associate with PCNA through its PCNA-binding motif whereas the major AP endonuclease, APEX1/APE1/HAP1/REF1,7,10-12 does not possess PCNA-binding motif. Therefore, we hypothesize that APEX2 is responsible for the incision of AP sites in replication-associated BER.8,9 APEX1 was reported to be essential for early embryonic development in mice.13 However, it has not yet been established that AP endonuclease activity is essential for mammals because, in addition to DNA repair, APEX1 also functions as a redox regulator of transcription factors.12 Investigating the functions of both APEX1 and APEX2 is considered to be essential to clarify the biologic importance of AP endonuclease activities. To explore the biologic significance of APEX2 in vivo, we established APEX2-null mice.14 We report herein that APEX2-null mice exhibit growth retardation and dyshematopoiesis accompanied by G2/M arrest.
Materials and methods
Cell culture
Isolated mouse splenocytes were cultured in RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Invitrogen), 50 μM 2-mercaptoethanol, 100 U/mL streptomycin, and 100 U/mL penicillin at 37°C in a humidified chamber with 5% CO2. APEX2-null mouse embryo fibroblasts (MEFs) were isolated from embryos (13.5 days postcoital), and were cultured in Dulbecco modified Eagle medium supplemented with 10% heat-inactivated fetal calf serum, 100 U/mL streptomycin, and 100 U/mL penicillin at 37°C in a humidified chamber with 5% CO2. The cells were routinely maintained by a standard 3T3 protocol, and spontaneously immortalized cell lines were thus established.
APEX2 expression plasmids
Two expression vectors for recombinant APEX2, pcDNA3.1/Hygro(+): mAPEX2 and pIRES1hyg:mAPEX2, were constructed by inserting DNA fragments encoding mouse APEX2 into the multiple cloning sites of pcDNA3.1/Hygro(+) (Invitrogen) and pIRES1hyg (Clontech, Palo Alto, CA), respectively.
Establishment of APEX2-null mice
Apex2-disrupted mice were established as described previously.14 Geno-types were analyzed by Southern blotting or by polymerase chain reaction (PCR) with the use of mouse tail DNA. PCR primers used to detect the wild-type allele were as follows: U796M, 5′GCAAGGCATCTCAACTATGGCTC3′; and L1321, 5′CTTCTCATCTTTGGACTCTGG3′. Mp2-5, 5′CTACGCATCGGTAATGAAGG3′, and L1321 were used to detect mutated allele. All primers were purchased from Hokkaido System Science (Sapporo, Japan). APEX2 protein was detected by Western blotting with the use of a specific antibody as described under “Western blotting.” Heterozygous female (Apex2+/-) mice were backcrossed with C57BL/6J males (Apex2+/Y). F9 or F10 male mice were used in most experiments unless stated otherwise in the text. APEX2-null female mice were obtained among offspring of Apex2 knock-out (KO) male mice and Apex2+/- female mice. All mice were maintained in an air-conditioned, light time-controlled, specific-pathogen-free room. The handling and killing of the animals were in accordance with the national prescribed guidelines, and ethical approval for the studies was granted by the Animal Experiment Committee of Kyushu University (Fukuoka, Japan).
Western blotting of APEX2
Cells were suspended in extract buffer (125 mM Tris-HCl [tris(hydroxymethyl)aminomethane-HCl], pH 6.8; 2% sodium dodecyl sulfate [SDS]; 5% glycerol) and disrupted by sonication. Cell lysates were then centrifuged at 100 000g. Protein concentrations of supernatant were analyzed by means of a DC Protein Assay Kit (Bio-Rad Laboratories, Mississauga, ON, Canada), and 25 μg protein per lane was subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting analysis with the use of anti-mAPEX2.
Body and organ weight analysis
All mice were measured for body weight 10 times simultaneously without anesthesia, and the mean value was calculated. To measure organ weights, mice were dissected under pentobarbital anesthesia (75 mg/kg), and abdominal vessels were cut for blood drainage. Each organ was carefully removed and immediately measured.
Thymocytes, splenocytes, and bone marrow cells
Thymus and spleen were ground with glass slides, and cells were suspended in ice-cold phosphate-buffered saline (PBS) or culture medium. Bone marrow cells were suspended in ice-cold PBS by means of a syringe with a needle. An aliquot of cell suspension was diluted, and cells were counted under a microscope. The remaining cells were subjected to other analyses.
Cell cycle analysis
Flow cytometric analysis of the cell cycle was performed as previously described.15 The cells were prepared by Nonidet P40 (NP40) treatment for the naked nuclei preparation. Then, 1 × 106 cells were centrifuged, washed with PBS, and suspended in fluorescence-activated cell sorter (FACS) buffer containing 3.4 mM sodium citrate, 10 mM NaCl, 0.1% NP40, and 50 μg/mL propidium iodide for 1 hour at 4 °C The data were analyzed by CellQuest and ModFit software (Becton Dickinson, San Jose, CA).
X-ray irradiation
Mice were irradiated with an MBR-1520R x-ray generator (Hitachi, Kashiwa, Chiba, Japan) mounted with a 1.0-mm aluminum filter. For cell count and thymocyte survival analysis, mice were irradiated at 0, 0.5, 1.0, 2.0, or 4.0 Gy and killed 21 hours after the irradiation. For cell cycle analysis, mice were irradiated at 0.5 Gy and killed 3 hours after irradiation. Thymocytes were collected, fixed with 70% of ethanol, and subjected to an analysis of DNA content.
Analysis of blood cells
Peripheral blood was collected from an axillary artery and promptly diluted with the same volume of PBS supplemented with 0.2% of EDTA-3K ([ethylenediaminetetraacetic acid]-3K). Densities of white blood cells, red blood cells, and platelets in peripheral blood were analyzed with a K-4500 hematology analyzer (Sysmex, Kobe, Japan). Surface marker molecules on peripheral white blood cells, thymocytes, and bone marrow cells were analyzed by means of an LSR flow cytometer (Becton Dickinson) after immunostaining with specific antibodies conjugated to fluorescent dye and treated with OptiLyse B reagent (Beckman Coulter, Hialeah, FL).
Transfection and establishment of stable transfectants of MEFs
Three cell lines of APEX2-null MEFs were transfected with pcDNA3.1/Hygro(+), pcDNA3.1/Hygro(+):mAPEX2, pIRES1hyg, or pIRES1hyg: mAPEX2 with the aid of LipofectAMINE (Invitrogen) according to the manufacturer's instructions, and stable transfectants were selected in the presence of 300 μg/mL hygromycin B. Expression of recombinant APEX2 protein was confirmed by Western blotting.
Colony formation assay
Exponentially growing MEFs were plated in 10-cm dishes at a density of 500 cells per dish. The cells attached to dishes by incubation for 6 hours were exposed to various doses of x-ray irradiation as described under “X-ray irradiation.” Each plate was incubated for 2 more weeks. Formed colonies were stained in 25% ethanol containing 0.3% crystal violet, and counted.
Splenocyte proliferation assay
Splenocytes were prepared from 6-week-old male mice with an Apex2- or Apex2+ genotype, treated with acetate kinase lysing buffer (pH 7.4, 150 mM NH4Cl 10 mM KHCO3, 0.1 mM Na2-EDTA), and suspended in culture medium supplemented with 20 μg/mL lipopolysaccharide (LPS) (from Escherichia coli, 055:B5; Sigma, St Louis, MO) or 4 μg/mL concanavalin A (ConA) (Wako, Osaka, Japan) at a cell density of 1.0 × 106/mL. Then, 200 μL cell suspension per well was dispensed in multiple-well plates and cultured for 72 hours. Every 24 hours, the cells from 3 wells subjected to each treatment were harvested, and then viable and dead cells were stained by the trypan blue exclusion method and counted under a microscope.
For cell cycle analysis, 2 mL splenocyte suspension per well was dispensed in a 24-well plate. Cells cultured with LPS or concanavalin A were harvested at 48 hours and 60 hours, respectively. Nuclei were isolated from the harvested cells, and the DNA contents were analyzed as described.14
To analyze expression of APEX2 protein, 10 mL splenocyte suspension per dish was placed in 90-mm-diameter dishes (Nunc, Rochester, NY) for 48 hours, and cells were harvested. Whole-cell extracts were prepared and subjected to Western blotting with anti-mAPEX2.
Analysis of the immune response to chicken egg ovalbumin
Eleven-week-old wild-type (n = 6) and APEX2-null mice (n = 6) were immunized with 3 intraperitoneal injections of chicken egg ovalbumin (OVA) (Sigma) (300 μg per injection) on days 0, 21, and 35. Animals were separated into 2 groups, and on alternate weeks, peripheral blood was collected from the tail veins of each group in biweekly sampling. Concentrations of total immunoglobulin M (IgM), IgA, and IgG in serum were analyzed by the single radial immunodiffusion method,16 and titers of OVA-specific antibodies were determined by an enzyme-linked immunosorbent assay using peroxidase-conjugated anti-mouse IgM (Rockland, Gilbertsville, PA) and anti-mouse IgG (Jackson ImmunoResearch Laboratories, Bar Harbor, ME) antibodies. Peroxidase activities were measured with a Sumilon peroxidase assay kit (Sumitomo Bakelite, Tokyo, Japan), according to the manufacturer's instructions.
Statistical analysis
To determine statistical significance, data of wild-type and APEX2-null mice were analyzed by the Welch t test, Mann-Whitney U test, or 2-way analysis of variance (ANOVA) with the use of StatView Software Ver. 5.0 (SAS Institute, Cary, NC).
Results
Growth retardation in APEX2-null mice
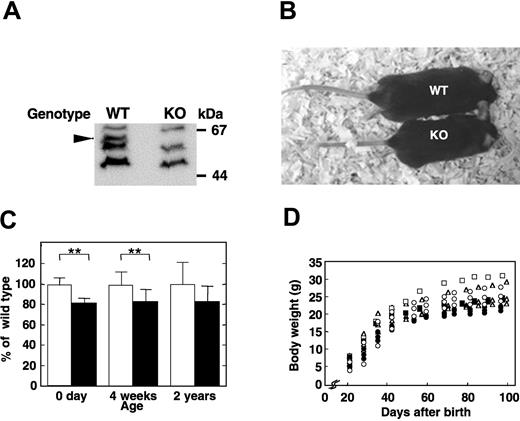
Heterozygous female (Apex2+/-) mice bearing a mutated Apex2 (Apex2-) allele on an X chromosome, in which a genomic region from the 3′ region of its intron 5 to 5′ region of its exon 6 was replaced with a neo cassette,14 were backcrossed with C57BL/6J males (Apex2+/Y). From the backcross, almost equal numbers of wild-type (Apex2+/Y) and knock-out (Apex2-/Y) males, and wild-type (Apex2+/+) and heterozygous females (Apex2+/-) were obtained, indicating that the Apex2 gene is not essential for viability (Table 1). The reverse-transcriptase polymerase chain reaction (data not shown) and Western blot analysis (Figure 1A) confirmed that thymocytes of Apex2-/Y mice are APEX2-null. The APEX2-null male mice showed moderate growth retardation (Figure 1B). Their body weights were about 80% those of the wild-type male littermates at birth, and this tendency persisted into childhood and adulthood (Figure 1C-D), indicating that all developing embryos, infants, and adults of APEX2-null mice may somehow be retarded in growth.
Genotypes of progeny from backcrosses of heterozygous female (Apex2-/+, F1) with wild-type male (Apex2+/Y, C57BL/6J) mice
. | Genotype, no. . | . | . | ||
|---|---|---|---|---|---|
| Sex . | WT . | Heterozygous . | KO . | ||
| Male | 13 | 0 | 15 | ||
| Female | 15 | 15 | 0 | ||
. | Genotype, no. . | . | . | ||
|---|---|---|---|---|---|
| Sex . | WT . | Heterozygous . | KO . | ||
| Male | 13 | 0 | 15 | ||
| Female | 15 | 15 | 0 | ||
Heterozygous, Apex2−/+; KO, Apex2−/− or Apex2−/Y.
Growth retardation of APEX2-null mice. (A) No APEX2 protein was detected in the Apex2 KO mouse. Whole-cell extracts of thymocytes from wild-type (WT) and Apex2 KO mice were prepared. APEX2 protein in extracts was detected by Western blotting using anti-APEX2 antibody as indicated by an arrowhead. (B) Photograph of an F6 15-week-old APEX2-null male mouse (KO) and its wild-type littermate (WT). Body weights of the KO and WT mice were 19.9 g and 27.2 g, respectively. (C) Mean values ± standard deviation (SD) of body weights of male mice with each genotype at an age of 0 days (F10 mice, 7 WT and 12 KO), 4 weeks (F9 mice, 10 WT and 13 KO), and 2 years (F3 mice, 12 WT and 7 KO) are shown. Open columns indicate WT; solid columns, KO mice. **P < .01. No asterisk indicates not statistically significant (P > .05). (D) Growth curves of APEX2-null males and their wild-type littermates. Each symbol tracks the growth of an individual mouse. Littermates with different genotypes are indicated with the same symbol. Open symbols indicate WT; closed symbols, KO mice.
Growth retardation of APEX2-null mice. (A) No APEX2 protein was detected in the Apex2 KO mouse. Whole-cell extracts of thymocytes from wild-type (WT) and Apex2 KO mice were prepared. APEX2 protein in extracts was detected by Western blotting using anti-APEX2 antibody as indicated by an arrowhead. (B) Photograph of an F6 15-week-old APEX2-null male mouse (KO) and its wild-type littermate (WT). Body weights of the KO and WT mice were 19.9 g and 27.2 g, respectively. (C) Mean values ± standard deviation (SD) of body weights of male mice with each genotype at an age of 0 days (F10 mice, 7 WT and 12 KO), 4 weeks (F9 mice, 10 WT and 13 KO), and 2 years (F3 mice, 12 WT and 7 KO) are shown. Open columns indicate WT; solid columns, KO mice. **P < .01. No asterisk indicates not statistically significant (P > .05). (D) Growth curves of APEX2-null males and their wild-type littermates. Each symbol tracks the growth of an individual mouse. Littermates with different genotypes are indicated with the same symbol. Open symbols indicate WT; closed symbols, KO mice.
Thymic atrophy
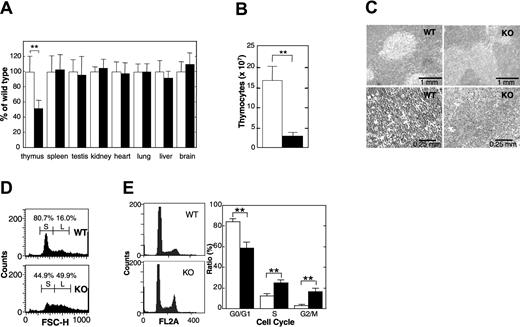
Thymus glands of APEX2-null mice exhibited statistically significant atrophy, and their relative weights were about 50% those of the wild-type thymus (Figure 2A). The total number of thymocytes in APEX2-null thymus decreased to about 20% of that in the wild-type thymus (Figure 2B). Histologic abnormalities in the cortex or medulla of thymi from APEX2-null mice were not apparent, however, APEX2-null thymocytes appeared to be larger than the wild-type cells (Figure 2C). Flow cytometric analysis clearly showed that a fraction of small cells abundant in wild-type thymocytes, which is likely to represent the resting CD4+CD8+ thymocytes,17 was largely reduced in the APEX2-null thymocytes (Figure 2D). Propidium iodide staining and flow cytometric analysis of isolated nuclei of thymocytes revealed that populations of APEX2-null thymocytes in both S and G2/M phases were statistically significantly increased compared with the wild type (Figure 2E), indicating a delayed progression of S phase and increased arrest in G2/M phase. APEX2-null female mice had essentially identical phenotypes, in terms of growth retardation and thymic atrophy, to those observed in APEX2-null male mice (data not shown).
Thymic atrophy in APEX2-null mice. (A) Organ weight. The body weight and weight of each organ of 32- or 33-day-old F7 male mice were measured, and the weight of each organ was normalized to the body weight of each mouse. The weight of each organ of APEX2-null mice (KO) was expressed relative to the weight of the corresponding organ of wild-type mice (WT) and shown as a percentage ± SD of the WT value. Open columns indicate WT (n = 11); solid columns, KO mice (n = 13). **P < .01. No asterisk indicates not statistically significant (P > .05) (B) Total number of thymocytes. Means ± SD of the total number of thymocytes in thymus from 4-week-old male mice are shown. Open column indicates WT (n = 7); solid column, KO mice (n = 7). **P < .01. (C) Histology. Thin sections of thymi of 4-week-old male mice were fixed with formaldehyde, embedded in paraffin, and stained with hematoxylin and eosin. Left panels are WT; right panels are KO mouse. (D) Flow cytometric analysis of thymocyte sizes. Thymocytes from WT (upper panel) and KO (lower panel) mice were analyzed by flow cytometry. Size distributions of thymocytes represented by forward scatter heights (FSC-Hs) are shown in histograms. Fractions of small and large thymocytes are indicated by S and L, respectively. (E) APEX2-null thymocytes show abnormal progression of the cell cycle. Isolated nuclei were stained with propidium iodide and cell cycle distribution was determined by flow cytometry. Left panels indicate representative histograms of DNA contents in isolated nuclei of thymocytes from WT (n = 7) and KO mice (n = 8). The distribution of isolated nuclei in each cell cycle phase was determined with the use of ModFit software and is shown with SD in the right panel. **P < .01.
Thymic atrophy in APEX2-null mice. (A) Organ weight. The body weight and weight of each organ of 32- or 33-day-old F7 male mice were measured, and the weight of each organ was normalized to the body weight of each mouse. The weight of each organ of APEX2-null mice (KO) was expressed relative to the weight of the corresponding organ of wild-type mice (WT) and shown as a percentage ± SD of the WT value. Open columns indicate WT (n = 11); solid columns, KO mice (n = 13). **P < .01. No asterisk indicates not statistically significant (P > .05) (B) Total number of thymocytes. Means ± SD of the total number of thymocytes in thymus from 4-week-old male mice are shown. Open column indicates WT (n = 7); solid column, KO mice (n = 7). **P < .01. (C) Histology. Thin sections of thymi of 4-week-old male mice were fixed with formaldehyde, embedded in paraffin, and stained with hematoxylin and eosin. Left panels are WT; right panels are KO mouse. (D) Flow cytometric analysis of thymocyte sizes. Thymocytes from WT (upper panel) and KO (lower panel) mice were analyzed by flow cytometry. Size distributions of thymocytes represented by forward scatter heights (FSC-Hs) are shown in histograms. Fractions of small and large thymocytes are indicated by S and L, respectively. (E) APEX2-null thymocytes show abnormal progression of the cell cycle. Isolated nuclei were stained with propidium iodide and cell cycle distribution was determined by flow cytometry. Left panels indicate representative histograms of DNA contents in isolated nuclei of thymocytes from WT (n = 7) and KO mice (n = 8). The distribution of isolated nuclei in each cell cycle phase was determined with the use of ModFit software and is shown with SD in the right panel. **P < .01.
General dyshematopoiesis
An analysis of peripheral blood cells of 6-week-old mice revealed general and moderate dyshematopoiesis in APEX2-null young mice (Table 2). The number of white blood cells in APEX2-null mice was significantly (P < .01) reduced to 42% of the wild-type level, and populations of CD3+ T lymphocytes and B220+ B lymphocytes were 44.8% and 33.3% of the wild-type levels, respectively, although the numbers of red blood cells, CD11b+/Dx5- monocytes/granulocytes, Dx5+/CD3-, natural killer cells, but not platelets, were only slightly reduced (Table 2). We also analyzed peripheral blood of 13-week-old adult APEX2-null mice, and observed decreased densities of white and red blood cells as compared with wild-type mice (Table 2). In thymus of 6-week-old APEX2-null mice, most fractions of thymocytes (CD4+CD8+, CD4-CD8+, and CD4+CD8-) were significantly (P < .01) reduced to about 20% of the wild-type levels (Table 3), as was observed in 4-week-old APEX2-null mice (Figure 2B). The number of CD4-CD8- cells in the APEX2-null thymus was reduced to only 50% of the wild-type level. The relative ratio of B220+ B cells in bone marrow cells was reduced to 75% of the wild-type level, irrespective of IgM expression (Table 4).
Moderate dyshematopoiesis in 6- and 13-week-old APEX2-null mice
. | Density, counts/μL* . | . | |
|---|---|---|---|
| Age of mice and cell type . | Wild type . | APEX2-null . | |
| 6 wks | |||
| WBC† | 5400 ± 1095 | 2275 ± 640 | |
| RBC, × 104‡ | 928 ± 41 | 771 ± 29 | |
| PLT, × 104 | 60 ± 17 | 73 ± 25 | |
| CD11b+/D×5−‡ | 877 ± 338 | 482 ± 154 | |
| D×5+/CD3− | 522 ± 151 | 391 ± 90 | |
| B220+† | 3064 ± 513 | 1021 ± 308 | |
| CD3+† | 1440 ± 344 | 643 ± 179 | |
| CD8+ CD3+† | 506 ± 109 | 227 ± 59 | |
| CD4+ CD3+† | 817 ± 150 | 345 ± 86 | |
| 13 wks | |||
| WBC† | 6800 ± 748 | 3640 ± 804 | |
| RBC, × 104† | 945 ± 42 | 833 ± 29 | |
| PLT, × 104 | 85 ± 24 | 105 ± 19 | |
. | Density, counts/μL* . | . | |
|---|---|---|---|
| Age of mice and cell type . | Wild type . | APEX2-null . | |
| 6 wks | |||
| WBC† | 5400 ± 1095 | 2275 ± 640 | |
| RBC, × 104‡ | 928 ± 41 | 771 ± 29 | |
| PLT, × 104 | 60 ± 17 | 73 ± 25 | |
| CD11b+/D×5−‡ | 877 ± 338 | 482 ± 154 | |
| D×5+/CD3− | 522 ± 151 | 391 ± 90 | |
| B220+† | 3064 ± 513 | 1021 ± 308 | |
| CD3+† | 1440 ± 344 | 643 ± 179 | |
| CD8+ CD3+† | 506 ± 109 | 227 ± 59 | |
| CD4+ CD3+† | 817 ± 150 | 345 ± 86 | |
| 13 wks | |||
| WBC† | 6800 ± 748 | 3640 ± 804 | |
| RBC, × 104† | 945 ± 42 | 833 ± 29 | |
| PLT, × 104 | 85 ± 24 | 105 ± 19 | |
WBC indicates white blood cells; RBC, red blood cells; PLT, platelets,
The mean ± standard deviation representing at least 5 mice.
P < .01 by Welch t test.
P < .05 by Welch t test.
Thymic dyslymphopoiesis in 6-week-old APEX2-null mice
. | Cell count, × 105 in thymus* . | . | |
|---|---|---|---|
| Types of cells† . | Wild type . | APEX2-null . | |
| Total cells | 1156 ± 125 | 234 ± 32 | |
| CD4−CD8− | 45 ± 7 | 25 ± 5 | |
| CD4+CD8+ | 982 ± 128 | 179 ± 24 | |
| CD4−CD8+ | 33 ± 3 | 10 ± 3 | |
| CD4+CD8− | 96 ± 8 | 20 ± 3 | |
| CD3+ | 88 ± 14 | 18 ± 3 | |
. | Cell count, × 105 in thymus* . | . | |
|---|---|---|---|
| Types of cells† . | Wild type . | APEX2-null . | |
| Total cells | 1156 ± 125 | 234 ± 32 | |
| CD4−CD8− | 45 ± 7 | 25 ± 5 | |
| CD4+CD8+ | 982 ± 128 | 179 ± 24 | |
| CD4−CD8+ | 33 ± 3 | 10 ± 3 | |
| CD4+CD8− | 96 ± 8 | 20 ± 3 | |
| CD3+ | 88 ± 14 | 18 ± 3 | |
The mean ± standard deviation representing 5 mice.
P < .01 by Welch t test.
Decreased population of IgM− B lymphocytes in 6-week-old APEX2-null mice
. | Percentage in bone marrow cells* . | . | |
|---|---|---|---|
| Type of cells . | Wild type . | APEX2-null . | |
| B220+† | 35.2 ± 1.6 | 26.3 ± 2.2 | |
| B220+/lgM+ | 14.7 ± 2.2 | 12.2 ± 0.8 | |
| B220+/lgM−† | 20.4 ± 1.4 | 14.1 ± 2.4 | |
. | Percentage in bone marrow cells* . | . | |
|---|---|---|---|
| Type of cells . | Wild type . | APEX2-null . | |
| B220+† | 35.2 ± 1.6 | 26.3 ± 2.2 | |
| B220+/lgM+ | 14.7 ± 2.2 | 12.2 ± 0.8 | |
| B220+/lgM−† | 20.4 ± 1.4 | 14.1 ± 2.4 | |
The mean ± standard deviation representing 5 mice.
P < .01 by Welch t test.
Sensitivity to x-ray exposure
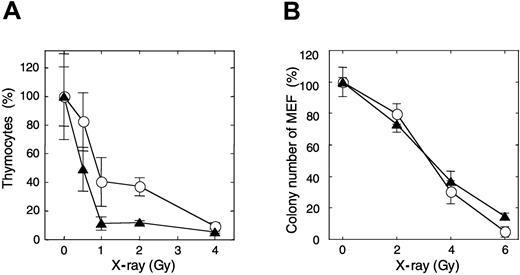
After mice were exposed to 1 Gy x-ray irradiation, about 11% of thymocytes in APEX2-null thymus survived, while more than 41% of thymocytes survived in the wild-type mice (Figure 3A). The sub-G1 fraction appeared in APEX2-null thymocytes 3 hours after the irradiation, but it was not so prominent in the wild type, indicating that APEX2-null thymocytes underwent apoptosis after x-ray exposure (data not shown). To examine whether the increased x-ray sensitivity of APEX2-null thymocytes is a general phenotype for any type of cells, we established immortalized MEFs from 3 APEX2-null embryos and introduced plasmid encoding mouse APEX2 protein to each cell line. Unexpectedly, each APEX2-null MEF line exhibited essentially the same colony-forming efficiency after exposure to various doses of x-ray irradiation, regardless of recombinant APEX2 expression (Figure 3B).
Increased sensitivity of thymocytes of APEX2-null mice to x-ray irradiation. (A) Mice were exposed to various x-ray doses. Thymus glands were collected 21 hours later, and viable thymocytes were counted. Ratios (percentages) of the numbers of thymocytes relative to those of an unirradiated control are shown with their mean values and SD. At least 3 mice were exposed to each x-ray dose. The difference between wild-type and APEX2-null mice was statistically significant (P = .0006, 2-way ANOVA). ○ indicates wild type; ▴, APEX2-null. (B) X-ray sensitivity of APEX2-null MEF lines. APEX2-null MEF lines with pIRES1hyg:mAPEX2 (○) or pIRES1hyg (▴) were plated in triplicate at a cell density of 500/10-cm dish and exposed to x-ray irradiation. After culture for 14 days, colonies were stained with crystal violet and counted. Ratios (percentages) of the numbers of colonies relative to those of an unirradiated control are shown with their mean values and SD.
Increased sensitivity of thymocytes of APEX2-null mice to x-ray irradiation. (A) Mice were exposed to various x-ray doses. Thymus glands were collected 21 hours later, and viable thymocytes were counted. Ratios (percentages) of the numbers of thymocytes relative to those of an unirradiated control are shown with their mean values and SD. At least 3 mice were exposed to each x-ray dose. The difference between wild-type and APEX2-null mice was statistically significant (P = .0006, 2-way ANOVA). ○ indicates wild type; ▴, APEX2-null. (B) X-ray sensitivity of APEX2-null MEF lines. APEX2-null MEF lines with pIRES1hyg:mAPEX2 (○) or pIRES1hyg (▴) were plated in triplicate at a cell density of 500/10-cm dish and exposed to x-ray irradiation. After culture for 14 days, colonies were stained with crystal violet and counted. Ratios (percentages) of the numbers of colonies relative to those of an unirradiated control are shown with their mean values and SD.
Abnormal proliferation and cell cycle progression of peripheral lymphocytes
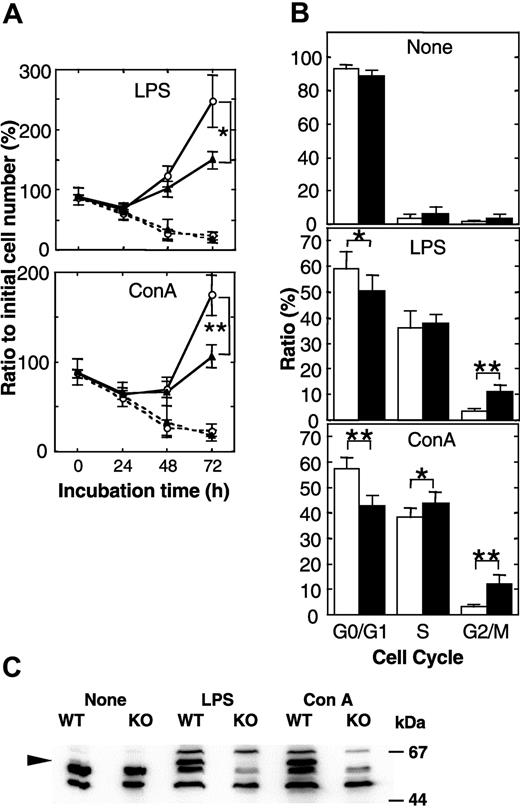
To examine whether the defects observed in lymphopoiesis in vivo are cell-autonomous defects, we further analyzed cultured primary splenocytes. In the absence of stimulation, splenocytes isolated from both APEX2-null and wild-type spleen were mostly in the quiescent state, and similarly underwent cell death during in vitro culture (Figure 4A). After stimulation with either LPS or ConA, APEX2-null splenocytes started to proliferate, but the extent of proliferation was much less than in wild-type splenocytes. The number of dead cells in the culture of APEX2-null splenocytes was almost equal to that of wild-type splenocytes (data not shown). The relative ratio of APEX2-null cells in G2/M phase was statistically significantly increased in comparison with the wild type (Figure 4B). Western blot analysis showed a notable induction of APEX2 expression in splenocytes after stimulation with these mitogens (Figure 4C).
Delayed progression of the cell cycle during proliferation of peripheral lymphocytes. (A) Mitogen-induced proliferation of splenocytes of wild-type (WT, n = 4) and APEX2-null (KO, n = 4) mice. Splenocytes were stimulated with LPS or ConA, and numbers of viable cells were counted under a microscope. Mean values ± SD are indicated. ○ indicates WT; ▴, KO. Solid lines represent cells incubated with mitogens; dotted lines indicate that no stimulant was used. **P < .01. *P < .05. (B) Cell cycle distribution of proliferating splenocytes. DNA contents of isolated nuclei stained with propidium iodide were analyzed by flow cytometry. Distribution of isolated nuclei in each cell cycle phase is shown as a percentage ± SD. Open columns indicate WT (n = 7); closed columns, KO mice (n = 10). **P < .01. *P < .05. (C) Induction of APEX2 expression by stimulation with LPS or ConA for 48 hours. APEX2 protein in whole-cell extracts of splenocytes from WT and KO mice was detected by Western blotting using anti-APEX2, as indicated by the arrowhead.
Delayed progression of the cell cycle during proliferation of peripheral lymphocytes. (A) Mitogen-induced proliferation of splenocytes of wild-type (WT, n = 4) and APEX2-null (KO, n = 4) mice. Splenocytes were stimulated with LPS or ConA, and numbers of viable cells were counted under a microscope. Mean values ± SD are indicated. ○ indicates WT; ▴, KO. Solid lines represent cells incubated with mitogens; dotted lines indicate that no stimulant was used. **P < .01. *P < .05. (B) Cell cycle distribution of proliferating splenocytes. DNA contents of isolated nuclei stained with propidium iodide were analyzed by flow cytometry. Distribution of isolated nuclei in each cell cycle phase is shown as a percentage ± SD. Open columns indicate WT (n = 7); closed columns, KO mice (n = 10). **P < .01. *P < .05. (C) Induction of APEX2 expression by stimulation with LPS or ConA for 48 hours. APEX2 protein in whole-cell extracts of splenocytes from WT and KO mice was detected by Western blotting using anti-APEX2, as indicated by the arrowhead.
Attenuated immune response in APEX2-null mice
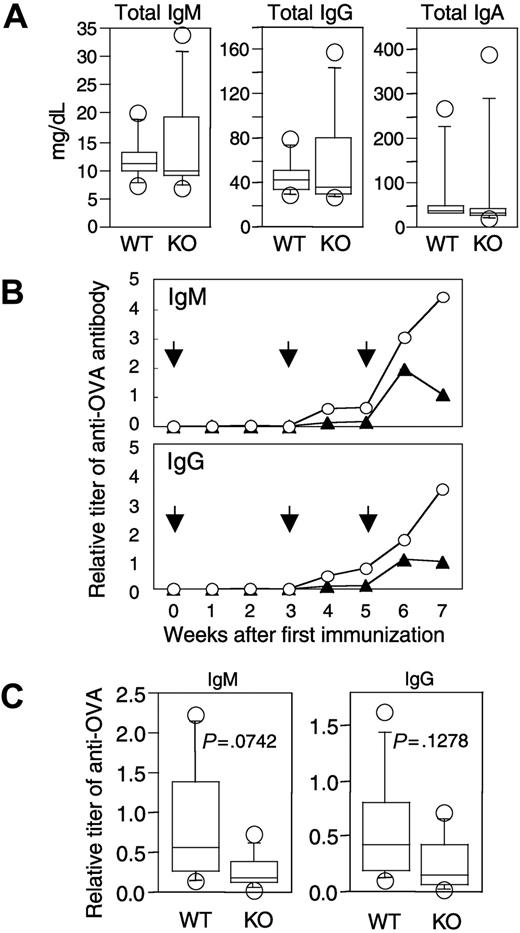
To evaluate the ability to produce functional antibodies, we initially analyzed levels of various immunoglobulins in peripheral blood of naive APEX2-null mice. Concentrations of total IgM, IgG, and IgA in peripheral blood of naive APEX2-null mice were essentially the same as in naive wild-type mice (Figure 5A). We then immunized APEX2-null and wild-type mice with OVA and collected peripheral blood from the immunized mice. From around 4 weeks after immunization, titers of anti-OVA IgM and IgG gradually increased in both APEX2-null and wild-type mice (Figure 5B); however, the mean titers of both anti-OVA IgM and anti-OVA IgG in the APEX2-null mice were always lower than those in the wild type, even at 7 weeks after the first immunization. We further immunized an additional 5 APEX2-null and 5 wild-type mice, collected their serum 4 weeks after the first immunization, and analyzed titers of anti-OVA antibodies. Although not of statistical significance (P > .05), mean titers of both anti-OVA IgM and anti-OVA IgG of APEX2-null mice were consistently lower than those of wild-type mice, as shown in Figure 5C.
Immunoglobulin levels in peripheral blood of naive and immunized APEX2-null mice. (A) Concentrations of IgM, IgG, and IgA of 11-week-old naive wild-type (WT, n = 7) and APEX2-null mice (KO, n = 8). Concentrations of immunoglobulins in serum are shown with box-and-whisker plots. In each plot, the box is bound top and bottom by upper and lower quartiles, and the statistical median is shown as a horizontal line within the box. The whiskers extend outward from the box to the farthest points that are not outliers (circles). The concentration of each immunoglobulin (IgM, IgG, or IgA) in serum of APEX2-null mice was not significantly different from that of wild-type mice (Mann-Whitney U test, P > .05). (B) Time-dependent induction of antigen-specific antibodies in wild-type and APEX2-null mice after immunization with OVA. Eleven-week-old wild-type (n = 6) and APEX2-null (n = 6) mice were immunized with 300 μg OVA on days 0, 21, and 35 as shown by arrows. Animals were separated into 2 groups, and serum was collected biweekly from each group of mice on alternate weeks. The mean titers of anti-OVA IgM and IgG of wild-type mice (○) and APEX2-null mice (▴) are shown as line graphs. (C) OVA-specific antibodies in wild-type and APEX2-null mice immunized with OVA for 4 weeks. A total of 8 mice were immunized with OVA as described in panel B. The titers of their serum anti-OVA IgM and IgG are shown with box-and-whisker plots. Differences between wild-type and APEX2-null mice were not statistically significant (Mann-Whitney U test, P > .05).
Immunoglobulin levels in peripheral blood of naive and immunized APEX2-null mice. (A) Concentrations of IgM, IgG, and IgA of 11-week-old naive wild-type (WT, n = 7) and APEX2-null mice (KO, n = 8). Concentrations of immunoglobulins in serum are shown with box-and-whisker plots. In each plot, the box is bound top and bottom by upper and lower quartiles, and the statistical median is shown as a horizontal line within the box. The whiskers extend outward from the box to the farthest points that are not outliers (circles). The concentration of each immunoglobulin (IgM, IgG, or IgA) in serum of APEX2-null mice was not significantly different from that of wild-type mice (Mann-Whitney U test, P > .05). (B) Time-dependent induction of antigen-specific antibodies in wild-type and APEX2-null mice after immunization with OVA. Eleven-week-old wild-type (n = 6) and APEX2-null (n = 6) mice were immunized with 300 μg OVA on days 0, 21, and 35 as shown by arrows. Animals were separated into 2 groups, and serum was collected biweekly from each group of mice on alternate weeks. The mean titers of anti-OVA IgM and IgG of wild-type mice (○) and APEX2-null mice (▴) are shown as line graphs. (C) OVA-specific antibodies in wild-type and APEX2-null mice immunized with OVA for 4 weeks. A total of 8 mice were immunized with OVA as described in panel B. The titers of their serum anti-OVA IgM and IgG are shown with box-and-whisker plots. Differences between wild-type and APEX2-null mice were not statistically significant (Mann-Whitney U test, P > .05).
Discussion
APEX2 is highly expressed in mouse thymus, spleen, and bone marrow, consistent with the significant abnormalities in these organs seen in APEX2-null mice.14 A major AP endonuclease, APEX1, is also highly expressed in these tissues; however, our results indicate that APEX2 deficiencies in these tissues are not compensated for by APEX1. Thus, results of the present study provide the first evidence for the biologic significance of APEX2 in mammals.
B lymphocytes were most severely reduced in peripheral blood of APEX2-null mice. Recently, it was reported that UNG-deficient mice and human patients exhibit abnormalities in immunoglobulin class-switch recombination and in somatic hypermutation in B lymphocytes.18,19 Nilsen et al20 reported the hyperplasia of lymphoid organs, including thymus in UNG-null mice; other mice deficient in any DNA glycosylase have never been reported to exhibit such hyperplasia of lymphoid organs. It has been shown that both UNG and APEX2 bind PCNA via their PCNA-binding motives, thus indicating that both play a crucial role during DNA replication or replication-associated BER.4 It is most likely that lack of UNG results in reduced generation of AP sites while lack of APEX2 in the presence of UNG results in increased accumulation of AP sites. Thus, we suggest that the large number of AP sites in lymphoid cells cause dyslymphopoiesis as we observed in APEX2-null mice, and that dyslymphopoiesis may be suppressed by simultaneous loss of UNG. The fact that the major AP endonuclease, APEX1, could not efficiently compensate for the lack of APEX2 clearly demonstrates the biologic significance of APEX2, especially in lymphoid organs. The highest expression of some other DNA glycosylases, including MUTYH and NEIL3 (endonuclease VIII-like 3), were also detected in thymus.1,21-23 MUTYH and NEIL3 have a PCNA-binding motif or a topoisomerase III homologous domain, respectively, which they shared in common with APEX2. Thus, we suggest that these DNA glycosylases may generate AP sites that are repaired by APEX2 in thymocytes. The difference in phenotypes of B lymphocytes and thymocytes may be due to accumulation of AP sites generated by UNG, MUTYH, NEIL3, or other DNA glycosylases. We did not observe any histologic abnormality in intestinal epithelium (data not shown), suggesting that the severely altered phenotype in APEX2-null mice is rather specific to lymphopoietic cells.
A decreased population of developing thymocytes as well as an abnormal proliferation of peripheral mature T lymphocytes was apparent in APEX2-null mice. Furthermore, various populations of hematopoietic lineages, which are derived from common stem cells in bone marrow, were significantly decreased in peripheral blood of APEX2-null mice. Thus, our data may suggest that there is an attenuated proliferation of bone marrow stem cells as well as abnormality in peripheral mature blood cells of APEX2-null mice.
Using spontaneously immortalized or simian virus 40 (SV40) T antigen-immortalized APEX2-null MEF lines, we analyzed their sensitivity to various DNA-damaging agents such as hydrogen peroxide, bleomycin, or x-ray irradiation; however, any protective effect of endogenous APEX2 in wild-type MEF lines or recombinant APEX2 protein expressed in all APEX2-null MEF lines was not evident (Figure 3B; also D.T., Y.I., Y.N., unpublished results, December 2002). Furthermore, after hydrogen peroxide challenge, we did not observe any difference in survival between APEX2-null and wild-type mouse embryonic stem cell lines.14 Thus, it is likely that APEX1 but not APEX2 plays major role in base excision repair against such DNA damaging agents in these cell lines, suggesting that APEX2 function is selectively required for proper cell cycle progression of activated T cells and B cells. The increased population of proliferating lymphocytes in both S and G2/M phases in APEX2-null mice suggests that an APEX2 deficiency increases the obstacles to be overcome for progression of the cell cycle. APEX2 but not APEX1 would be required in replication-associated BER to incise AP sites in the nascent strand because of the ability of APEX2 to bind PCNA. Thus, the lack of APEX2 most likely results in a delayed progression of replication-associated BER. We hypothesize 2 models for a mechanism of replication-associated BER. The first is a replication-coupled BER, in which misincorporated bases such as uracil or adenine opposite 8-oxoguanine are repaired immediately after their incorporation by replicative DNA polymerases. The other is a postreplicative BER, in which misincorporated bases left behind replication forks are repaired before completion of S phase. In either model, the inefficient PCNA-anchored BER machinery itself might be an obstacle to DNA replication and cause delayed S phase and G2/M phase arrest. We did not detect any increased apoptosis in thymocytes and stimulated splenocytes, and this may indicate that the remaining AP sites in APEX2-null cells are likely to be slowly repaired by APEX1. It has been reported that Saccharomyces cerevisiae also possesses a APEX2 homolog, Apn2.24 Recently, Guillet et al25 reported that an S cerevisiae mutant strain lacking apn1, rad1, ung1, and apn2, which possesses decreased AP endonuclease activities, exhibits growth delay owing to a G2/M checkpoint arrest. Our data observed in APEX2-null mice are in good agreement with the phenotype.
After immunization with OVA, production of anti-OVA antibodies in APEX2-null mice was somehow retarded in comparison with wild-type mice. Notably, anti-OVA IgG was apparently induced in APEX2-null mice as well as anti-OVA IgM, indicating that immunoglobulin class-switch recombination (CSR) was achieved. UNG, in addition to its general role in replication-associated BER, is thought to play a specific role in CSR by excising the uracil base produced by activation-induced cytidine deaminase (AID).18,19 This function of UNG might be independent of PCNA because generation of uracil base in DNA by AID should be independent of DNA replication. APEX2 is the PCNA-associated AP endonuclease, and thus may participate in replication-associated BER initiated by UNG or other DNA glycosylases, but not in the AID-initiated CSR during which UNG produces AP sites independent of PCNA.
An APEX2 deficiency in mice is not lethal and may be associated with certain human hereditary diseases. The human APEX2 gene is also located on the X chromosome9 ; thus, a mutation in one APEX2 allele of a germ line might easily lead to the birth of APEX2-deficient male offspring. The human diseases anticipated from an APEX2 deficiency might be more prevalent in males and be associated with decreased body size, moderate immunodeficiency, and a high sensitivity of lymphocytes to x-ray irradiation.
Prepublished online as Blood First Edition Paper, August 19, 2004; DOI 10.1182/blood-2004-04-1476.
Supported by grants from CREST, Japan Science and Technology Agency, the Ministry of Education, Culture, Sports, Science, and Technology of Japan (no. 15025257) and the Japan Society for the Promotion of Science (nos. 15590347 and 16390119).
Y.I. and D.T. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank M. Furuichi and H. Sumichika for their helpful discussions; A. Matsuyama, S. Kitamura, J. Ikeda, and Y. Yamada for their technical assistance; and W. Campbell for comments on the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal