Abstract
In the current report, we investigated the possibility of a cross-talk between receptor activator of NF-κB ligand (RANKL) and tumor necrosis factor α (TNF-α) using macrophage cell lines derived from wild-type mice and from mice with genetic deletion of the type 1 TNF receptor (p60-/-), the type 2 TNF receptor (p80-/-), or both receptors (p60-/-p80-/-). Deletion of TNF receptors sensitized the cells to RANKL-induced NF-κB activation, in order from least to most sensitive of p60-/- less than p80-/- less than p60-/-p80-/-. The effect on nuclear factor-κB (NF-κB) activation correlated with RANKL-induced IκBα kinase activation. Deletion of both TNF receptors also potentiated RANKL-induced c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase 1 and 2 (ERK1/2), and p38 mitogen-activated protein kinase (MAPK) activations in a dose- and time-dependent manner. Nitric oxide (NO) production and expression of inducible NO synthase (iNOS) and cyclooxygenase 2 (COX-2) induced by RANKL was also maximally induced in double knock-out cells. RANKL had no effect on the proliferation of wild-type cells, but deletion of TNF receptors induced growth modulatory effects. We also found that tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6), which mediates RANKL signaling, was constitutively bound to RANK in TNF receptor-deleted cells but not in wild-type cells, and this binding was enhanced by RANKL. Overall our results show that RANKL signaling is modulated by the TNF receptors and thus provide evidence of cross-talk between the receptors of 2 cytokines. (Blood. 2004;104: 4113-4121)
Introduction
Two decades ago, our group first purified and determined the structure of 2 tumor necrosis factors (TNF-α and TNF-β; also called lymphotoxin or LT),1-5 and these cytokines have now been shown to be highly pleiotropic.6 Since then 19 different members of the TNF superfamily have been identified, all characterized by their ability to modulate cell growth and to activate nuclear factor-κB (NF-κB), c-Jun N-terminal kinase (JNK), and p38 mitogen-activated protein kinase (MAPK).
The receptor for activation of NF-κB (RANK) ligand (RANKL), a member of the TNF superfamily, interacts with the cell surface receptor RANK and mediates dendritic cell survival and osteoclastogeneic signals7,8 through interaction with TNF receptor-associated factors (TRAF) 1, 2, 3, 5, and 6.9-12 That RANKL can activate NF-κB, JNK, extracellular signal-regulated kinase 1 and 2 (ERK1/2), and p38 MAPK is well established.13 We have recently shown that, like other members of the TNF superfamily, RANKL can modulate cell growth.13 We and others have shown that through the sequential recruitment of TRAF6 and NF-κB-inducing kinase (NIK), RANKL can activate NF-κB, and recruitment of TRAF2 leads to JNK activation.9-12
In contrast to RANKL, TNF binds to 2 distinct receptors, TNFR1 (also called p60) and TNFR2 (also called p80).6 Although TNFR2 recruits TRAF1 and TRAF2 directly, TNFR1 recruits only TRAF2 through TRADD (TNF receptor-associated death domain), an adaptor protein.6 Although most reports suggest that TNF mediates its signaling primarily though TNFR1, our report using TNFR-deleted cells suggests that both receptors are needed for optimum signaling.14 Using TNFR-deleted cells, we have also recently provided evidence of a cross-talk between TNF signaling and lipopolysaccharide (LPS) signaling.15 The cell signaling induced by both LPS and RANKL require the recruitment of TRAF6.
TNF-α and TNF-β, although products of distinct genes, have been found to bind to a common receptor and thus mediate similar cellular responses.16 There are several lines of evidence to show that either TNF-α-induced cellular responses overlap with those of RANKL or that the 2 cytokines mediate synergistic responses. These responses include osteoclastogenesis and bone resorption,17,18 dendritic cell survival, immune regulation, and inflammation.17-27 It has also been reported that TNFR1 and TNFR2 differentially regulate osteoclastogenesis.28 Besides cellular responses, the production of TNF is regulated by the RANKL.17 Whether there is cross-talk between TNF signaling and that induced by RANKL, however, is not known. To investigate the RANKL signaling, we used macrophages derived from mice in which either TNFR1 or TNFR2 or both receptors had been genetically deleted. We provide evidence that RANKL-induced activation of NF-κB, NF-κB-regulated gene expression, MAPK activation, and cellular growth are all modulated in cells with deleted TNF receptors, and this modulation correlated with enhanced association of TRAF6 with RANK.
Materials and methods
Materials
RANKL was kindly provided by Bryant Darnay (M. D. Anderson Cancer Center, Houston, TX). 3-(4, 5-Dihydro-6-(4-(3, 4-dimethoxy benzoyl)-1-piperazinyl)-2(1 H)-quinolinone (MTT) and anti-β-actin antibody were purchased from Sigma Chemical (St Louis, MO). NG-Nitro-L-argininemethyl ester (L-NAME) was purchased from Alexis Biochemical (San Diego, CA). Penicillin, streptomycin, RPMI 1640 medium, and fetal bovine serum (FBS) were obtained from Invitrogen (Grand Island, NY). Antibodies to p50, p65, cyclin D1, p38 MAPK, extracellular signal-regulated kinase (ERK)2, JNK1, inducible NO synthase (iNOS), NF-κB-inducing kinase (NIK), TRAF1, TRAF2, TRAF6, and poly(adenosine diphosphate [ADP]-ribose) polymerase (PARP) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against phospho-p38 MAPK and phospho-ERK1/2 were obtained from Cell Signaling Technology (Beverly, MA). Cyclooxygenase 2 (COX-2) antibody was purchased from BD Biosciences (San Diego, CA). Antibody against IκBα kinase (IKK)-α, IKK-β, RANK, cell-permeable TRAF6 binding peptide with or without a Kaposi fibroblast growth factor leader sequence (AAVALLPAVLLALLAP-RKIPTEDEYTDRPSQPST; leader sequence in italics), and mutant TRAF6 binding peptide (AAVALLPAVLLALLAP-RKIATADEATDRPSQPST; leader sequence in italics and mutated amino acids underlined) were kind gifts from Imgenex (San Diego, CA). N-acetyl-leucyl-leucyl-norleucinal (ALLN) was purchased from Calbiochem (San Diego, CA)
Cell lines and culture
Production of mice with genetic deletions of p60, p80, or both have been described.29,30 Briefly, immortalized macrophage cell lines were established from the bone marrow of wild-type (wt) C57BL/6J mice and its TNFR knock-out homozygous mice (p60-/-, p80-/-, and p60-/-p80-/-) as previously described.14,15 By using reverse transcriptase-polymerase chain reaction (RT-PCR), fluorescence-activated cell sorting (FACS) analysis, and Western blot analysis, the cells were shown to lack expression of TNF receptors.14 All cell lines were cultured in RPMI 1640 medium supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin.
NF-κB assay
NF-κB activation was analyzed by electrophoretic mobility shift assay (EMSA) as described previously.31 In brief, 15 μg nuclear extracts prepared from TNF-treated or untreated cells were incubated with 32 P end-labeled 45-mer double-stranded NF-κB oligonucleotide from human immunodeficiency virus-1 long terminal repeat (5′-TTGTTACAAGGGACTTTCCGCTGGGGACTTTCCAG GGAGGCGTGG-3′; underlined sequence is binding site) for 30 minutes at 37°C, and the DNA-protein complex was resolved in a 6.6% native polyacrylamide gel. The specificity of binding was examined by competition with unlabeled 100-fold excess oligonucleotide and with mutant oligonucleotide. The composition and specificity of binding was also determined by supershift of the DNA-protein complex using specific and irrelevant antibodies. For supershift experiments, the antibody-treated samples of NF-κB were resolved by 5.0% native polyacrylamide gel. The radioactive bands from the dried gels were visualized and quantitated by PhosphorImager (Molecular Dynamics, Sunnyvale, CA) using ImageQuant software.
IKK assay
The IKK assay was performed by a method described previously.14 Briefly, IKK complex from whole-cell extracts was incubated with antibody against IKK-α and precipitated with protein A/G-Sepharose beads (Pierce, Rockford, IL). The beads were washed with lysis buffer and then assayed in kinase assay mixture containing 50 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES; pH 7.4), 20 mM MgCl2, 2 mM dithiothreitol (DTT), 20 μCi (0.74 MBq) γ-32P] adenosine triphosphate (ATP), 10 μM unlabeled ATP, and 2 μg substrate glutathione S-transferase (GST)-IκBα. After incubation at 30°C for 30 minutes, the reaction was terminated by boiling with sodium dodecyl sulfate (SDS) sample buffer for 5 minutes. Finally, the protein was resolved on 10% SDS-PAGE (polyacrylamide gel electrophoresis), the gel was dried, and the radioactive bands were visualized by PhosphorImager. To determine the total amounts of IKK in each sample, same whole-cell extracts were resolved by 7.5% SDS-PAGE, electrotransferred to nitrocellulose membrane, and then blotted with either anti-IKK-α or IKK-β antibodies.
JNK assay
The JNK kinase assay was performed by a method described previously.32 Briefly, whole-cell extracts were incubated with antibody against JNK1, and precipitated with protein A/G-Sepharose beads (Pierce). The beads were washed with lysis buffer, and then assayed in kinase assay mixture containing 2 μg substrate GST-c-Jun (aa1-79). After incubation at 30°C for 30 minutes, the reaction was terminated by boiling with SDS sample buffer for 5 minutes. Finally, the protein was resolved on 10% SDS-PAGE, the gel was dried, and the radioactive bands were visualized by PhosphorImager. To determine the total amounts of IKK in each sample, same whole-cell extracts were resolved by 10% SDS-PAGE, electrotransferred to nitrocellulose membrane, and then blotted with anti-JNK1 antibody.
Western blot analysis
Whole-cell extracts (30-50 μg) were prepared as described32 and resolved by SDS-PAGE. Then the proteins were electrotransferred to a nitrocellulose membrane, blocked with 5% nonfat dry milk, and probed with primary antibodies for 2 hours at 4°C. The blotting membrane was washed, exposed to horseradish peroxidase-conjugated secondary antibodies for 1 hour, and detected by chemiluminescence (ECL; Amersham Pharmacia Biotech, Arlington Heights, IL).
NO production assay
The concentration of stable nitrite, the end product from NO generation by macrophages, was determined by Griess reaction.15 Equal volumes of test supernatant and Griess reagent (Sigma Chemical) were mixed and kept at room temperature for 15 minutes in a 96-well plate. The absorbance at 530 nm was then determined and quantified by extrapolation from a sodium nitrite standard curve in each experiment. Percentages of NO production in each macrophages control were set as 0%.
Cytotoxicity assay
The cytotoxic effects of RANKL were determined by the MTT uptake method as described.33 Briefly, 5000 cells were incubated in triplicate in 96-well plates with the indicated RANKL for 72 hours at 37°C. Thereafter, MTT solution was added to each well. After a 2-hour incubation at 37°C, extraction buffer (20% SDS, 50% dimethylformamide) was added, the mixture incubated overnight at 37°C, and the optical density measured at 570 nm using a 96-well multiscanner (Dynex Tech; MRX Revelation, Chantilly, VA).
NF-κB-dependent reporter assay
To study RANKL signaling, we examined the effects of TRAF2-, TRAF6-, TRAF5-DN (dominant negative), and RANK-, TRAF2-, TRAF6-wild-type expression vectors on the expression of a NF-κB-dependent reporter gene, secretory alkaline phosphatase (SEAP), as previously described.34 Macrophages, deficient in either or both types of TNF receptor, were plated in 6-well plates (0.5 million cells/well) and transiently transfected by the calcium phosphate method with pNF-κB-SEAP (0.5 μg), the inducer plasmid (1 μg), and the control plasmid pCMVFLAG1 DNA (1 μg). After 24 hours, cells were then incubated at the absence or presence of 5 nM RANKL for an additional 24 hours, and the cell culture medium harvested and analyzed for SEAP activity according to the protocol essentially as described by the manufacturer (Clontech, Palo Alto, CA). We used a 96-well fluorescence plate reader (Fluoroscan II; Labsystems, Chicago, IL) with excitation set at 360 nm and emission at 460 nm.
Immunoprecipitation
Cells were washed with ice-cold phosphate-buffered saline (PBS) and lysed in a buffer containing 50 mM HEPES (pH 7.4), 150 mM NaCl, 1 mM ethylene glycol tetraacetic acid (EGTA), 10 mM NaF, 10% glycerol, 0.2% Triton X-100, 2 mM sodium orthovanadate, 2 μg/mL aprotinin, and 2 μg/mL leupeptin. The whole-cell extracts were incubated with primary antibody for 2 hours and precipitated using protein A/G-Sepharose beads. After 1 hour of incubation, the immunocomplex was washed with lysis buffer, boiled with SDS sample buffer for 5 minutes, resolved on SDS-PAGE, and then analyzed by Western blotting.
Results
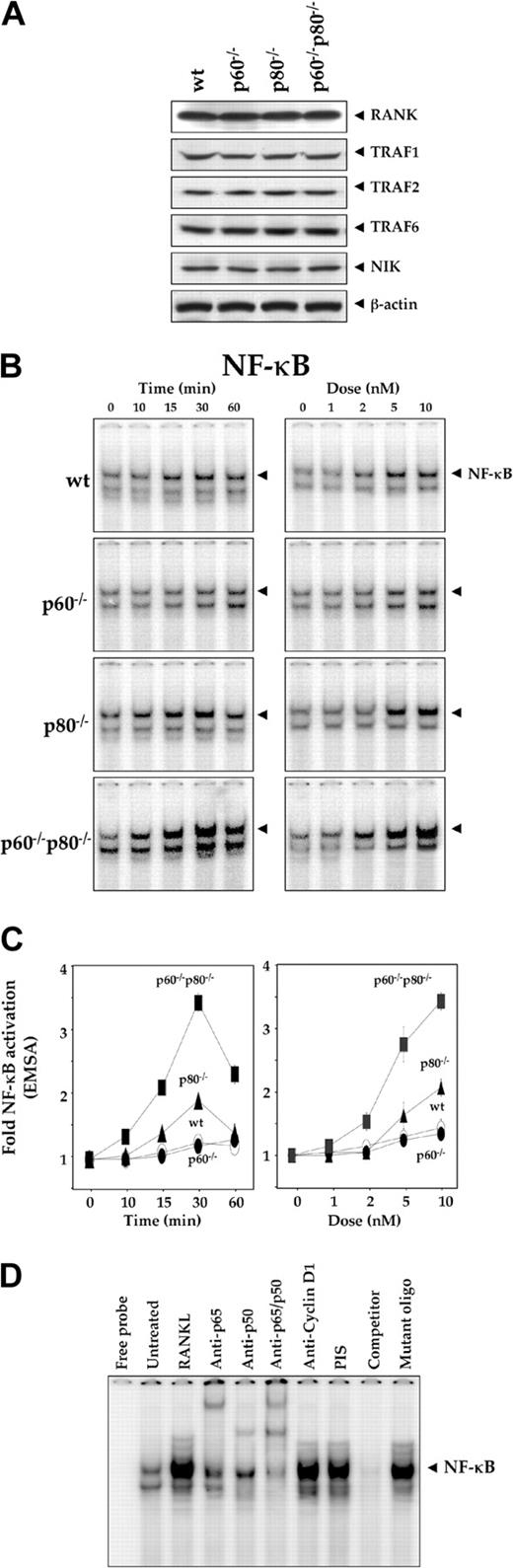
The aim of the present study was to investigate the role of TNF receptor in RANKL-induced cell signaling. To understand the role of each type of TNF receptor, we used macrophage cell lines isolated from mice in which the genes for either or both the TNF receptors have been deleted. We have reported the characterization of these cells for TNF signaling.14 Both RANKL and TNF have been shown to interact with macrophages and induce differentiation into osteoclast. Thus, to investigate the effect of TNFR on RANKL signaling in macrophages is preferred. Western blot analysis revealed that deletion of either or both TNF receptors had no effect on the expression of either RANK or the RANK-signaling proteins TRAF1, TRAF2, and TRAF6 (Figure 1A). NIK, which mediates RANK signaling, was also unaltered.
Deletion of TNF receptors enhances RANKL-induced activation of NF-κB. (A) Deletion of TNF receptors has no effect on the expression of RANKL signaling-related proteins. Whole-cell extracts were prepared from wild-type and TNF receptor-deleted macrophages, resolved by SDS-PAGE, and electrotransferred to a nitrocellulose membrane. Western blot analysis was performed using anti-RANKL, TRAF1, TRAF2, TRAF6, and NIK antibodies as described in “Materials and methods.” The same membrane was reblotted with anti-β-actin antibody. (B) Time- and dose-dependent NF-κB activation by RANKL in wild-type and TNF receptor-deleted macrophages. 1.0 × 106 cells were treated with 5 nM RANKL for the indicated times or with the indicated concentrations of RANKL for 30 minutes. Nuclear extracts were prepared and analyzed for NF-κB activation by EMSA as described in “Materials and methods.” (C) Graphical representation of the results shown in panel B. Results are expressed as fold activation over the untreated control; bars represent standard deviation. (D) Supershift and specificity of NF-κB. Nuclear protein was extracted from untreated or 5-nM RANKL-treated p60-/-p80-/- macrophages, incubated for 30 minutes with different antibodies, probed with nonlabeled NF-κB oligo, and then assayed for NF-κB activity by EMSA as described.
Deletion of TNF receptors enhances RANKL-induced activation of NF-κB. (A) Deletion of TNF receptors has no effect on the expression of RANKL signaling-related proteins. Whole-cell extracts were prepared from wild-type and TNF receptor-deleted macrophages, resolved by SDS-PAGE, and electrotransferred to a nitrocellulose membrane. Western blot analysis was performed using anti-RANKL, TRAF1, TRAF2, TRAF6, and NIK antibodies as described in “Materials and methods.” The same membrane was reblotted with anti-β-actin antibody. (B) Time- and dose-dependent NF-κB activation by RANKL in wild-type and TNF receptor-deleted macrophages. 1.0 × 106 cells were treated with 5 nM RANKL for the indicated times or with the indicated concentrations of RANKL for 30 minutes. Nuclear extracts were prepared and analyzed for NF-κB activation by EMSA as described in “Materials and methods.” (C) Graphical representation of the results shown in panel B. Results are expressed as fold activation over the untreated control; bars represent standard deviation. (D) Supershift and specificity of NF-κB. Nuclear protein was extracted from untreated or 5-nM RANKL-treated p60-/-p80-/- macrophages, incubated for 30 minutes with different antibodies, probed with nonlabeled NF-κB oligo, and then assayed for NF-κB activity by EMSA as described.
Deletion of TNF receptors sensitizes macrophages to RANKL-induced activation of NF-κB
Activation of NF-κB is one of the earliest events induced by RANKL. Whether RANKL-induced NF-κB activation is modulated by individual TNF receptors was investigated. We treated wt macrophages and their TNF receptor-deficient variants with RANKL, prepared the nuclear extracts, and analyzed them for NF-κB activation by EMSA. We found that both dose-dependent and time-dependent activation of NF-κB occurred in all cell types, but the levels of NF-κB activation varied with the receptor deletion (Figure 1B). Both dose-response and time course results indicated maximum RANKL-induced NF-κB activation in macrophages deficient in both TNF receptor (p60-/-p80-/-) cells and minimum activation in wt cells (Figure 1C). The deletion of the p80 receptor affected response at 15 and 30 minutes only; the p60-deletion had no significant effect.
EMSA of nuclear extracts prepared from RANKL-treated macrophages showed that either anti-p50 or anti-p65 antibodies abrogated/supershifted the NF-κB/DNA complex, whereas preimmune sera (PIS) or irrelevant anti-cyclin D1 antibodies had no effect (Figure 1D). Thus, NF-κB induced by RANKL in macrophages contained both the p50 and p65 (RelA) subunits. The specificity of the RANKL-induced NF-κB/DNA complex was also confirmed by demonstrating that the binding was disrupted in the presence of a 100-fold excess of unlabeled κB-oligonucleotide (Figure 1D, Competitor) but not by mutant oligonucleotide (Mutant oligo).
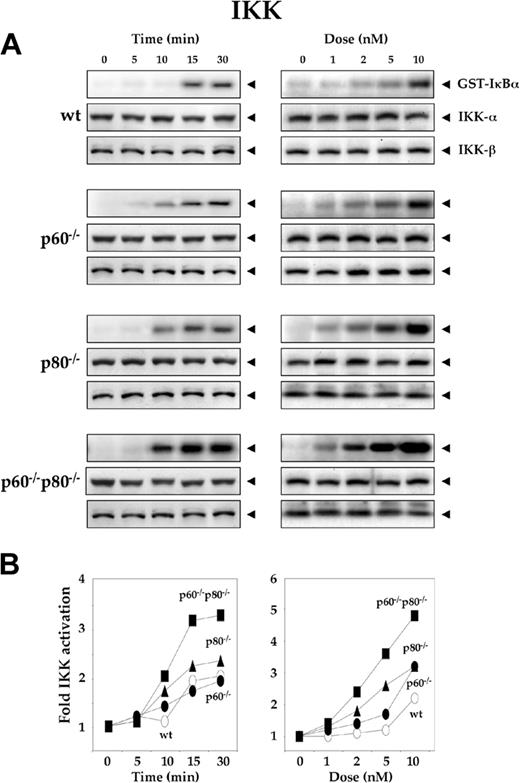
Deletion of TNF receptors sensitizes macrophages to RANKL-induced activation of IκBα kinase
Activation of NF-κB requires the activation of IKK. To determine whether RANKL-induced IKK activation was modulated by individual TNF receptors, we treated wt macrophages and TNF receptor-deficient variants with 5 nM RANKL for the indicated times and analyzed whole-cell extracts for IKK activity by immunecomplex kinase assay. The kinetics of IKK activation was slightly slower in wt macrophages (30 minutes) than in TNF receptor-deficient variants (10 minutes) (Figure 2A, left panel). In every case, activation of NF-κB was dose dependent, although the activation levels of IKK varied (Figure 2A, right panel). The wt macrophages were less sensitive to RANKL (10 nM) than in both TNF receptors-deficient (p60-/-p80-/-, 2 nM). Like NF-κB activation, these results also suggest that maximum RANKL-induced IKK activation occurs in macrophages deficient in both TNF receptors (p60-/-p80-/-) and minimum in wt cells (Figure 2B). Deletion of p60 receptor had minimal effect on RANKL-induced IKK activation. The deletion of p80 receptor had somewhat more effect than deletion of the p60 receptor.
Deletion of TNF receptors enhances the RANKL-induced activation of IκBα kinase. (A) Time- and dose-dependent IKK activation by RANKL in wild-type and TNF receptor-deleted macrophages. 1.0 × 106 cells were pretreated with 50 μg/mL ALLN and then treated with 5 nM RANKL for the indicated times or with the indicated concentrations of RANKL for 15 minutes. Whole-cell extracts were prepared, incubated with anti-IKK-α antibody, and immunoprecipitated with protein A/G-Sepharose beads. The beads were washed and subjected to kinase assay as described in “Materials and methods.” Fifty micrograms of the same protein extracts was resolved by 7.5% SDS-PAGE and electrotransferred to a nitrocellulose membrane. Western blot analysis was performed using anti-IKK-α and IKK-β antibodies. (B) Graphical representation of the results shown in panel A.
Deletion of TNF receptors enhances the RANKL-induced activation of IκBα kinase. (A) Time- and dose-dependent IKK activation by RANKL in wild-type and TNF receptor-deleted macrophages. 1.0 × 106 cells were pretreated with 50 μg/mL ALLN and then treated with 5 nM RANKL for the indicated times or with the indicated concentrations of RANKL for 15 minutes. Whole-cell extracts were prepared, incubated with anti-IKK-α antibody, and immunoprecipitated with protein A/G-Sepharose beads. The beads were washed and subjected to kinase assay as described in “Materials and methods.” Fifty micrograms of the same protein extracts was resolved by 7.5% SDS-PAGE and electrotransferred to a nitrocellulose membrane. Western blot analysis was performed using anti-IKK-α and IKK-β antibodies. (B) Graphical representation of the results shown in panel A.
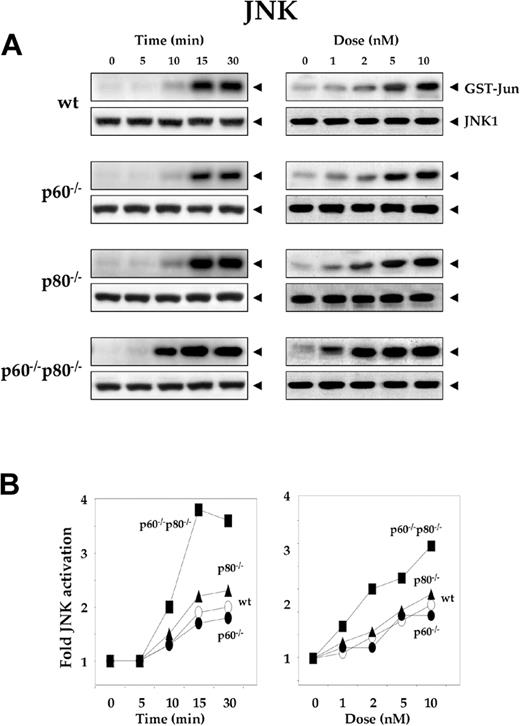
Deletion of TNF receptors sensitizes macrophages to RANKL-induced activation of JNK
Activation of JNK is another early cellular response induced by RANKL.23 To explore the specific role of TNF receptors in RANKL-induced JNK activation, we treated wt macrophages and their TNF receptor-deficient variants with 5 nM RANKL for the indicated times, and analyzed whole-cell extracts for JNK activity by immunecomplex kinase assay. Time-dependent activation of JNK occurred in wt and the TNF receptor-deficient macrophages, although the level of JNK activation varied (Figure 3A, left panel). The kinetics of JNK activation was slightly slower in wt macrophages (15 minutes) than in its TNF receptor-deleted variants (10 minutes) (Figure 3A, left panel). Activation of JNK was dose dependent in all 4, but the activation levels varied. The sensitivity to RANKL in JNK activation was less in wt macrophages (5 nM) than in both its TNF receptor-deficient variants (p60-/-p80-/-, 1 nM). Maximum RANKL-induced JNK activation occurred in macrophages lacking both TNF receptors (p60-/-p80-/-) and minimum in wt cells (Figure 3B). Deletion of p60 receptor once again had minimal effect on RANKL-induced NF-κB activation. The deletion of the p80 receptor had more effect than deletion of the p60 receptor.
Deletion of TNF receptors sensitizes macrophages to RANKL-induced activation of JNK. (A) Time- and dose-dependent JNK activation by RANKL in wild-type and TNF receptor-deleted macrophages. 1.0 × 106 cells were treated with 5 nM RANKL for the indicated times or with the indicated concentrations of RANKL for 15 minutes. Whole-cell extracts were prepared, incubated with anti-JNK1 antibody, and immunoprecipitated with protein A/G-Sepharose beads. The beads were washed and subjected to kinase assay as described in “Materials and methods.” Fifty micrograms of the same protein extract was resolved by 10% SDS-PAGE and electrotransferred to a nitrocellulose membrane. Western blot analysis was performed using anti-JNK1 antibody. (B) Graphical representation of the results shown in panel A.
Deletion of TNF receptors sensitizes macrophages to RANKL-induced activation of JNK. (A) Time- and dose-dependent JNK activation by RANKL in wild-type and TNF receptor-deleted macrophages. 1.0 × 106 cells were treated with 5 nM RANKL for the indicated times or with the indicated concentrations of RANKL for 15 minutes. Whole-cell extracts were prepared, incubated with anti-JNK1 antibody, and immunoprecipitated with protein A/G-Sepharose beads. The beads were washed and subjected to kinase assay as described in “Materials and methods.” Fifty micrograms of the same protein extract was resolved by 10% SDS-PAGE and electrotransferred to a nitrocellulose membrane. Western blot analysis was performed using anti-JNK1 antibody. (B) Graphical representation of the results shown in panel A.
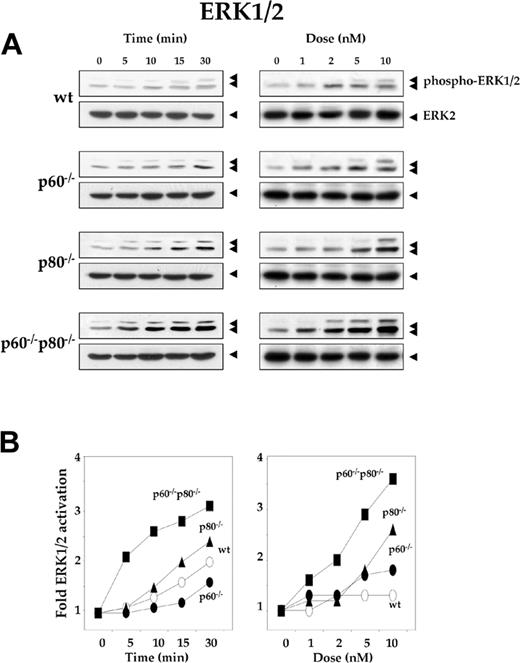
Deletion of TNF receptors sensitizes macrophages to RANKL-induced activation of ERK1/2
Through the Ras/Raf/MAPK kinase (MEK) cascade, RANKL can activate ERK1/2.22 To explore the specific role of TNF receptors in RANKL-induced ERK activation, we treated the wt macrophages and their TNF receptor-deficient variants with 5 nM RANKL for the indicated times, resolved whole-cell extracts by SDS-PAGE, and performed Western blot analysis using phospho-specific anti-ERK1/2 antibody. Time-dependent phosphorylation of ERK occurred in wt macrophages and in the TNF receptor-deficient variants (Figure 4A). Maximum activation occurred in p60-/-p80-/- macrophages. The kinetics of ERK phosphorylation was slightly slower in wt macrophages (30 minutes) than in their TNF receptor-deleted variants (5 minutes) (Figure 4A, left panel). As shown in Figure 4B, right panel, dose-dependent phosphorylation of ERK occurred in wt macrophages and their variants, but the activation levels varied. Maximum phosphorylation was observed with p60-/-p80-/- macrophages. The sensitivity to RANKL for ERK phosphorylation was less in wt macrophages (10 nM) than in both their TNF receptor-deficient variants (p60-/-p80-/-, 1 nM). Both dose-response and time course results indicated that maximum RANKL-induced ERK activation occurred in macrophages deficient in both TNF receptors (p60-/-p80-/-) and minimum activation occurred in wt cells (Figure 4B).
Deletion of TNF receptors sensitizes macrophages to RANKL-induced activation of ERK1/2. (A) Time- and dose-dependent ERK1/2 activation by RANKL in wild-type and TNF receptor-deleted macrophages. 1.0 × 106 cells were treated with 5 nM RANKL for the indicated times or with the indicated concentrations of RANKL for 15 minutes. Whole-cell extract (50 μg) was resolved by 10% SDS-PAGE and electrotransferred to a nitrocellulose membrane. Western blot analysis was performed using phospho-specific anti-ERK1/2 antibody as described in “Materials and methods.” The same membrane was reblotted with anti-ERK2 antibody. (B) Graphical representation of the results shown in panel A.
Deletion of TNF receptors sensitizes macrophages to RANKL-induced activation of ERK1/2. (A) Time- and dose-dependent ERK1/2 activation by RANKL in wild-type and TNF receptor-deleted macrophages. 1.0 × 106 cells were treated with 5 nM RANKL for the indicated times or with the indicated concentrations of RANKL for 15 minutes. Whole-cell extract (50 μg) was resolved by 10% SDS-PAGE and electrotransferred to a nitrocellulose membrane. Western blot analysis was performed using phospho-specific anti-ERK1/2 antibody as described in “Materials and methods.” The same membrane was reblotted with anti-ERK2 antibody. (B) Graphical representation of the results shown in panel A.
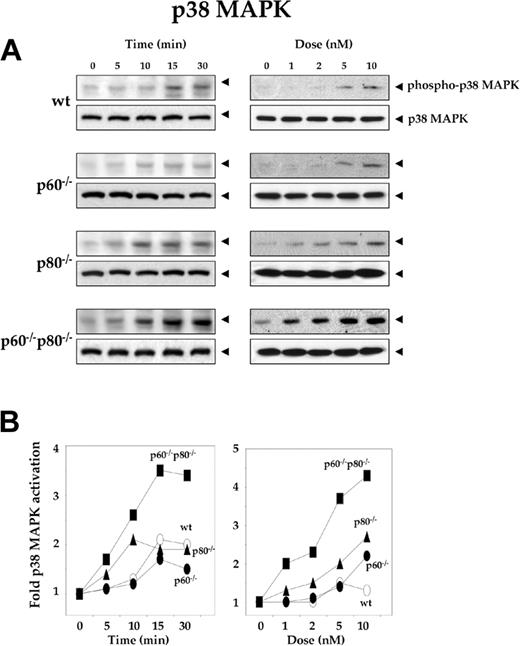
Deletion of TNF receptors sensitizes macrophages to RANKL-induced activation of p38 MAPK
Like JNK, p38MAPK is a Ser/Thr protein kinase activated rapidly by RANKL.17 To explore the specific role of TNF receptors in RANKL-induced p38 MAPK activation, we treated the wt macrophages and their receptor-deficient variants with 5 nM RANKL for the indicated times, resolved whole-cell extracts by SDS-PAGE, and performed Western blot analysis using phospho-specific anti-p38 MAPK antibody. Time-dependent phosphorylation of p38 MAPK occurred in wt macrophages and their TNF receptor-deficient variants, but the level of p38 MAPK phosphorylation varied. Maximum activation occurred in p60-/-p80-/- macrophages. The kinetics of p38 MAPK phosphorylation was slightly slower in wt macrophages (15 minutes) than in their TNF receptor-deleted variants (10 minutes) (Figure 5A, left panel). As shown in Figure 5B right panel, dose-dependent phosphorylation of p38 MAPK occurred in wt macrophages and their TNF receptor-deficient variants, but the phosphorylation levels varied. Both dose-response and time course results indicated that maximum RANKL-induced p38 MAPK activation occurred in macrophages deficient in both TNF receptors (p60-/-p80-/-) and the minimum activation occurred in wt cells (Figure 5B). Deletion of p60 receptor had minimal effect on RANKL-induced NF-κB activation. The deletion of p80 receptor had more effect than p60 receptor.
Deletion of TNF receptors sensitizes macrophages to RANKL-induced activation of p38 MAPK. (A) Time- and dose-dependent p38 MAPK activation by RANKL in wild-type and TNF receptor-deleted macrophages. 1.0 × 106 cells were treated with 5 nM RANKL for the indicated times or with the indicated concentrations of RANKL for 15 minutes. Whole-cell extract (50 μg) was resolved by 10% SDS-PAGE and electrotransferred to a nitrocellulose membrane. Western blot analysis was performed using phospho-specific anti-p38 MAPK antibody as described in “Materials and methods.” The same membrane was reblotted with anti-p38 MAPK antibody. (B) Graphical representation of the results shown in panel A.
Deletion of TNF receptors sensitizes macrophages to RANKL-induced activation of p38 MAPK. (A) Time- and dose-dependent p38 MAPK activation by RANKL in wild-type and TNF receptor-deleted macrophages. 1.0 × 106 cells were treated with 5 nM RANKL for the indicated times or with the indicated concentrations of RANKL for 15 minutes. Whole-cell extract (50 μg) was resolved by 10% SDS-PAGE and electrotransferred to a nitrocellulose membrane. Western blot analysis was performed using phospho-specific anti-p38 MAPK antibody as described in “Materials and methods.” The same membrane was reblotted with anti-p38 MAPK antibody. (B) Graphical representation of the results shown in panel A.
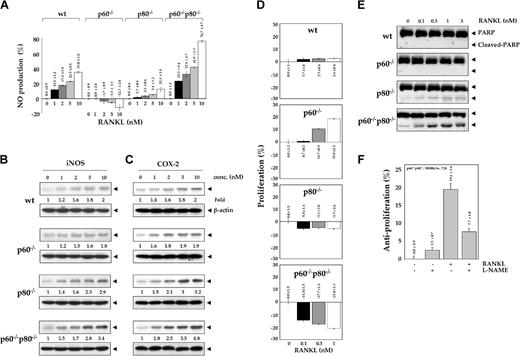
Deletion of TNF receptors sensitizes macrophages to RANKL-induced NO production
Inducible NO synthase (iNOS) in macrophages is known to be regulated by NF-κB.35 To determine whether TNF receptors have any effect on RANKL-induced NO production in macrophages, we cultured macrophages for 12 hours in the presence of indicated concentrations of RANKL and assayed NO production by using Griess reagent. RANKL induced NO production in a dose-dependent manner in all macrophage cell lines, but the induction was lowest in p60-/- macrophages and highest in macrophages lacking both TNF receptors (Figure 6A). The induction was minimal in cells with either TNF receptor deletion than in those with both TNF receptors deleted.
Deletion of TNF receptors potentiates RANKL-induced NO production and induction of iNOS expression. (A) Effect of RANKL on the production of NO in wild-type and TNF receptor-deleted macrophages. 1.0 × 106 cells were treated with the indicated concentrations of RANKL for 12 hours. Culture medium was collected, and NO production was determined by using Griess reagent as described in “Materials and methods.” Numbers on top of the bars indicate percentage of change ± SD. (B) Effect of RANKL on the expression of iNOS in wild-type and TNF receptor-deleted macrophages. One million cells were treated with various concentrations of RANKL for 12 hours. Whole-cell extract (50 μg) was resolved by 7.5% SDS-PAGE and electrotransferred to a nitrocellulose membrane. Western blot analysis was performed using anti-iNOS antibody as described in “Materials and methods.” The same membrane was reblotted with anti-β-actin antibody. (C) Effect of RANKL on the expression of COX-2 in wild-type and TNF receptor-deleted macrophages. 1.0 × 106 cells were treated with the indicated concentrations of RANKL for 12 hours. Whole-cell extract (50 μg) was resolved by 7.5% SDS-PAGE and electrotransferred to a nitrocellulose membrane. Western blot analysis was performed using anti-COX-2 antibody as described in “Materials and methods.” The same membrane was reblotted with anti-β-actin antibody. (D) Effect of RANKL on the cell viability of wild-type and TNF receptor-deleted macrophages. 5.0 × 103 were seeded in 0.1 mL culture media in 96-well plates and exposed to the indicated concentrations of RANKL for 72 hours in triplicate, and then cell viability was determined using the MTT assay as described in “Materials and methods.” Numbers indicate percentage of change ± SD. (E) RANKL induces caspase-activated PARP cleavage in TNF receptor-deleted macrophages. 1.0 × 106 cells were treated with the indicated concentrations of RANKL for 24 hours. Whole-cell extract (50 μg) was resolved by 7.5% SDS-PAGE, electrotransferred to a nitrocellulose membrane, and probed for PARP using anti-PARP antibody as described in “Materials and methods.” (F) Antiproliferative effects of RANKL require NO production. 1.0 × 104 p60-/-p80-/- macrophages were seeded in 0.1 mL culture media in 96-well plates, preincubated with 100 μM L-NAME for 1 hour, and then exposed to 1 nM RANKL in triplicate. After 72 hours, cell viability was determined by the MTT method as described in “Materials and methods.” Numbers on top of the bars indicate percentage of change ± SD.
Deletion of TNF receptors potentiates RANKL-induced NO production and induction of iNOS expression. (A) Effect of RANKL on the production of NO in wild-type and TNF receptor-deleted macrophages. 1.0 × 106 cells were treated with the indicated concentrations of RANKL for 12 hours. Culture medium was collected, and NO production was determined by using Griess reagent as described in “Materials and methods.” Numbers on top of the bars indicate percentage of change ± SD. (B) Effect of RANKL on the expression of iNOS in wild-type and TNF receptor-deleted macrophages. One million cells were treated with various concentrations of RANKL for 12 hours. Whole-cell extract (50 μg) was resolved by 7.5% SDS-PAGE and electrotransferred to a nitrocellulose membrane. Western blot analysis was performed using anti-iNOS antibody as described in “Materials and methods.” The same membrane was reblotted with anti-β-actin antibody. (C) Effect of RANKL on the expression of COX-2 in wild-type and TNF receptor-deleted macrophages. 1.0 × 106 cells were treated with the indicated concentrations of RANKL for 12 hours. Whole-cell extract (50 μg) was resolved by 7.5% SDS-PAGE and electrotransferred to a nitrocellulose membrane. Western blot analysis was performed using anti-COX-2 antibody as described in “Materials and methods.” The same membrane was reblotted with anti-β-actin antibody. (D) Effect of RANKL on the cell viability of wild-type and TNF receptor-deleted macrophages. 5.0 × 103 were seeded in 0.1 mL culture media in 96-well plates and exposed to the indicated concentrations of RANKL for 72 hours in triplicate, and then cell viability was determined using the MTT assay as described in “Materials and methods.” Numbers indicate percentage of change ± SD. (E) RANKL induces caspase-activated PARP cleavage in TNF receptor-deleted macrophages. 1.0 × 106 cells were treated with the indicated concentrations of RANKL for 24 hours. Whole-cell extract (50 μg) was resolved by 7.5% SDS-PAGE, electrotransferred to a nitrocellulose membrane, and probed for PARP using anti-PARP antibody as described in “Materials and methods.” (F) Antiproliferative effects of RANKL require NO production. 1.0 × 104 p60-/-p80-/- macrophages were seeded in 0.1 mL culture media in 96-well plates, preincubated with 100 μM L-NAME for 1 hour, and then exposed to 1 nM RANKL in triplicate. After 72 hours, cell viability was determined by the MTT method as described in “Materials and methods.” Numbers on top of the bars indicate percentage of change ± SD.
We also investigated the effect of TNF receptors on RANKL-induced iNOS protein expression. Cells were treated with the indicated concentrations of RANKL for 12 hours, and iNOS expression was determined by Western blot analysis. RANKL induced iNOS expression in a dose-dependent manner (Figure 6B). The induction was minimal in wt and in p60-/- macrophages and highest in cells lacking both TNF receptors. These results are in agreement with those for NO production. These results demonstrate that TNF receptors suppressed RANKL-induced activation of macrophages.
Deletion of TNF receptors sensitizes macrophages to RANKL-induced COX-2 expression
COX-2 is another inflammatory gene that is regulated by NF-κB36 and plays an important role in osteoblast differentiation through prostaglandin production.37,38 Whether RANKL-induced COX-2 expression is also modulated by TNF receptors was investigated. Western blot analysis indicated that RANKL induced COX-2 expression in a dose-dependent manner (Figure 6C). The induction was least in wt and p60-/- macrophages and greatest in their p60-/-p80-/- variant cells. The maximum COX-2 expression observed on treatment of wt, p60-/-, p80-/-, and p60-/-p80-/- macrophages with 10 nM RANKL was 2-, 1.9-, 3.2-, and 4.8-fold, respectively. These results demonstrate that TNF receptors suppress RANKL-induced COX-2 expression, possibly through down-modulation of NF-κB activation.
Deletion of TNF receptors alters macrophages to RANKL-induced cytotoxicity and cell proliferation in macrophages
Besides NF-κB, JNK, ERK, and p38 MAPK, RANKL can also suppress cell proliferation and induce apoptosis.13 To determine the effect of TNF receptors on RANKL-induced proliferation of cells, all cell types were incubated for 72 hours with the indicated concentrations of RANKL and then assayed for cell viability by MTT uptake. RANKL induced proliferation of p60-/- cells by almost 20% but inhibited the proliferation of p60-/-p80-/- cells by 21% (Figure 6D), and this effect was dose dependent. The effect on wt and p80-deleted cells was minimal. Whether the antiproliferative effects of RANKL correlate with apoptosis of cells was examined by caspase-mediated PARP cleavage. We found that RANKL-induced significant cleavage of PARP substrate in p60-/-p80-/- cells (Figure 6E), indicating apoptosis. Because RANKL was found to induce nitric oxide (NO), whether NO is required for the antiproliferative effects of RANKL was also investigated. We found that L-NAME, a specific inhibitor of NOS, reversed the antiproliferative effects of RANKL in p60-/-p80-/- cells (Figure 6F). These results clearly show that the TNF receptors modulate RANKL-induced cellular proliferation.
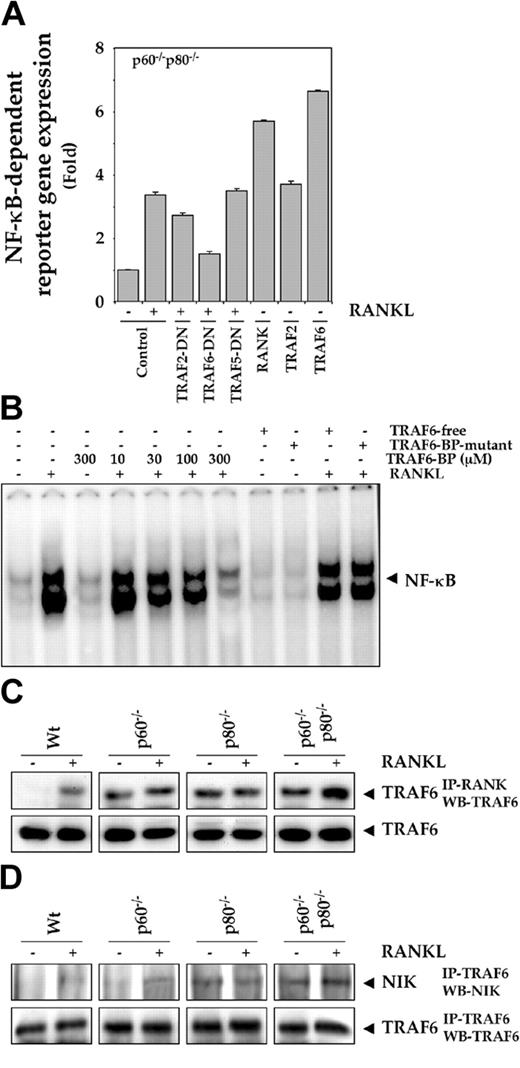
Dominant-negative TRAF6 and TRAF2, but not TRAF5, suppresses RANKL-induced NF-κB reporter gene expression in p60-/-p80-/- macrophages
TRAF6 and TRAF2 play a critical role in RANKL signaling downstream from RANK,9,39 so we assayed the ability of TRAFs to modulate RANKL signaling in TNF receptor-deficient macrophages. Because RANKL signaling was maximally affected in cells in which TNF receptors were deleted, we used these cells. Cells were transiently transfected with the NF-κB-regulated SEAP reporter plasmid along with various expression vectors and then stimulated with RANKL. The dominant-negative protein expression vectors we used were ones for TRAF2-, TRAF6-, and TRAF5-DN; we also used expression vectors for RANK, TRAF2, and TRAF6, and then monitored NF-κB-dependent SEAP expression. As shown in Figure 7A, RANKL-induced NF-κB-regulated reporter gene expression was suppressed maximally by DN-TRAF6 and minimally by DN-TRAF2. DN-TRAF5 had no effect. These results suggest that TRAF2 and TRAF6, but not TRAF5, play a role in RANKL-induced NF-κB activation in macrophages. Wild-type RANK-, TRAF2-, and TRAF6-expressing vectors also increased NF-κB-regulated SEAP reporter expression, further suggesting that TRAF2 and TRAF6 can induce NF-κB activation in macrophages.
Role of TRAF6 and NIK in RANKL signaling. (A) Dominant-negative TRAF6 and TRAF2, but not TRAF5, suppress RANKL-induced NF-κB reporter gene expression in p60-/-p80-/- macrophages. 0.5 × 106 cells were transiently transfected with a NF-κB-containing plasmid alone or with indicated plasmids for 24 hours. After transfection, cells were washed and treated with 10 nM RANKL for an additional 24 hours. The supernatants of the culture medium were assayed for SEAP activity as described in “Materials and methods.” (B) TRAF6-binding protein suppresses RANKL-induced NF-κB activation in p60-/-p80-/- macrophages. 1.0 × 106 cells were pretreated with the indicated concentrations of TRAF6-binding protein (TRAF6-BP), 300 μM TRAF6-free, or 300 μM TRAF6-BP mutant peptide and then treated with 5 nM RANKL for 30 minutes. Nuclear extracts were prepared and analyzed for NF-κB activation by EMSA as described in “Materials and methods.” (C) Deletion of TNF receptors potentiates recruitment of TRAF6 into RANK complex. 1.0 × 107 cells were treated with 10 nM RANKL for 15 minutes; whole-cell extracts were prepared, incubated with anti-RANK antibody for 2 hours, and then immunoprecipitated with protein A/G-Sepharose beads. The beads were washed and resolved by 10% SDS-PAGE and electrotransferred to a nitrocellulose membrane. Western blot analysis was performed using anti-TRAF6 antibody. Fifty micrograms of the same protein extract was resolved by 10% SDS-PAGE and electrotransferred to a nitrocellulose membrane. Western blot analysis was performed using anti-TRAF6 antibody. (D) Deletion of TNF receptors potentiates association of NIK to TRAF6. 1.0 × 107 cells were either untreated or treated with 10 nM RANKL for 15 minutes; whole-cell extracts were prepared, incubated with anti-TRAF6 antibody for 2 hours, and then immunoprecipitated with protein A/G-Sepharose beads. The beads were washed and resolved by 7.5% SDS-PAGE and electrotransferred to a nitrocellulose membrane. Western blot analysis was performed using anti-NIK antibody. The same membrane was also blotted with anti-TRAF6 antibody.
Role of TRAF6 and NIK in RANKL signaling. (A) Dominant-negative TRAF6 and TRAF2, but not TRAF5, suppress RANKL-induced NF-κB reporter gene expression in p60-/-p80-/- macrophages. 0.5 × 106 cells were transiently transfected with a NF-κB-containing plasmid alone or with indicated plasmids for 24 hours. After transfection, cells were washed and treated with 10 nM RANKL for an additional 24 hours. The supernatants of the culture medium were assayed for SEAP activity as described in “Materials and methods.” (B) TRAF6-binding protein suppresses RANKL-induced NF-κB activation in p60-/-p80-/- macrophages. 1.0 × 106 cells were pretreated with the indicated concentrations of TRAF6-binding protein (TRAF6-BP), 300 μM TRAF6-free, or 300 μM TRAF6-BP mutant peptide and then treated with 5 nM RANKL for 30 minutes. Nuclear extracts were prepared and analyzed for NF-κB activation by EMSA as described in “Materials and methods.” (C) Deletion of TNF receptors potentiates recruitment of TRAF6 into RANK complex. 1.0 × 107 cells were treated with 10 nM RANKL for 15 minutes; whole-cell extracts were prepared, incubated with anti-RANK antibody for 2 hours, and then immunoprecipitated with protein A/G-Sepharose beads. The beads were washed and resolved by 10% SDS-PAGE and electrotransferred to a nitrocellulose membrane. Western blot analysis was performed using anti-TRAF6 antibody. Fifty micrograms of the same protein extract was resolved by 10% SDS-PAGE and electrotransferred to a nitrocellulose membrane. Western blot analysis was performed using anti-TRAF6 antibody. (D) Deletion of TNF receptors potentiates association of NIK to TRAF6. 1.0 × 107 cells were either untreated or treated with 10 nM RANKL for 15 minutes; whole-cell extracts were prepared, incubated with anti-TRAF6 antibody for 2 hours, and then immunoprecipitated with protein A/G-Sepharose beads. The beads were washed and resolved by 7.5% SDS-PAGE and electrotransferred to a nitrocellulose membrane. Western blot analysis was performed using anti-NIK antibody. The same membrane was also blotted with anti-TRAF6 antibody.
TRAF6-binding peptide (TRAF6-BP) suppresses RANKL-induced NF-κB activation in macrophages
Whether TRAF6 mediates RANKL-induced NF-κB activation was also investigated using synthetic peptide inhibitors. A synthetic TRAF6-binding peptide (TRAF6-BP) with a Kaposi fibroblast growth factor leader sequence that makes cells permeable to it has been shown to suppress RANKL signaling.13,40 TRAF6-BP inhibited RANKL-induced NF-κB activation in our system (Figure 7B). The TRAF6-BP that lacks the delivery sequence (TRAF6-free) and the TRAF6-BP mutant, which has mutation in TRAF6-BP, had no effect. These results again indicate the critical role of TRAF6 in RANKL-induced NF-κB activation in macrophages.
Deletion of TNF receptors potentiates recruitment of TRAF6 and NIK into the RANK complex
The TNF receptor deletion could have altered the interaction between TRAF6 and RANK upon RANKL stimulation, thus explaining the results obtained here. But TNF receptor-deficient macrophages were treated with RANKL, and immunoprecipitated with anti-RANK antibody displayed enhanced association of TRAF6 with RANK as compared with the control wt cells (Figure 7C, left panel).
Previous studies from our laboratory have indicated that NIK is required for RANKL-induced NF-κB activation.12 Therefore, we examined the association between TRAF6 and NIK in untreated cells and in cells after treatment with RANKL to address the question of cross-talk at a step downstream of RANK. We found that TNF receptor-deficient cells (p80-/- cells and p60-/-p80-/-cells) exhibit constitutive binding of TRAF6 to NIK. This association was significantly enhanced on treatment with RANKL, especially in cells lacking both the TNF receptors (Figure 7D). These results suggest that the interaction between RANK, TRAF6, and NIK in RANKL signaling is modulated by the TNF receptors.
Discussion
The aim of the current study was to determine whether there is a cross-talk between RANKL and TNF signaling For this we used macrophage cell lines derived from wt mice and from mice with genetically deleted type 1 TNF receptor (p60-/-), the type 2 TNF receptor (p80-/-), or both receptors (p60-/-p80-/-). We demonstrate that, despite similar expression levels of RANK, TRAF1, TRAF2, and TRAF6, the 4 cell lines differed with respect to RANKL-induced NF-κB, IKK, JNK, ERK1/2, and p38 MAPK activation. The cells in which both receptors were deleted were maximally sensitive to RANKL. Deletion of the TNF receptors also enhanced RANKL-induced NO production and iNOS and COX-2 expression. Deletion of both receptors also switched the RANKL-induced cellular proliferation to antiproliferative response. TRAF6, which mediates RANKL signaling, was constitutively bound to RANK in TNF receptor-deleted cells but not in wt cells, and this binding was enhanced by RANKL. Similarly, a constitutive interaction between NIK and TRAF6 was found especially in p60-/-p80-/- cells, and this binding was enhanced by RANKL.
The mechanism by which TNFR regulates RANKL-induced signaling is uncertain. RANKL activates NF-κB through sequential interaction with RANK, TRAF6, NIK, and IKK.12 In contrast TNF activates NF-κB through sequential interactions with TNFR1, TRADD, receptor-interacting protein (RIP), and IKK (46-49). In our studies, deletion of TNFR1 had no significant effect on RANKL-induced NF-κB, IKK, ERK, and JNK activation, whereas Zhang et al18 reported that deletion of TNFR1 reduced RANKL-induced NF-κB, ERK, and JNK activation. In our studies, TNFR2 and the 2 receptors together had a strong effect on RANKL signaling, but these responses were not examined by Zhang et al.18 They did show that the expression of TRAF2 and TRAF6 were reduced in TNFR1-deleted macrophages, which may explain why they found decreased responsiveness to RANKL.18 In our studies, we found that these 2 TRAFs were unchanged in all cell lines, and the responses were strictly linked to the depletion of TNF receptor. As for RANKL-induced osteoclastogenesis, Zhang et al,18 like us, showed that it was enhanced in TNFR2-deleted cells. Abu-Amer et al28 also showed that basal osteoclastogenesis was significantly enhanced in TNFR2-deleted cells as compared with wt or TNR1-deleted cells. They also did not examine RANKL-induced NF-κB, JNK, and ERK response in TNFR2-deleted cells. They did not examine RANKL-induced p38 MAPK induction in receptor-deleted cells. Besides sensitizing of cells to RANKL-induced NF-κB activation, we found that TNFR deletion increased the production of NF-κB-regulated gene products such as COX-2 and iNOS.
Our results also indicate that deletion of TNFR1 sensitized the cells to RANKL-induced proliferation, whereas deletion of both receptors sensitized them to apoptosis. Although RANKL is a cell survival factor for dendritic cells, it has been shown to induce apoptosis in RAW 264.7 cells.13 How deletion of the p60 receptor induces proliferation and deletion of p80 induces apoptosis is not clear, but the p60 TNF receptor mediates apoptosis through the presence of the death domain (DD) in its cytoplasmic portion and p80 receptor, which lacks the DD, mediates cell proliferation.6
Our results also indicate that TRAF6 is essential for RANKL-induced NF-κB activation. These results are consistent with previous reports.9-12,25 We also found that all 4 cell lines expressed similar levels of TRAF6, but the level of RANK-associated TRAF6 protein varied. No constitutive interaction between TRAF6 and RANK was found in wt cells, but interaction was induced by RANKL. TNFR-deleted cells, however, had a constitutive level of RANK-bound TRAF6, and it was further enhanced by RANKL in cells lacking both receptors. Similarly, NIK, which is required for RANKL-induced NF-κB activation,12 was constitutively bound to TRAF6 in p60-/-p80-/- cells but not in wt cells, and also this binding was enhanced by RANKL.
There are reports that suggest that RANKL and TNF display synergistic effects,19-21 whereas others suggest they mediate their signals independent of each other.21,24 That TNF mediates RANKL-induced cellular responses has also been demonstrated.17,18 TRAF5 functions in both RANKL- and TNF-induced cellular responses.26 Our results clearly show that the TNF receptors play an important role in RANKL signaling. The sensitization of RANKL signaling by deletion of TNFR may be mediated through the competition of a limited pool of TRAF2, TRAF5, and TRAF6. Interestingly, RANKL signaling was more substantially affected by deletion of TNFR2 than by deletion of TNFR1. Both TNFR2 and RANK are known to lack the DD and exhibit structural homology not only in their extracellular domain but also in their intracellular domain. The intracellular domain of both binds TRAF2 directly.6-9 Overall, our studies provide evidence for a cross-talk between TNF and RANKL signaling.
Prepublished online as Blood First Edition Paper, August 17, 2004; DOI 10.1182/blood-2004-04-1607.
Supported by the Clayton Foundation for Research (B.B.A.), the Department of Defense US Army Breast Cancer Research Program (grant BC010610) (B.B.A.), PO1 grant on lung chemoprevention from the National Institutes of Health (CA91844) (B.B.A.), and a P50 Head and Neck SPORE (Specialized Programs of Research Excellence) grant from the National Institutes of Health (B.B.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Mr Walter Pagel for carefully proofreading the manuscript and providing valuable comments. Dr Aggarwal is a Ransom Horne, Jr, Distinguished Professor of Cancer Research.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal