Abstract
Fetal liver tyrosine kinase 3 internal tandem duplication (FLT3 ITD) mutations are the most common molecular abnormality associated with adult acute myeloid leukemia (AML). To exploit this molecular target, a number of potent and specific FLT3 kinase inhibitors have been developed and are currently being tested in early phase clinical trials of patients with refractory AML. To explore the efficacy of combining a FLT3 inhibitor with standard AML chemotherapy drugs, we tested the effect of combining the FLT3 inhibitor SU11248 with cytarabine or daunorubicin on the proliferation and survival of cell lines expressing either mutant (FLT3 ITD or FLT3 D835V) or wild-type (WT) FLT3. SU11248 had additive-to-synergistic inhibitory effects on FLT3-dependent leukemic cell proliferation when combined with cytarabine or daunorubicin. The synergistic interaction of SU11248 and the traditional antileukemic agents was more pronounced for induction of apoptosis. SU11248 inhibited the proliferation of primary AML myeloblasts expressing mutant FLT3 ITD but not WT FLT3 protein. Combining SU11248 and cytarabine synergistically inhibited the proliferation of primary AML myeloblasts expressing FLT3 ITD but not WT FLT3 protein. These data suggest that the addition of potent FLT3 inhibitors such as SU11248 to AML chemotherapy regimens could result in improved treatment results.
Introduction
Acute myeloid leukemia (AML) remains a difficult disease to treat. Patients typically respond to initial treatment with anthracycline and cytarabine-based induction chemotherapy, but most patients ultimately relapse and die of their disease. Internal tandem duplications (ITDs) of the juxtamembrane domain of the fetal liver tyrosine kinase 3 (FLT3) receptor tyrosine kinase are found in 20% to 30% of cases of de novo AML. FLT3 ITD mutations are the most common molecular abnormality associated with AML and presence of such a mutation predicts for a worse outcome for patients,1-3 particularly those with a high mutant-to-normal FLT3 allelic ratio.4 An additional 5% to 7% of AML patients have an activating mutation of the FLT3 kinase activation loop.5
There is substantial experimental and clinical evidence to support the hypothesis that FLT3 mutations are important in the initiation or maintenance of AML in some patients. Activating mutations of FLT3 result in constitutive activation of FLT3 tyrosine kinase activity and can transform factor-dependent hematopoietic cells as evidenced by conversion to factor-independent growth and formation of tumors in immunodeficient mice.6-8 In addition, retroviral transduction of primary murine bone marrow with an AML patient–derived FLT3 ITD cDNA results in a lethal myeloproliferative syndrome.9 Finally, retroviral transduction of bone marrow derived from promyelocytic leukemia/retinoic acid receptor α (PML-RARα) transgenic mice with FLT3 ITD results in a marked increase in the incidence of acute progranulocytic (APL)–like leukemia in such mice when compared with mice that received a transplant of mock-transduced bone marrow.10
These findings suggest that FLT3 may be a target for molecular therapy, analogous to that of BCR-ABL in chronic myeloid leukemia (CML). In CML, the use of single-agent imatinib (STI571, Gleevec) is associated with impressive response rates and is now considered the standard frontline nontransplantation medical therapy.11,12 Agents capable of inhibiting FLT3 kinase have been developed and display promising preclinical activity.13-19 These agents are capable of halting leukemic cell proliferation and inducing apoptosis in vitro and in different animal models. Several of these FLT3 inhibitors are currently being studied in phase-1 and -2 clinical trials of patients with AML but thus far have demonstrated only modest single-agent activity.20-22 These results are somewhat analogous to those seen using imatinib to treat patients with CML blast crisis, where the rate of complete hematologic or cytogenetic response, as well as the duration of such responses, were markedly inferior to the results obtained in treating chronic-phase patients. This experience suggests that FLT3 inhibitors may need to be combined with other antileukemic treatments to obtain the best clinical results. Theoretically, it would be advantageous to add chemotherapy agents that act synergistically with FLT3 inhibitors for antileukemic effect. Ideally, such agents should not have similar or overlapping toxicities.
We sought to profile the activity of a FLT3 inhibitor in combination with traditional antileukemic agents. For our studies we selected SU11248, a small-molecule FLT3 kinase inhibitor with potent effects in vivo and in vitro.17 This agent was studied in combination with cytarabine and daunorubicin, current corner-stones of therapy for patients with AML.23 SU11248 was found to have additive-to-synergistic effects in inhibiting FLT3 ITD leukemia cell proliferation when combined with the traditional cytotoxic agents. Similar results were obtained using cells that were dependent upon either FLT3 ligand (FL) activation of wild-type (WT) FLT3 or the kinase activity of a FLT3 activation loop mutant isoform (FLT3 D835V). A stronger synergistic effect of combining SU11248 with single-agent chemotherapy was seen when cellular death (apoptosis) was the experimental end point. SU11248 significantly inhibited the proliferation of FLT3 ITD–positive primary AML myeloblasts and the combination of SU11248 and cytarabine had a greater antiproliferative effect than either agent used alone. In contrast, SU11248 had minimal activity against primary AML myeloblasts expressing WT FLT3 and the antiproliferative effects of a combination of SU11248 and cytarabine were not significantly different than that seen using cytarabine as single agent. Our findings suggest that combining a FLT3 inhibitor such as SU11248 with standard antileukemia chemotherapy may improve clinical outcomes in AML.
Patients, materials, and methods
Cell culture
MV4-11 and BAF3 cell lines were obtained through the American Type Culture Collection (ATCC, Manassas, VA) and the MOLM14 cell line was acquired through the Fujisaki Cell Center (Okayama, Japan). Wild-type BAF3 cells were grown in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) and 10% WEHI-3B supernatant. MOLM14 cells were maintained in RPMI 1640 supplemented with 10% FBS. MV4-11 cells were maintained in RMPI 1640 supplemented with 10% FBS. MV4-11 cells are hemizygous for an ITD of amino acids VDFREYEYDH at position 592-601,16 whereas MOLM14 cells (sister cell line to MOLM 13) are heterozygous for an ITD of DFREYE at amino acid position 593-598.24 The cDNA for human FLT3 was cloned into the pLXSN retroviral vector.25 Mutations encoding for a FLT3 ITD (reduplication of YEYDLK at amino acid positions 597-602)16 and D835V were produced by site-directed mutagenesis and confirmed by bidirectional sequencing. Retroviral packaging cells were transfected with plasmids encoding WT, ITD, or D835V FLT3 and retroviral supernatants were used to transduce BAF3 cells.26 Cells were selected for factor-independent growth (ITD FLT3 or D835V) or FL-dependent growth (WT FLT3), as previously described.16 WT FLT3 BAF3 cells were maintained in RPMI 1640 + 10% fetal calf serum (FCS) supplemented with 5 ng/mL of recombinant human FL (R&D Systems, Minneapolis, MN). Factor-independent BAF3 ITD FLT3 and BAF3 D835V cells were maintained in RPMI 1640 supplemented with 10% FBS.
Reagents
The indolinone compounds SU6668 and SU11248 were obtained from SUGEN (South San Francisco, CA), dissolved in dimethyl sulfoxide (DMSO) to create 10-mM stock solutions, and kept frozen at -20°C. SU14816 (imatinib, STI571) was also obtained from SUGEN, dissolved in DMSO to create a 10-mM stock solution, and kept frozen at -20°C. Cytarabine (cytosine β-d-arabinofuranoside) and daunorubicin hydrochloride were obtained from Sigma (St Louis, MO).
Proliferation assays
Cells were added to 96-well plates at densities of 50 000 cells/well for MV4-11, 50 000 cell/well for MOLM14, and 20 000 cells/well for BAF3-derived cells. Test compounds were added and proliferation was measured at 72 hours using a 2,3-bis[2-methoxyl-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide (XTT)–based assay (Roche Molecular Biochemicals, Indianapolis, IN).27 In the experiments involving a combination of SU11248 and cytarabine or daunorubicin, the 2 agents were added simultaneously to the cells.
Informed consent for the use of residual blood specimens remaining after completion of clinical testing to determine FLT3 ITD status was given by AML patients. The Institutional Review Boards of both the Oregon Health & Science University and the Portland VA Medical Center approved the studies involving patient specimens. Informed consent was obtained in accordance with the Declaration of Helsinki. Samples were deidentified prior to use in the in vitro experiments and the FLT3 ITD status of the deidentified specimens was independently determined by denaturing high-performance liquid chromatography (D-HPLC) and direct sequencing as previously described.16 Low-density mononuclear cells were isolated by Ficoll-Hypaque density centrifugation and were plated in a 96-well plate in the presence or absence of SU11248 and/or cytarabine. The effect of SU11248 and/or cytarabine on the proliferation of primary AML myeloblasts was determined after 72 to 96 hours of treatment using an XTT-based assay. In the experiments involving a combination of SU11248 and cytarabine, the 2 agents were added simultaneously to the cells.
Immunoblotting
MV4-11 cells (5 × 107) were grown in RPMI 1640 and 10% FBS for 24 hours in the presence of study drugs, pelleted, and lysed with 100 μL of protein lysis buffer (50 mM Tris [tris(hydroxymethyl)aminomethane], 150 mM NaCl, 1% nonidet P–40 [NP-40], 0.25% deoxycholate with added inhibitors: aprotinin, AEBSF [4-(2-aminoethyl)-benzenesulfonyl fluoride], leupeptin, pepstatin, sodium orthovanadate, and sodium pyruvate). Apoptosis was assessed by immunoblotting with a monoclonal antibody against cleaved poly–adenosine diphosphate (ADP) ribose polymerase (PARP; Cell Signaling Technology, Beverly, MA) used at 1:1000 dilution and a peroxidase-conjugated goat antimouse antibody was used at 1:5000 dilution (BioRad, Hercules, CA).17
Apoptosis assays
Cells were rinsed with phosphate-buffered saline (PBS) and incubated in RPMI supplemented with 10% FCS. Cells (1 × 106) were incubated with test compounds for 48 hours. Apoptosis was assessed using a fluorescein isothiocyanate (FITC)–labeled annexin V/propidium iodide assay (Immunotech, Marseille, France).27 In the experiments involving a combination of SU11248 and cytarabine or daunorubicin, the 2 agents were added simultaneously to the cells.
Data analysis
Results from experiments were analyzed using the median effect method of Chou and Talalay28 (Calcusyn Software available from Biosoft, Cambridge, United Kingdom). Individual agents were tested to determine the dose required to inhibit proliferation of cells to 50% of untreated controls (ED50). Doses of 4 times, 2 times, 1 times, 0.5 times, and 0.25 times the ED50 were then analyzed using the Calcusyn software program to create isobolograms and calculate combination indices (CIs). The combination index was calculated using the more stringent statistical assumption of mutually nonexclusive modes of action. Specifically, CI = D1/(Dx)1 + D2/(Dx)2 + (D1 × D2)/((Dx)1 × (Dx)2), where D1 is the dose of drug 1, D2 is the dose of drug 2, and (Dx)1 and (Dx)2 represent the estimated dose of drug 1 and 2 alone, respectively, inhibiting x%. The CI provides a numeric description of the effects of a combination treatment. Specifically, a CI less than 1 indicates synergy, a CI = 1 indicates an additive effect, and a CI more than 1 indicates antagonism between the 2 agents.
For the experiments examining the effects of SU11248 ± cytarabine on the proliferation of primary AML myeloblasts, an analysis of variance factorial treatment design framework was used to examine differences in inhibition of cellular proliferation based on 3 factors: AML FLT3 genotype (FLT3 ITD vs FLT3 WT); SU11248 concentration (0, 1, 10, 50, 100 nM); and cytarabine concentration (0, 250, 500, 1000, 2000, 4000, 8000 nM). Specific contrasts were made comparing SU11248 (0 nM) with SU11248 (50 nM) for the different concentrations of cytarabine by FLT3 ITD status separately.
Results
Effects of combinations of cytarabine and SU11248 on cellular proliferation
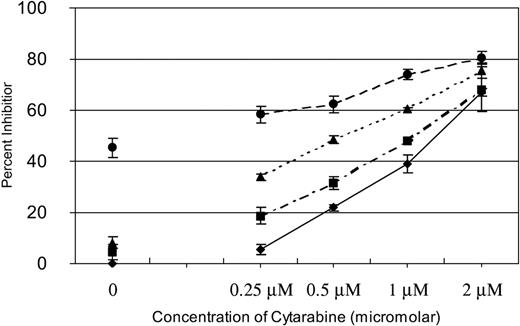
We have previously reported that SU11248 inhibits FLT3 kinase activity in AML cell lines and selectively inhibits proliferation and induces apoptosis in cell lines with a FLT3 ITD mutation.17 Cytarabine is one of the most active chemotherapeutic agents for the treatment of AML and it remains the backbone of induction and consolidation regimens.23,29 To determine if inhibition of FLT3 kinase in AML cells might potentiate the effects of cytarabine, we performed experiments in which fixed doses of SU11248 were added to increasing doses of cytarabine to test if a combination of the agents would prove more effective than either agent used alone. MV4-11 cells, known to be hemizygous for a FLT3 ITD mutation,16 were treated with 0, 0.25, 0.5, 1, or 2 μM of cytarabine. Zero, 2, 4, or 8 nM SU11248 was then added at each dose of cytarabine. As shown in Figure 1, SU11248 increased the inhibitory effects of cytarabine on cellular proliferation across the entire dose range studied. It should be noted that 2 nM and 4 nM of SU11248 as a single agent had minimal effects on the proliferation of MV4-11 cells (4% and 8% inhibition of proliferation, respectively) and, similarly, single-agent cytarabine at the 0.25-μM dose only inhibited proliferation by 6%. The 8-nM dose of SU11248 inhibited proliferation of MV4-11 cells by approximately 45%, consistent with our previous report.17 Notably, combining 0.25 μM cytarabine with either 2 or 4 nM SU11248 inhibited cellular proliferation by 19% and 34%, respectively.
Effects of SU11248 and/or cytarabine on proliferation of MV4-11 cells. Cells were exposed to cytarabine alone (♦) or in combination with 2 (▪), 4 (▴), or 8 nM (•) SU11248. Proliferation was assessed with an XTT-based assay at 48 hours. The effects of SU11248 without cytarabine are shown on the left side of the graph. Each data point represents the mean across 3 identical wells. Results from 1 of 3 separate experiments are shown. Percent inhibition is plotted on the y-axis (errors bars indicate standard deviation). The addition of SU11248 increased the dose-dependent inhibition of proliferation by cytarabine at all concentrations tested. Higher doses of SU1148 increased the efficacy of the combinations.
Effects of SU11248 and/or cytarabine on proliferation of MV4-11 cells. Cells were exposed to cytarabine alone (♦) or in combination with 2 (▪), 4 (▴), or 8 nM (•) SU11248. Proliferation was assessed with an XTT-based assay at 48 hours. The effects of SU11248 without cytarabine are shown on the left side of the graph. Each data point represents the mean across 3 identical wells. Results from 1 of 3 separate experiments are shown. Percent inhibition is plotted on the y-axis (errors bars indicate standard deviation). The addition of SU11248 increased the dose-dependent inhibition of proliferation by cytarabine at all concentrations tested. Higher doses of SU1148 increased the efficacy of the combinations.
For a more quantitative analysis of the apparent synergistic interaction between cytarabine and SU11248, we performed fixed-ratio dilution experiments. The results from these experiments were used to create isobolograms and calculate combination indices (CIs) for various levels of drug activity. Three separate cell lines with known juxtamembrane tandem duplications in FLT3 were used in our combination studies: MOLM14 cells (heterozygous for an ITD mutation), a BAF3 cell line engineered to express human FLT3 with an ITD mutation (BAF3 ITD FLT3), and MV4-11 cells (hemizygous for an ITD mutation). The different mutations of these cell lines provide a spectrum of ITD mutations and also reduce potential bias from idiosyncrasies of any individual cell line. In addition, we performed similar experiments using factor-independent BAF3 D835V FLT3 and FL-dependent BAF3 WT FLT3.
Experiments were performed with each cell line to determine the concentrations of cytarabine or SU11248 capable of decreasing proliferation to 50% of untreated control cells (ED50). As noted previously, the ED50 for SU11248 inhibition of proliferation was significantly lower for cells expressing FLT3 ITD compared with FL-activated WT FLT3 or D835V. Specifically, the ED50 for inhibition of MV4-11, MOLM14, and BAF3 ITD FLT3 were 8 nM, 12 nM, and 8 nM, respectively (x-intercept values in Figure 2; Table 1). In contrast, the ED50 values for SU11248 inhibition of the proliferation of FL-activated WT FLT3– and factor-independent FLT3 D835V–expressing cells were 50 and 110 nM, respectively.
Isobolograms for the effects of SU11248 and cytarabine on cellular proliferation. Cells were incubated with cytarabine, SU11248, or both for 72 hours and proliferation was assessed using an XTT-based assay. Results were analyzed using Calcusyn software for (A) MV4-11, (B) MOLM14, (C) BAF3 ITD FLT3, (D) FL-stimulated BAF3 WT FLT3, and (E) BAF3 D835V FLT3 cell lines (concentrations of agents are expressed in nM). For 90% inhibition (ED90), the points fall to the left of the predicted line of additive effect, suggesting a synergistic interaction of cytarabine and SU11248. The drugs appear to have an additive effect for doses necessary to inhibit proliferation at 50% of untreated control. • indicates ED50; ▪, ED75; and ▾, ED90. See Table 1 for CI values.
Isobolograms for the effects of SU11248 and cytarabine on cellular proliferation. Cells were incubated with cytarabine, SU11248, or both for 72 hours and proliferation was assessed using an XTT-based assay. Results were analyzed using Calcusyn software for (A) MV4-11, (B) MOLM14, (C) BAF3 ITD FLT3, (D) FL-stimulated BAF3 WT FLT3, and (E) BAF3 D835V FLT3 cell lines (concentrations of agents are expressed in nM). For 90% inhibition (ED90), the points fall to the left of the predicted line of additive effect, suggesting a synergistic interaction of cytarabine and SU11248. The drugs appear to have an additive effect for doses necessary to inhibit proliferation at 50% of untreated control. • indicates ED50; ▪, ED75; and ▾, ED90. See Table 1 for CI values.
CI values for the effects of SU11248 and cytarabine on cellular proliferation
. | ED50 . | ED75 . | ED90 . |
|---|---|---|---|
| MV4-11 | 0.93 | 0.86 | 0.82 |
| MOLM14 | 1.00 | 0.86 | 0.85 |
| BAF3 ITD FLT3 | 1.35 | 1.09 | 0.91 |
| BAF3 WT FLT3 + FL | 0.71 | 0.74 | 0.76 |
| BAF3 D835V FLT3 | 0.59 | 0.71 | 0.87 |
. | ED50 . | ED75 . | ED90 . |
|---|---|---|---|
| MV4-11 | 0.93 | 0.86 | 0.82 |
| MOLM14 | 1.00 | 0.86 | 0.85 |
| BAF3 ITD FLT3 | 1.35 | 1.09 | 0.91 |
| BAF3 WT FLT3 + FL | 0.71 | 0.74 | 0.76 |
| BAF3 D835V FLT3 | 0.59 | 0.71 | 0.87 |
CI values significantly less than 1 indicate synergy; values close to 1, an additive effect and values significantly greater than 1, an antagonistic effect of the 2 agents.
Cells were treated with cytarabine, SU11248, or both agents at 4 times, 2 times, 1 times, 0.5 times, and 0.25 times the ED50. Analysis of the data using the method of Chou and Talalay28 produced isobolograms for the 5 cell lines (Figure 2; Table 1). The simulated isobolograms define a “dose of equivalence” for the agents. Experimental results that fall near the line suggest that combinations of the 2 agents have an additive effect. Experimental results falling to the left of the line indicate better-than-additive effects of combining the agents (ie, synergy). Experimental results that fall far to the right of the line indicate that the 2 agents have antagonistic effects. In our experiments, the observed effect for the concentration of the 2 agents necessary to inhibit cells by 90% (ED90) fell to the left of the line of dose equivalence for all 5 tested cell lines. Adding SU11248 at low nM doses markedly reduced the amount of cytarabine necessary to achieve any given amount of antiproliferative effect. CI values for the observed effects on proliferation were also computed. For the experiments with the 5 cell lines, the ED90 CI was less than 1, suggesting a better-than-additive effect of combining SU11248 and cytarabine.
SU11248 is a multitargeted kinase inhibitor, with potent activity against KIT, platelet-derived growth factor receptor A (PDGFRA), PDGFRB, FLT3, colony-stimulating factor 1R (CSF-1R), and vascular endothelial growth factor receptor 2 (VEGFR2).17,30 To confirm that the observed antiproliferative effects were solely due to the activity of SU11248 against FLT3 and not one or more other SU11248-target kinases, we compared the effects of SU11248, SU6668, and SU14816 (imatinib) on the proliferation of the different cell lines. SU6668 inhibits KIT, PDGFRA, PDGFRB, and VEGFR2 but not FLT3,31,32 whereas SU14816 inhibits KIT, PDGFRA, and PDGFRB but not FLT3, CSF-1R, or VEGFR2.27,33 All 5 cell lines were inhibited by SU11248 but not by other potent inhibitors targeting KIT, PDGFRA, PDGFRB, or VEGFR2 (SU14816 at 1000 nM, SU6668 at 500-3000 nM; data not shown). The effect of SU11248 on the proliferation of BAF3-derived cell lines expressing mutant or WT FLT3 was completely abrogated when interleukin 3 (IL-3) was provided as alternative growth factor. In addition, SU11248 in doses up to 3000 nM had no effect on the proliferation of the parental BAF3 cell line grown in the presence of IL-3 (data not shown). Based on these results, we conclude that antiproliferative effects of SU11248 on the factor-independent growth of the mutant FLT3 cell lines and the FL-dependent growth of WT FLT3 BAF3 cell line used in our experiments are mediated entirely by inhibition of FLT3 kinase.
Effects of combinations of daunorubicin and SU11248 on cellular proliferation
Anthracyclines are another class of chemotherapeutic agents commonly used in the treatment of AML.23 Therefore, we performed experiments to determine the efficacy of the combination of SU11248 and daunorubicin. The ED50 for daunorubicin was determined separately for each cell line. Cells were then treated with daunorubicin, SU11248, or both agents at 4 times, 2 times, 1 times, 0.5 times, and 0.25 times the ED50.
All 5 of the cell lines were sensitive to daunorubicin in a 10- to 80-nM dose range. In the isobolograms (Figure 3; Table 2), the actual experimental points lie very near the lines of equivalence for the MV4-11 and MOLM14 cell lines, indicating that the combination of daunorubicin and SU11248 has an additive rather than synergistic effect on the proliferation of MV4-11 and MOLM-14 lines. This is also reflected in the calculated CI values that are approximately equal to 1. In contrast, the experimental values for the combination of SU11248 and daunorubicin fall significantly to the left of the lines of equivalence for all 3 BAF3-derived cell lines, indicating that the combination has a synergistic effect. This is also reflected in the calculated CI values that are significantly less than 1.
Isobolograms for the effects of daunorubicin and SU11248 on cellular proliferation. Cells were incubated with daunorubicin, SU11248, or both for 72 hours and proliferation was assessed using an XTT-based assay. Results were analyzed using Calcusyn software for (A) MV4-11, (B) MOLM14, (C) BAF3 ITD FLT3, (D) BAF3 WT FLT3 + FL, and (E) BAF3 D835V FLT3 cell lines (concentration of agents are expressed in nM). Observations lie near the lines of equivalence, suggesting an additive effect for the combination SU11248 and daunorubicin on MV4-11 and MOLM14 cells. For the 3 BAF3-derived cell lines, the combination results are more clearly to the left of the lines of equivalence, suggesting a synergistic effect. The CI values are listed in Table 2. • indicates ED50; ▪, ED75; and ▾, ED90.
Isobolograms for the effects of daunorubicin and SU11248 on cellular proliferation. Cells were incubated with daunorubicin, SU11248, or both for 72 hours and proliferation was assessed using an XTT-based assay. Results were analyzed using Calcusyn software for (A) MV4-11, (B) MOLM14, (C) BAF3 ITD FLT3, (D) BAF3 WT FLT3 + FL, and (E) BAF3 D835V FLT3 cell lines (concentration of agents are expressed in nM). Observations lie near the lines of equivalence, suggesting an additive effect for the combination SU11248 and daunorubicin on MV4-11 and MOLM14 cells. For the 3 BAF3-derived cell lines, the combination results are more clearly to the left of the lines of equivalence, suggesting a synergistic effect. The CI values are listed in Table 2. • indicates ED50; ▪, ED75; and ▾, ED90.
CI values for the effects of daunorubicin and SU11248 on cellular proliferation
. | ED50 . | ED75 . | ED90 . |
|---|---|---|---|
| MV4-11 | 1.22 | 1.09 | 0.98 |
| MOLM14 | 1.15 | 1.07 | 1.00 |
| BAF3 ITD FLT3 | 1.16 | 1.00 | 0.87 |
| BAF3 WT FLT3 + FL | 0.73 | 0.72 | 0.72 |
| BAF3 D835V FLT3 | 0.82 | 0.72 | 0.64 |
. | ED50 . | ED75 . | ED90 . |
|---|---|---|---|
| MV4-11 | 1.22 | 1.09 | 0.98 |
| MOLM14 | 1.15 | 1.07 | 1.00 |
| BAF3 ITD FLT3 | 1.16 | 1.00 | 0.87 |
| BAF3 WT FLT3 + FL | 0.73 | 0.72 | 0.72 |
| BAF3 D835V FLT3 | 0.82 | 0.72 | 0.64 |
CIs are near 1 for MV4-11 and MOLM14 cell lines for all effective doses for the combination, suggesting an additive effect for the combination. In contrast, CIs were significantly < 1 for ED75 and ED90 for all 3 BAF3-derived cell lines, indicating a synergistic effect for the combination.
Effects of combinations of cytarabine and SU11248 on induction of apoptosis
Our XTT-based “proliferation” assays directly measure the number of metabolically viable cells for each treatment condition. Thus, both cytostatic and cytotoxic effects of the single agents and combinations are reflected in the assay results. Previous studies have demonstrated that potent FLT3 inhibitors are capable of selectively inducing apoptosis in cell lines containing FLT3 ITD mutations.13,15,17,19 Since the combination of SU11248 with traditional cytotoxic agents increased the “antiproliferative” effects of chemotherapy, we sought to determine whether the combination of SU11248 with antileukemia chemotherapy drugs actually increased cell killing when compared with single-agent therapy. MV4-11 cells were treated with single-agent SU11248 (25 nM or 50 nM) or single-agent cytarabine (2 μM or 4 μM). Additionally, cells were treated with combinations of these agents: 25 nM SU11248 with either 2 μM or 4 μM cytarabine. Apoptosis was assessed at 24 hours using immunoblotting for proteolytic cleavage of poly (ADP-ribose) polymerase (PARP; Figure 4).
Combination of cytarabine and SU11248 on apoptosis. MV4-11 cells cultured in the presence of SU11248, cytarabine, or both were analyzed for PARP cleavage by Western blot after 24 hours of exposure to agents. Representative results from 1 of 3 experiments are displayed. Increasing doses of either single agent increased the amount of cleaved PARP detected. The combination of agents produced a dramatic increase in the cleavage product with almost no full-length PARP detected.
Combination of cytarabine and SU11248 on apoptosis. MV4-11 cells cultured in the presence of SU11248, cytarabine, or both were analyzed for PARP cleavage by Western blot after 24 hours of exposure to agents. Representative results from 1 of 3 experiments are displayed. Increasing doses of either single agent increased the amount of cleaved PARP detected. The combination of agents produced a dramatic increase in the cleavage product with almost no full-length PARP detected.
Cytarabine and SU11248 were both capable of inducing apoptosis in a dose-dependent manner, as assessed by PARP cleavage. However the combination of the lowest doses of both agents (2 μM cytarabine and 25 nM SU11248) produced a greater effect than those seen using twice the dose of either agent alone, suggesting a synergistic interaction. The small amount of full-length PARP detectable after treatment with either agent alone was absent when the agents were combined.
In order to more precisely evaluate synergy using a mathematical model, we used a more quantitative assessment of apoptosis in our subsequent experiments. Analysis of apoptosis with flow cytometry using annexin V–FITC and propidium iodide provides a convenient method to quantify apoptotic and/or nonviable cell populations. For these experiments we used MV4-11, MOLM14, and BAF3 ITD cells as models of the FLT3 ITD mutations commonly found in AML. Isobolograms from these experiments demonstrate dramatic synergistic induction of apoptosis for combination of cytarabine and SU11248 (Figure 5; Table 3).
Isobolograms for the apoptotic effects of cytarabine and SU11248 of FLT3 ITD cell lines. Cells were incubated with cytarabine, SU11248, or both for 48 hours and apoptosis was assessed with a flow cytometric assay. Results were used to construct isobolograms for (A) MV4-11, (B) MOLM14, and (C) BAF3 ITD FLT3 cells with concentrations expressed in nM. Experimental results fall far to the left of the lines of equivalence, suggesting potent synergy between cytarabine and SU11248. The experimental CI values are tabulated in Table 3. • indicates ED50; ▪, ED75; and ▾, ED90.
Isobolograms for the apoptotic effects of cytarabine and SU11248 of FLT3 ITD cell lines. Cells were incubated with cytarabine, SU11248, or both for 48 hours and apoptosis was assessed with a flow cytometric assay. Results were used to construct isobolograms for (A) MV4-11, (B) MOLM14, and (C) BAF3 ITD FLT3 cells with concentrations expressed in nM. Experimental results fall far to the left of the lines of equivalence, suggesting potent synergy between cytarabine and SU11248. The experimental CI values are tabulated in Table 3. • indicates ED50; ▪, ED75; and ▾, ED90.
CI values for the apoptotic effects of cytarabine and SU11248 of FLT3 ITD cell lines
. | ED50 . | ED75 . | ED90 . |
|---|---|---|---|
| MV4-11 | 0.83 | 0.34 | 0.20 |
| MOLM14 | 0.89 | 0.66 | 0.52 |
| BAF3 ITD FLT3 | 1.86 | 1.07 | 0.73 |
. | ED50 . | ED75 . | ED90 . |
|---|---|---|---|
| MV4-11 | 0.83 | 0.34 | 0.20 |
| MOLM14 | 0.89 | 0.66 | 0.52 |
| BAF3 ITD FLT3 | 1.86 | 1.07 | 0.73 |
Synergy for ED90 (CI < 1) is seen for all 3 tested cell lines.
The ED90 experimental values for the combination of SU11248 and cytarabine fall well to the left of the line of equivalence, demonstrating a strong synergy between cytarabine and SU11248 in inducing apoptosis. Calculated CI values were less than 1 at almost all dose effects for the 3 cell lines, indicating that a combination of cytarabine and SU11248 had a much greater-than-additive effect in inducing apoptosis of these leukemic cell lines.
Effects of combinations of daunorubicin and SU11248 on induction of apoptosis
Similar to the apoptosis experiments with cytarabine, cells were treated with daunorubicin and SU11248 either as individual agents or in combination and then analyzed using a flow cytometry–based assay. Results were analyzed using the median effect method to generate isobolograms and CI values (Figure 6; Table 4).
Isobolograms for the apoptotic effects of daunorubicin and SU11248 on FLT3 ITD–positive cell lines. Cells were incubated with daunorubicin, SU11248, or both for 48 hours and apoptosis was assessed with a flow cytometric assay. Results were used to construct isobolograms for (A) MV4-11, (B) MOLM14, and (C) BAF3 ITD FLT3 cells, with concentrations for each agent expressed in nM. Again, the doses necessary to produce a given effect fall far to the left of the lines of equivalence, suggesting a potent synergy between daunorubicin and SU11248. The experimental CI values are tabulated in Table 4. • indicates ED50; ▪, ED75; and ▾, ED90.
Isobolograms for the apoptotic effects of daunorubicin and SU11248 on FLT3 ITD–positive cell lines. Cells were incubated with daunorubicin, SU11248, or both for 48 hours and apoptosis was assessed with a flow cytometric assay. Results were used to construct isobolograms for (A) MV4-11, (B) MOLM14, and (C) BAF3 ITD FLT3 cells, with concentrations for each agent expressed in nM. Again, the doses necessary to produce a given effect fall far to the left of the lines of equivalence, suggesting a potent synergy between daunorubicin and SU11248. The experimental CI values are tabulated in Table 4. • indicates ED50; ▪, ED75; and ▾, ED90.
CI values for the apoptotic effects of daunorubicin and SU11248 on FLT3 ITD–positive cell lines
. | ED50 . | ED75 . | ED90 . |
|---|---|---|---|
| MV4-11 | 1.00 | 0.67 | 0.49 |
| MOLM14 | 0.98 | 0.57 | 0.34 |
| BAF3 ITD FLT3 | 0.96 | 0.72 | 0.55 |
. | ED50 . | ED75 . | ED90 . |
|---|---|---|---|
| MV4-11 | 1.00 | 0.67 | 0.49 |
| MOLM14 | 0.98 | 0.57 | 0.34 |
| BAF3 ITD FLT3 | 0.96 | 0.72 | 0.55 |
The experimental CI values are less than 1 for all given dose effects, with the lowest CI values (indicating the greatest degree of synergy) seen when ED90 was used as the experimental end point.
The actual experimental combination results for ED50, ED75, and ED90 fell far to the left of the lines of equivalence. These results indicate a strong synergistic interaction of daunorubicin and SU11248 in inducing apoptosis of these FLT3 ITD–positive cell lines. CI values were less than one for all dose effects with greatest impact at ED90, indicating potent synergy for the combination of SU11248 with this traditional cytotoxic agent.
In vitro effects of SU11248 and cytarabine on primary human AML cells
To further test whether our cell line model observations might be relevant to the treatment of human AML, we isolated low-density peripheral blood mononuclear cells from 5 patients with refractory and/or multiply relapsed AML. The FLT3 ITD status of the 5 patients was determined by D-HPLC and DNA sequencing: 3 patients had unique FLT3 ITDs (REYEYDL595-601 after L601, FREYEYDLKWEFPRENL594-610 after N609, YEYDLKWEFPRENLEF597-612 after F612, respectively) and 2 patients had no mutation of the FLT3 juxtamembrane or activation loop domains.16,34 Low-density peripheral blood mononuclear cells were cultured for 72 to 96 hours in the presence or absence of SU11248 and/or cytarabine. The effects of single-agent or combined treatment on cellular proliferation, as judged by an XTT-based assay, are graphically depicted in Figure 7. SU11248, at a concentration of 50 nM, significantly inhibited the proliferation of primary AML cells with a mutant FLT3 ITD (P < .0001). In contrast, this dose of SU11248 had no significant effect on the proliferation of primary AML cells lacking any detectable FLT3 mutation. The addition of 250 or 500 nM cytarabine to SU11248 significantly inhibited the proliferation of FLT3 ITD AML cells compared with the effect of cytarabine alone (P = .0085 and P = .0493, respectively). At higher doses of cytarabine, the addition of SU11248 did not significantly further inhibit proliferation of AML cells with FLT3 ITD. However, the small number of patient samples and the near-maximal antiproliferative effects of the higher doses of cytarabine may have obscured any additional effects of the combination treatment. In contrast to the results obtained using FLT3 ITD AML specimens, the addition of SU11248 at a concentration of 50 nM did not increase the antiproliferative effects of cytarabine doses of 250 to 8000 nM on the proliferation of FLT3 WT AML cells. The limited numbers of AML cells obtained from the patient samples precluded our ability to perform the multiple fixed-ratio combinations of SU11248 and cytarabine required for the generation of isobolograms. Likewise, the number of cells was insufficient to allow us to test the effects of combinations of SU11248 and daunorubicin on primary AML specimens.
Effects of SU11248 and/or cytarabine on the proliferation of primary AML cells. Low-density peripheral blood mononuclear cells from patients with refractory and/or relapsed AML were incubated with cytarabine (0-8000 nM) and/or SU11248 (50 nM) for 48 hours and cellular proliferation was assessed with an XTT-based assay (error bars indicate standard error of the mean values). The results for cells treated with SU11248 are shown with the solid-line plots, and the results for cells treated with cytarabine alone are shown with the dashed-line plots. Single-agent SU11248 (50 nM) significantly inhibited the proliferation of FLT3 ITD AML cells (top) but not FLT3 WT AML cells (bottom). The addition of SU11248 to 250 or 500 nM cytarabine significantly inhibited proliferation of FLT3 ITD AML cells compared with cytarabine alone. In contrast, the effect of the combination of SU11248 and cytarabine on proliferation of FLT3 WT AML cells was not significantly different than that of single-agent cytarabine. **P < .01 compared with single-agent cytarabine (0 nM or 250 nM); *P < .05 compared with single-agent cytarabine (500 nM cytarabine).
Effects of SU11248 and/or cytarabine on the proliferation of primary AML cells. Low-density peripheral blood mononuclear cells from patients with refractory and/or relapsed AML were incubated with cytarabine (0-8000 nM) and/or SU11248 (50 nM) for 48 hours and cellular proliferation was assessed with an XTT-based assay (error bars indicate standard error of the mean values). The results for cells treated with SU11248 are shown with the solid-line plots, and the results for cells treated with cytarabine alone are shown with the dashed-line plots. Single-agent SU11248 (50 nM) significantly inhibited the proliferation of FLT3 ITD AML cells (top) but not FLT3 WT AML cells (bottom). The addition of SU11248 to 250 or 500 nM cytarabine significantly inhibited proliferation of FLT3 ITD AML cells compared with cytarabine alone. In contrast, the effect of the combination of SU11248 and cytarabine on proliferation of FLT3 WT AML cells was not significantly different than that of single-agent cytarabine. **P < .01 compared with single-agent cytarabine (0 nM or 250 nM); *P < .05 compared with single-agent cytarabine (500 nM cytarabine).
Discussion
AML is the most common type of acute leukemia in adults. Patients typically respond to initial treatment with anthracycline and cytosine arabinoside–based induction chemotherapy, but most patients ultimately relapse and die of refractory disease. The prognosis is worse in elderly patients who typically experience substantially greater treatment-related toxicities with few durable remissions and are rarely cured. In addition, the elderly are usually not candidates for more intensive therapy using autologous or allogeneic stem cell transplantation. Although advances in supportive care have improved treatment-related mortality, the overall cure rate in adult AML has not improved significantly in the last decade. This plateau has lead to a search for newer therapies that might target fundamental molecular abnormalities in AML cells.35-38
The recent success of imatinib in treating CML has fueled enthusiasm for the further development of targeted therapies for AML.11,12 Logically, such therapies should be most efficacious if the target is a critical molecular mechanism initiating or maintaining the leukemic state. Although the weight of evidence suggests that translocations involving transcriptional machinery are the initiating event in many cases of AML, in most instances it is not yet possible to effectively target these abnormalities.39 The lone exception has been the development of all trans-retinoic acid (ATRA) therapy for the treatment of APL.40 New approaches to selective inhibition of specific histone deacetylases and disruption of pathologic transcription factor complexes are urgently needed. In contrast, substantial progress has been made in the development of specific tyrosine kinase inhibitors.
To date, FLT3 mutations are one of the most attractive AML targets yet identified, as these mutations are one of the most common molecular abnormalities found in cases of AML.1-3 A number of potent and selective FLT3 tyrosine kinase inhibitors have been developed and characterized in preclinical models.13-18,34 Several of these FLT3 inhibitors are being testing in ongoing phase-1 and -2 trials for patients with refractory AML.20-23 In preliminary reports, these inhibitors appear to have some activity as single agents but to date responses have tended to be incomplete or of limited duration. Although not definitive, such studies suggest the possibility that FLT3 inhibitors may have only a limited role as single-agent therapy, at least in patients with refractory or multiply relapsed AML.
These results are consistent with proposed models of AML leukemogenesis in which multiple different abnormalities are hypothesized to be necessary to produce leukemia. Existing evidence suggests that hematopoietic stem or progenitor cell expression of BCR-ABL,41,42 Ets variant gene 6 (ETV6)–PDGFRB (ETV6–PDGFRB),43-45 or FIP1L1-PDGFRA46 are sufficient to induce a myeloproliferative disease in mice, consistent with these abnormalities being the initiating molecular event in CML, chronic myelomonocytic leukemia, and hypereosinophilic syndrome, respectively. In contrast, FLT3 mutations appear to be a later event that increases the proliferation of a preexisting neoplastic clone.39,47
The most successful application of molecularly targeted therapy in AML to date has been the use of ATRA in the treatment of APL. Treatment of APL with ATRA as a single agent results in early remission but unacceptably high relapse rates. However, the use of ATRA in combination with traditional chemotherapeutic agents produces the highest sustained remissions rate of any French-American-British (FAB) subtype of AML.40,48 This example suggests that the most effective use of targeted agents in AML may be in combination with standard AML chemotherapy agents.
In our experiments we have shown the feasibility of combining a FLT3 inhibitor, SU11248, with standard AML chemotherapy drugs. SU11248 increased the antiproliferative effects of cytarabine and daunorubicin on FLT3 ITD–containing cell lines. Low concentrations of this agent reduced the dose of cytotoxic agent necessary to produce any given antiproliferative effect. More importantly, adding SU11248 to cytotoxic agents resulted in strong synergistic effects for the induction of apoptosis of the leukemic cell lines. The ability of SU11248 to induce apoptosis in leukemia cells when added to traditional chemotherapy drugs demonstrates that these combinations are potently cytotoxic rather then merely cytostatic.
We attempted to validate the potential clinical relevance of these observations using cultured primary AML myeloblasts. In these experiments, SU11248 significantly inhibited the proliferation of FLT3 ITD–positive AML myeloblasts and the combination of SU11248 and cytarabine had a greater antiproliferative effect than either agent used alone. In contrast, SU11248 had minimal activity against primary AML myeloblasts expressing WT FLT3, and the antiproliferative effects of a combination of SU11248 and cytarabine were not significantly different than that seen using cytarabine as single agent.
Levis et al34 have recently reported that combining an alternative FLT3 inhibitor, CEP-701, with various chemotherapy agents including cytarabine or anthracyclines synergistically induced cell killing of FLT3 ITD–positive cells. In their experiments, CEP-701 synergized with chemotherapy if used simultaneously or immediately following exposure to cytotoxic chemotherapy agents. In contrast, pretreatment with CEP-70 was generally antagonistic, especially when used with cytarabine.34 In our experiments, SU11248 was used simultaneously with cytarabine or daunorubicin. Overall, our results with SU11248 are consistent with the results obtained using CEP-701. Taken together, these 2 studies suggest that inhibition of FLT3 kinase activity and not effects on other drug targets (eg, KIT) is responsible for the observed synergistic proapoptotic effects when these inhibitors are used with standard antileukemic chemotherapy drugs.
The major side effects of standard AML chemotherapy are mucositis, cardiac toxicity–prolonged myelosuppression, bleeding, and infection.23 Extensive testing of single-agent FLT3 inhibitors in animals and limited testing in humans does not indicate that these agents cause mucositis or cardiac toxicity. In animal models, myelosuppression does not appear to be a significant side effect of FLT3 inhibitors. Grade 3 or 4 myelosuppression in solid-tumor patients being treated with SU11248 was uncommon; fatigue and hypertension were the dose-limiting toxicities of this agent.49-51 The effects of SU11248 on the blood counts of AML patients may be more difficult to interpret as myelosuppression may result from suppression of the leukemic clone rather than an effect on normal hematopoietic cells. However, it seems feasible that a combination of standard AML chemotherapy agents and a FLT3 inhibitor such as SU11248 could result in increased clinical efficacy without necessarily increasing the toxicity of either agent. Based on our results, further clinical development of such an approach seems warranted.
In addition to the possible use of full-dose chemotherapy regimens in combination with FLT3 inhibitors such as SU11248, our data also suggest another potential strategy to improve outcomes in elderly patients with AML. Due to the toxicities of standard-intensity chemotherapy, many elderly patients are not viewed as candidates for treatment with regimens capable of inducing a complete remission and are instead treated with supportive care only.35 Our results suggest that the addition of a FLT3 inhibitor to a given chemotherapy regimen might allow a 2- to 4-fold reduction in chemotherapy doses without impacting the antileukemic effect. Such dose reductions could increase the proportion of elderly patients with AML who are candidates for induction and/or consolidation treatments.
Early-phase clinical studies to characterize the role of FLT3 inhibitors in AML are currently under way. It is hoped that these kinase inhibitors will prove to be a useful addition to standard treatments of AML, ultimately leading to more effective and less toxic treatment regimens for patients with this challenging disease.
Prepublished online as Blood First Edition Paper, August 10, 2004; DOI 10.1182/blood-2003-10-3381.
Supported in part by a Merit Review Grant from the Department of Veterans Affairs (M.C.H.), the Doris Duke Charitable Foundation (M.C.H., K.W.H.Y.), and Deutsche Krebshilfe (M.S.).
Two of the authors (A.O., J.M.C.) are employed by a company (SUGEN) whose product was studied in the present work.
K.W.H.Y. and M.S. made an equal contribution to the authorship of this manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Biostatistical support was provided by the Biostatistics Shared Resource of the Oregon Health & Science University (OHSU) Cancer Institute (P30 CA69533).







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal