Abstract
Imatinib (STI571, Gleevec) is a tailored drug for chronic myelogenous leukemia (CML), whereas arsenic compounds were used as ancient remedies for CML with certain efficacy. The aim of this study was to investigate the potential benefit of combination therapy with imatinib and arsenic sulfide (As4S4). Analysis of cell proliferation and clonogenic ability showed that As4S4 and imatinib exerted synergistic effects on both K562 cells and fresh CML cells. The effective concentrations on fresh CML cells were pharmacokinetically available in vivo but had much less inhibitory effect on CD34+ cells from the nonleukemic donors. Examination of cell cycles showed that As4S4 induced G2/M arrest whereas imatinib induced G1 arrest. Using a number of parameters such as morphology, annexin V/propidium iodide (PI), mitochondrial transmembrane potential, caspase-3 activity, and Fas/Fas-L, the synergistic effects were revealed on induction of cell apoptosis, largely through the mitochondrial pathway. The 2 drugs also exhibited a synergistic effect in targeting BCR-ABL protein. While As4S4 triggered its degradation and imatinib inhibited its tyrosine kinase activity, combined use of the 2 led to lower protein/enzymatic activity levels of BCR-ABL. Our in vitro data thus strongly suggest a potential clinical application of imatinib and As4S4 combination on CML.
Introduction
Chronic myeloid leukemia (CML) is a clonal pluripotent hematopoietic stem cell disorder. Leukemic cells from more than 95% of patients with CML carry the Philadelphia (Ph) chromosome, resulting from t(9;22) reciprocal chromosome translocation, and express a BCR-ABL fusion protein with a relative molecular mass of 210 kDa. BCR-ABL protein has more potent tyrosine kinase activity than the wild-type 145-kDa ABL, leading its downstream signal molecules to be phosphorylated and activated, constituting the molecular basis of CML,1-3 although the critical pathways of CML still remain to be further defined. Targeted treatment against BCR-ABL and/or its downstream effector molecules has become a new hot spot and the introduction of imatinib (STI571, Gleevec, CGP57148B) into CML treatment during the past few years represents the most significant example in this progress.4-7
Imatinib competes the adenosine triphosphate (ATP) binding site of the kinase domain of ABL, which allows a selective inhibition of the action of BCR-ABL and thereby blocks the activation of its downstream effector molecules.8 Clinical trials have shown that imatinib has very good effects on patients with chronic phase (CP), but its effects on patients with accelerated phase or blast phase (advanced phase, AP) are unsatisfactory since many patients relapse after transient remission.9-12 In addition, even among patients at CP, imatinib seems unable to eradicate the malignant progenitors and a significant portion of patients develops drug resistance after long-time use.13-16 Hence, to improve response rates and to circumvent resistance, additional drugs are needed in CML-targeted therapy.
The antileukemic activity of arsenic (Fowler solution) was first reported in the late 1800s.17 Recently, arsenic compounds have regained attention due to the discovery of their clinical effects and unique mode of action on acute promyelocytic leukemia (APL).18-20 Moreover, it was reported that As2O3 could enhance the selective cytotoxic effects of imatinib against BCR-ABL–positive leukemia cells.21,22 A drawback of As2O3 is that it must be administered by intravenous infusion on a daily basis to achieve a good therapeutic result and to avoid gastrointestinal side effects. More recently, arsenic sulfide (As4S4), another arsenic compound, has been shown to be equally effective in the treatment of APL, with fewer side effects and requiring only oral administration.23 Some preliminary data from our group suggested As4S4 could induce apoptosis of CML primary cells and the K562 cell line.24 This orally administered agent would thus contribute to quality of life and also provide easy access to consolidation and maintenance therapy in leukemia therapy.
The aim of this study is to investigate the combined effects of imatinib and As4S4 on BCR-ABL and CML cells. The K562 cell line was used since it has been considered as a cellular model of CML for drug screening. On the other hand, in order to address the potential clinical application of the drugs, we used fresh CD34+ hematopoietic progenitor cells isolated from patients with CML, which have stronger clonogenic ability and also higher drug sensitivity than their progenies.25,26 By a variety of assays for cell proliferation and clonogenic potential, cell cycle distribution, programmed cell death, and protein turnover/activity of BCR-ABL tyrosine kinase, we studied the effect of imatinib and/or As4S4.
Patients, materials, and methods
Patients
Heparin-treated bone marrow (BM) samples were obtained from 22 patients with Ph+ CML and 8 nonleukemic donors. Informed consent was obtained according to institutional guidelines. The 15 men and 7 women with CML ranged in age from 32 years to 63 years and had either CP (14 patients) or AP (8 patients). The diagnosis of CML was established on the basis of morphologic examination, presence of Ph chromosome, and positive reverse transcriptase–polymerase chain reaction (RT-PCR) results for BCR-ABL fusion transcripts. At the time of investigation, 8 patients had received no previous treatment (6 at CP and 2 at AP), whereas 14 had been treated with hydroxyurea, including 4 who also received interferon. Patients were off therapy for a week or on hydroxyurea as the only treatment when the samples were collected.
Selection and culture of CD34+
BM mononuclear cells (BMMNCs) were isolated by means of Ficoll density gradient centrifugation (specific gravity, 1.077; Shanghai Second Reagent Factory, Shanghai, China). CD34+ cells were selected from BMMNCs by using positive immunomagnetic column separation (Miltenyi Biotech, Auburn, CA) according to the manufacturer's instructions. The purity of CD34+ cells ranged from 88% to 96% as determined by flow cytometry (EPICS XL; Coulter, Hialeah, FL), and the viability of CD34+ cells was above 96% by trypan blue exclusion assay.
CD34+ cells were cultured in multiwell tissue-culture plates in serumfree medium (StemPro; Gibco BRL, Gaithersburg, MD) supplemented with growth factors (200 ng/L granulocyte macrophage–colony-stimulating factor, 1 μg/L granulocyte colony-stimulating factor, 200 ng/L stem cell factor, 50 ng/L leukemia inhibitory factor, 200 ng/L macrophage inflammatory protein-1 alpha, 1 μg/L interleukin-6 [PeproTech, London, United Kingdom]).13
Cell lines
The BCR-ABL–positive K562 cell line was derived from a patient with CML in erythroid blast phase. HL60 is a BCR-ABL–negative acute myeloid leukemia cell line. U937 cells are BCR-ABL–negative myeloid precursors blocked at the promonocytic stage, which derived from a patient with histiocytic lymphoma. K562, HL60, and U937 cells were cultured in RPMI 1640, supplemented with 10% fetal bovine serum (PAA, Linz, Austria), in a humidified atmosphere with 5% CO2 at 37°C. Morphology was determined with Wright staining of cells centrifuged onto slides by cytospin (100 g, 4 minutes; Shandon, Runcorn, United Kingdom).
Drugs
Imatinib was kindly provided by Novartis Pharma (Basel, Switzerland), and prepared as one mM stock solution in sterile distilled water at -20°C. As4S4 (Alfa Aesar, Ward Hill, MA) was dissolved in 0.1 M sodium hydroxide to make a stock solution of one mM. Stock solutions were diluted in RPMI 1640 medium to achieve the final concentration.
Proliferation and viability assay
K562, HL60, U937, and CD34+ cells were plated in triplicate at 1 × 105 cells/mL to 2 × 105 cells/mL. After being incubated with 0 μM to 1 μM imatinib and/or 0 μM to 4 μMAs4S4 for one to 3 days at 37°C, viable cells were counted by 0.4% trypan blue exclusion.
Analysis of dose-effect curves
The dose-effect curves of single or combined drug treatment were analyzed by the median-effect method of Chou and Talalay27 using the Calcusyn Software (Biosoft, Cambridge, United Kingdom). The combination indexes (CI) less than 1, equal to 1, and greater than 1 indicate synergistic, additive, and antagonistic effects, respectively.28
Progenitor assay
Mixed with 0 μM to 2 μM imatinib and/or 0 μM to 2 μM As4S4, 103 CD34+ cells were plated in semisolid methylcellulose progenitor culture (Methocult H4434; Stem Cell Technologies, Vancouver, BC, Canada). After 14 days incubation at 37°C in a fully humidified atmosphere of 5% CO2, granulocyte macrophage–colony-forming units (CFU-GMs) and erythroid burst-forming units (BFU-Es) were counted. Only clusters with more than 50 cells were counted as a colony. Total CFUs, CFU-GMs, and BFU-Es were expressed as the percentage of inhibition versus control. All clonogenic assays were done in triplicate.
DNA content analysis by flow cytometry
Cells were collected, washed in phosphate-buffered saline (PBS), and fixed in cold 70% ethanol for at least one hour at 4°C. Shortly before flow cytometry analysis, cells were rinsed with PBS, treated by 50 mg/L RNAse A for at least 15 minutes at 37°C, and stained with 50 mg/L propidium iodide (PI). Distribution of cells with different DNA contents was analyzed by flow cytometry. Ten thousand cells were analyzed in each sample.
Apoptosis assessment by annexin V staining
After drug treatment for 24 to 72 hours, 1 × 105 cells to 5 × 105 cells were washed in PBS and resuspended in 200 μL staining solution containing 5 μL of annexin V–fluorescein isothiocyanate (FITC) and 10 μLof 20 μg/mL PI, according to the protocol of the annexin V staining kit (Clontech, Palo Alto, CA). Flow cytometry was used to analyze 5000 cells. This allowed the discrimination of live cells (unstained with either fluorochrome) from apoptotic cells (stained with annexin V) and necrotic cells (stained with PI). All data were collected, stored, and analyzed by Multigraph software (Coulter, Miami, FL).
Fas receptor (CD95) assay
After drug treatment for 48 hours, 1 × 105 cells to 5 × 105 cells were washed in PBS and resuspended in 100 μL PBS, containing 5 μL phycoerythrin (PE)–conjugated CD95 antibody (Coulter Immunotech, Margency, France) or PE-conjugated nonspecific immunoglobulin G (IgG). The cells were incubated in the dark at 4°C for 30 minutes and the samples were analyzed by flow cytometry.
Determination of mitochondrial transmembrane potentials (ΔΨm)
After being washed twice with PBS, about 106 cells were incubated with 10 mg/L rhodamine 123 (Rh123) at 37°C for 30 minutes, a cationic lipophilic fluorochrome taken up by mitochondria in proportion to the ΔΨm. Then, 50 mg/L PI, a membrane-impermeable DNA binding dye, was added to cells. Flow cytometry was used to determine 104 cells.
Caspase-3 activity assay
Caspase-3 activity was measured using a kit (Clontech). Briefly, 2 × 106 cells treated by As4S4 and/or imatinib were lysed. Assays were performed by incubating 100 μg of cell lysates in 100 μL reaction buffer (1% Nonidet P-40 [NP40], 20 mM Tris-HCl, pH 7.5, 137 mM NaCl, 10% glycerol) containing 5 μL caspase-3 substrate DEVD-pNA at 37°C for 2 hours. Thereafter, the absorbance at 405 nm was measured by a spectrophotometer.
Western blot analysis
After drug treatment for 48 hours, 5 × 106 cells were washed in PBS, then lysed using 200 μL radioimmunoprecipitation assay (RIPA) buffer (containing 50 mM Tris pH 8.0, 150 mM NaCl, 0.1% SDS, 0.5% deoxycholate, 1% NP-40, 1 mM DTT, 1 μM sodium vanadate, and 0.2 mM phenylmethyl sulfonic fluoride [PMSF]). Protein concentrations were determined with the Bradford method (Dc Protein Assay; BIO-RAD, Hercules, CA). Total protein (100 μg) was run on 8% sodium dodecyl sulfate (SDS)–polyacrylamide gels, then transferred to polyvinylidene difluoride membranes (Amersham, Buckinghamshire, United Kingdom), probed with individual antibodies, and visualized by an enhanced chemiluminescence (ECL) system (Pierce, Rockford, IL). The following antibodies were used: anti-retinoblastoma (anti-Rb), anti–phospho-Rb, anti-cyclin–dependent kinase1 (anti-CDK1), anti–phospho-CDK1, anti–poly adenosine diphosphate (ADP)–ribose polymerase (anti-PARP), anti-FasL, anti–Bcl-2, anti-abl (Santa Cruz Biotechnology, Santa Cruz, CA), and anti-actin (Oncogene, Boston, MA). Secondary antibodies were antirabbit peroxidase–conjugated antibody (New England Biolabs, Beverly, MA), and antimouse or antigoat peroxidase–conjugated antibody (Oncogene).
Protein tyrosine kinase (PTK) activity assay
c-ABL and BCR-ABL in samples containing 100 μg total protein were obtained by immunoprecipitation (Santa Cruz Biotechnology). Immunoprecipitates were washed with RIPA buffer and then resuspended in the assay buffer (Tris-HCl 50 mM pH 7.4, MgCl2 40 mM, sodium vanadate 50 mM, dithiothreitol [DTT] 2 mM, MnCl2 1 mM). After centrifugation, the precipitated protein was directly assayed for the PTK activity according to the protocol recommended by manufacturer (SGT410; CHEMICON International, Temecula, CA). Each test was triplicated and the results were calibrated with a corresponding phosphopeptide standard curve and control. The absorbance at 450 nm was measured on a spectrophotometer.
Statistical analysis
Data were reported as the mean plus or minus the standard deviation (SD). Significance levels were determined by SPSS 10.0 for Windows (SPSS, Chicago, IL).
Results
The influence of As4S4 and/or imatinib on the proliferation of the K562 cell line and CD34+ cells of patients with CML
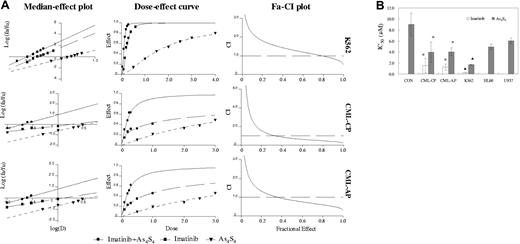
In this study, the K562 cell line was used as a reference since data from several groups, including our own, showed that these cells were sensitive to the imatinib and arsenic trioxide.21,22,24 Here, we examined the combined effects of As4S4 and imatinib on the growth inhibition of K562 cells. Next, we observed the effect of As4S4 and imatinib on fresh CML CD34+ cells for 48 hours. The median-effect principle was applied not only to analyze dose-response curves for each drug alone and combined but also to quantify synergism or antagonism at various proliferation inhibition levels. Median-effect plot showed the value of linear correlation coefficient of As4S4 and/or imatinib was superior to 0.95 (Figure 1A). Fa-CI plot indicated synergistic and additive effects between 2 μM and 4 μMAs4S4 and 0.2 μM and 0.4 μM imatinib after 48 hours on K562 cells. However, synergistic effects were mainly observed between 1 μM and 3 μM As4S4 and 0.1 μM and 0.3 μM imatinib on CD34+ cells among most primary CML samples.
Effects of As4S4 and/or imatinib on various human leukemic cell lines and CD34+ cells from patients with CML and from nonleukemic donors. (A) Median-effect plot, dose-effect curve, and Fa-CI plot of K562 and primary CD34+ cells treated with imatinib and/or As4S4 for 48 hours. (B) IC50 values in various human leukemic cell lines and CD34+ cells from patients with CML and nonleukemic donors treated with imatinib and/or As4S4 for 48 hours. K562, HL60, and U937, results from 3 separate experiments; CML-CP, results of CD34+ cells from 9 CP patients; CML-AP, results of CD34+ cells from 7 AP patients; CON, results of CD34+ cells from 4 nonleukemic donors. Data analysis was performed for the mutual nonexclusive assumption. The black broken line in the Fa-CI plots represents CI equal to 1. Fa values of 0.2, 0.4, or 0.6 correspond to 20%, 40%, or 60% growth inhibition. The concentration ratio of imatinib in combination with As4S4 was 1:10. Statistical analysis using a one-side paired t test. *, P < .01 versus primary nonleukemic cells; ▴, P < .01 versus HL60 or U937 cells.
Effects of As4S4 and/or imatinib on various human leukemic cell lines and CD34+ cells from patients with CML and from nonleukemic donors. (A) Median-effect plot, dose-effect curve, and Fa-CI plot of K562 and primary CD34+ cells treated with imatinib and/or As4S4 for 48 hours. (B) IC50 values in various human leukemic cell lines and CD34+ cells from patients with CML and nonleukemic donors treated with imatinib and/or As4S4 for 48 hours. K562, HL60, and U937, results from 3 separate experiments; CML-CP, results of CD34+ cells from 9 CP patients; CML-AP, results of CD34+ cells from 7 AP patients; CON, results of CD34+ cells from 4 nonleukemic donors. Data analysis was performed for the mutual nonexclusive assumption. The black broken line in the Fa-CI plots represents CI equal to 1. Fa values of 0.2, 0.4, or 0.6 correspond to 20%, 40%, or 60% growth inhibition. The concentration ratio of imatinib in combination with As4S4 was 1:10. Statistical analysis using a one-side paired t test. *, P < .01 versus primary nonleukemic cells; ▴, P < .01 versus HL60 or U937 cells.
The inhibitory concentration (IC50) values (50% inhibition of proliferation) of As4S4 and imatinib in various cells are shown in Figure 1B. Imatinib produced no measurable effect in BCR-ABL–negative HL60 cells, U937 cells, and CD34+ cells from nonleukemic donors at therapeutically relevant concentrations (IC50 > 10 μM).
The effect of As4S4 and/or imatinib on the progenitor-colony forming of CML primary CD34+ cells
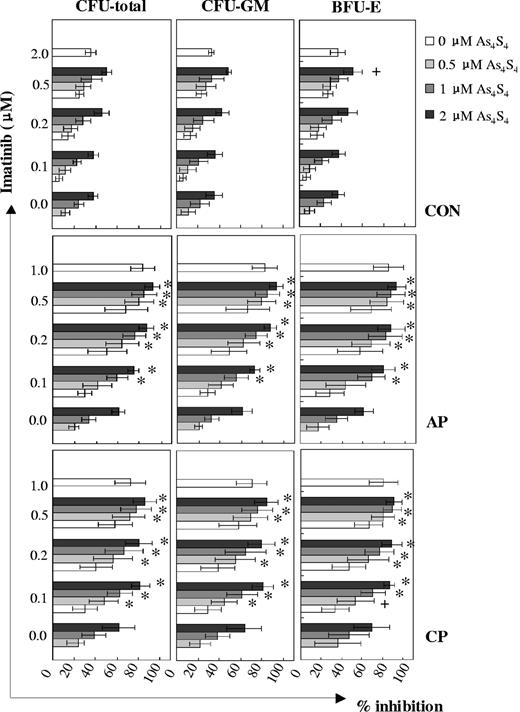
As4S4 and imatinib showed different degrees of synergistic inhibitory effects on the growth of progenitors from distinct disease phases of CML (Figure 2). Among samples at CML-CP, additive and synergistic effects were observed between 0.5 μM and 2 μM As4S4 and 0.1 μM and 0.5 μM imatinib combination groups, whereas among those at CML-AP, a synergism was observed between all combinations except for the 0.5 μM As4S4/0.1 μM imatinib group. With regard to CFU total inhibition, the effects of As4S4 at distinct concentrations combined with 0.1 μM imatinib were more powerful in the CML-CP group than in the CML-AP group. However, when 2 μM As4S4 was combined with 0.2 μM imatinib, CML-AP cells responded in a similar way as CML-CP cells to the inhibitory effect of the 2 agents. In addition, the effect of the 2 μM As4S4 and 0.2 μM imatinib combination was stronger than that of 1 μM imatinib in the CML-CP group (P < .01, n = 13). It is interesting to note that the sensitivity of the colony-forming ability of normal CD34+ progenitors to As4S4 and/or imatinib was significantly lower than that of CML CD34+ cells.
Inhibition of CML progenitor cell growth following exposure to As4S4 and/or imatinib. Patients at CP: n = 13 (except that the number of 0.1 μM imatinib/0.5 μM to 2 μM As4S4 combination groups was 8). Patients at AP: n = 6. Controls: n = 8 (except that the number of 0.5 μM imatinib/0.5 μM to 2 μM As4S4 combination groups was 7). +, additive; *, synergism. Error bars indicate 1 SD.
Inhibition of CML progenitor cell growth following exposure to As4S4 and/or imatinib. Patients at CP: n = 13 (except that the number of 0.1 μM imatinib/0.5 μM to 2 μM As4S4 combination groups was 8). Patients at AP: n = 6. Controls: n = 8 (except that the number of 0.5 μM imatinib/0.5 μM to 2 μM As4S4 combination groups was 7). +, additive; *, synergism. Error bars indicate 1 SD.
Effects of imatinib and/or As4S4 on cell cycle distribution and cell cycle–related protein in K562 cells
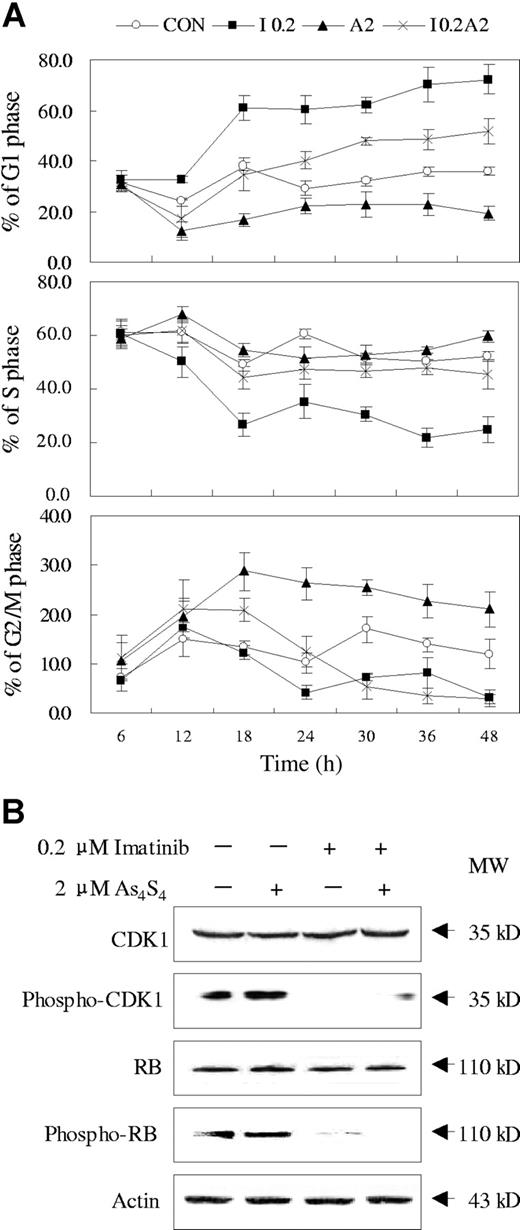
The effects of imatinib and As4S4 on the cell cycle distribution of K562 cells were evaluated. As shown in Figure 3A, after 12 hours of exposure to 0.2 μM imatinib, K562 cells began to be arrested at G1 phase. In contrast, an accumulation of cells arrested at G2/M phase was observed when K562 cells were exposed to 2 μM As4S4 for 12 hours. Interestingly, after combined treatment of imatinib and As4S4, K562 cells were arrested at G2/M phase before 18 hours, while a substantial number of cells were arrested at G1 phase after 24 hours. To address the mechanism of cell cycle regulation under the 2 drugs, we examined some related protein. As shown in Figure 3B, no significant changes were observed of total CDK1 and Rb proteins upon effect of both compounds. However, imatinib profoundly reduced the phosphorylation of CDK1 and Rb, in contrast to As4S4, which exerted no obvious effect on the phosphorylation of the 2 proteins. When cells were exposed to combination treatment, the levels of phosphorylated CDK1 and Rb proteins showed similar changes as those under imatinib. Therefore, the regulatory effect of As4S4 on K562 cell cycle may involve a different set of proteins.
Effects of imatinib and/or As4S4 on cell cycle distribution of K562 cells. (A) The cell cycle distribution analysis of K562 cells incubated with As4S4 and/or imatinib at different time points (n = 3, mean ± SD). CON indicates control; I 0.2, 0.2 μM imatinib; A2, 2 μM As4S4; and I 0.2A2, 0.2 μM imatinib and 2 μM As4S4. (B) Western blot analysis of some cell cycle–related proteins in K562 cells treated with imatinib and/or As4S4 for 48 hours.
Effects of imatinib and/or As4S4 on cell cycle distribution of K562 cells. (A) The cell cycle distribution analysis of K562 cells incubated with As4S4 and/or imatinib at different time points (n = 3, mean ± SD). CON indicates control; I 0.2, 0.2 μM imatinib; A2, 2 μM As4S4; and I 0.2A2, 0.2 μM imatinib and 2 μM As4S4. (B) Western blot analysis of some cell cycle–related proteins in K562 cells treated with imatinib and/or As4S4 for 48 hours.
As4S4 and/or imatinib induced apoptosis of the K562 cell line and CML primary CD34+ cells
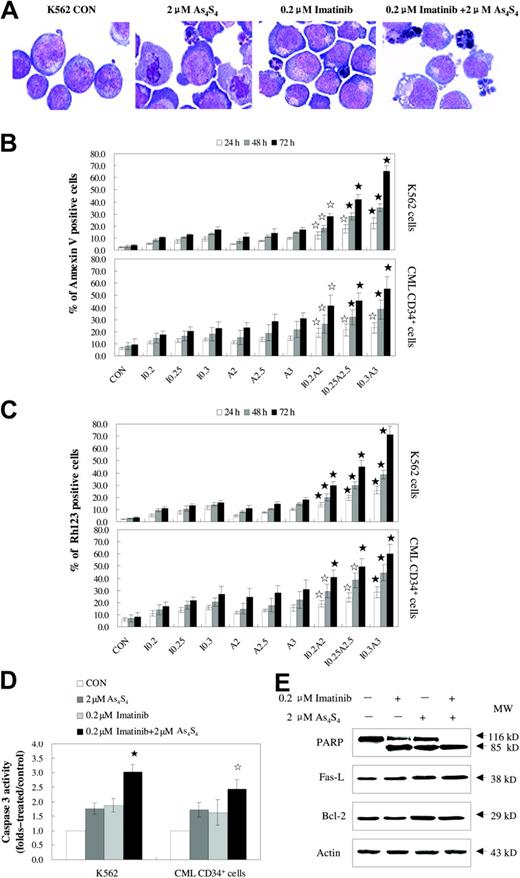
The combination of imatinib and As4S4 induced more apoptosis than either agent alone (Figure 4). Typical morphologic features of apoptosis, such as nuclear fragmentation and apoptotic bodies, were observed under light microscopic examination (Figure 4A). Since annexin V is the early indicator of apoptosis, the percentages of apoptotic cells were determined using annexin V/PI staining, after K562 cells and CD34+ cells from patients with CML were exposed to imatinib and/or As4S4 for 24 to 72 hours. Compared with either 0.2 μM to 0.3 μM imatinib or 2 μM to 3 μM As4S4 alone for 24 to 72 hours, combined treatment with these agents resulted in significantly more apoptosis of K562 cells in a dose- and time-dependent manner. The CD34+ cells from patients with CML with similar treatment exhibited significant apoptotic changes as well, and the results suggested synergistic effects of the drugs (Figure 4B). Parallel results were also obtained when loss of mitochondrial potential was monitored on K562 cells and CML CD34+ cells (Figure 4C). As shown in Figure 4D, combination treatment activated caspase-3 in a higher percentage of cells as compared with the result obtained with either drug alone (P < .05, n = 4). More 116 kDa PARPs were cleaved into an 85-kDa fragment by caspase-3 in the combination group as compared with the single treatment group (Figure 4E). Moreover, Western blot showed that Fas-L was up-regulated in the 2 μM As4S4 group and the cotreatment group with 0.2 μM imatinib and 2 μM As4S4, whereas no obvious change was found in CD95 expression in K562 cells (2.2% ± 0.2%, 2.4% ± 1.0%, 3.3% ± 0.9% versus 3.6% ± 1.5%, for control, 0.2 μM imatinib, 2 μMAs4S4 versus 0.2 μM imatinib and 2 μM As4S4 combination, respectively; n = 4, P > .05). In addition, Western blot results suggested that the apoptosis induced by 0.2 μM imatinib and 2 μM As4S4 was independent of Bcl-2 protein level.
As4S4 and/or imatinib induce the apoptosis of the K562 cell line and primary CML cells. (A) The morphology of K562 cells untreated or treated with 2 μM As4S4 and/or 0.2 μM imatinib for 48 hours. The K562 cells were stained with Wright staining and observed on an Olympus BX 50 light microscope (Olympus, Tokyo, Japan) provided with MCDS-20/0 Super Bone Marrow Cell Analysis System (Chongqing, China). Original magnification, ×1000 (objective × 100). Images were captured using a Polaroid Dmc (Polaroid, Miami, FL). (B) Annexin V–PI assessment of the K562 cells and CD34+ cells from patients with CML. Cells were treated with As4S4 and/or imatinib for 24 to 72 hours and measured for annexin V positivity (n = 3, mean ± SD). (C) The reduction of mitochondrial transmembrane potential in K562 cells and CML CD34+ cells after treatment with As4S4 and/or imatinib for 24 to 72 hours (n = 3, mean ± SD). (D) Caspase-3 activity of K562 cells and CML CD34+ cells after 48 hours of incubation with As4S4 and/or imatinib (n = 4, mean ± SD). (E) Western blot analysis of some proteins related to apoptosis after K562 cells, after 48 hours of exposure to As4S4 and/or imatinib. Statistical analysis using a one-side paired t test (☆, P < .05 versus imatinib, As4S4 group, and control; ⋆, P < .01 versus imatinib group, As4S4 group, and control). CON indicates control; I 0.2, 0.2 μM imatinib; I 0.25, 0.25 μM imatinib; I 0.3, 0.3 μM imatinib; A2, 2 μM As4S4; A2.5, 2.5 μM As4S4; A3, 3 μM As4S4; I 0.2A2, 0.2 μM imatinib and 2 μM As4S4; I 0.25A2.5, 0.25 μM imatinib and 2.5 μM As4S4; and I 0.3A3, 0.3 μM imatinib and 3 μM As4S4.
As4S4 and/or imatinib induce the apoptosis of the K562 cell line and primary CML cells. (A) The morphology of K562 cells untreated or treated with 2 μM As4S4 and/or 0.2 μM imatinib for 48 hours. The K562 cells were stained with Wright staining and observed on an Olympus BX 50 light microscope (Olympus, Tokyo, Japan) provided with MCDS-20/0 Super Bone Marrow Cell Analysis System (Chongqing, China). Original magnification, ×1000 (objective × 100). Images were captured using a Polaroid Dmc (Polaroid, Miami, FL). (B) Annexin V–PI assessment of the K562 cells and CD34+ cells from patients with CML. Cells were treated with As4S4 and/or imatinib for 24 to 72 hours and measured for annexin V positivity (n = 3, mean ± SD). (C) The reduction of mitochondrial transmembrane potential in K562 cells and CML CD34+ cells after treatment with As4S4 and/or imatinib for 24 to 72 hours (n = 3, mean ± SD). (D) Caspase-3 activity of K562 cells and CML CD34+ cells after 48 hours of incubation with As4S4 and/or imatinib (n = 4, mean ± SD). (E) Western blot analysis of some proteins related to apoptosis after K562 cells, after 48 hours of exposure to As4S4 and/or imatinib. Statistical analysis using a one-side paired t test (☆, P < .05 versus imatinib, As4S4 group, and control; ⋆, P < .01 versus imatinib group, As4S4 group, and control). CON indicates control; I 0.2, 0.2 μM imatinib; I 0.25, 0.25 μM imatinib; I 0.3, 0.3 μM imatinib; A2, 2 μM As4S4; A2.5, 2.5 μM As4S4; A3, 3 μM As4S4; I 0.2A2, 0.2 μM imatinib and 2 μM As4S4; I 0.25A2.5, 0.25 μM imatinib and 2.5 μM As4S4; and I 0.3A3, 0.3 μM imatinib and 3 μM As4S4.
Effects of imatinib and/or As4S4 on BCR-ABL protein and its PTK activity
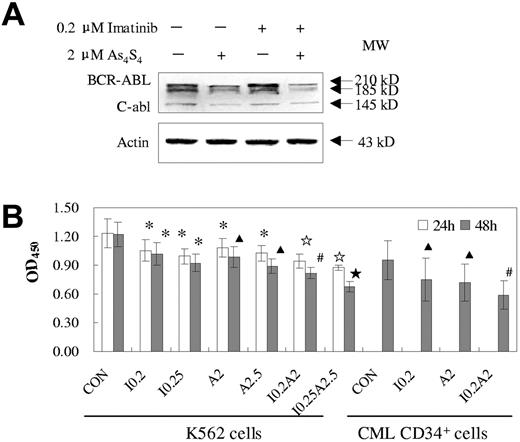
After exposure to As4S4 and/or imatinib for 24 hours or 48 hours, the BCR-ABL protein levels and their PTK activities were assessed in K562 cells or primary cells. The 2 drugs exhibited synergistic effects not only on the degradation of BCR-ABL protein but also on the reduction of tyrosine kinase activity. Figure 5A demonstrated that exposure to 2 μM As4S4 for 48 hours reduced BCR-ABL protein levels in K562 cells whereas 0.2 μM imatinib could not. However, the cotreatment seemed to induce more degradation of BCR-ABL than did As4S4 alone. On the other hand, cotreatment led to more decline of PTK activity than either drug alone (P < .05, n = 3) and the synergistic effects were dose- and time-dependent. To test the effect of combined treatment in primary cells, we used fresh BMMNCs from 3 patients with CML-AP that contained a relatively high proportion of CD34+ cells (30%, 65%, and 75%, respectively). Similar results were observed in those samples as compared with K562 cells.
Effects of imatinib and/or As4S4 on BCR-ABL oncoprotein and PTK activity in K562 cells and primary CML cells. (A) Western blot analysis of BCR-ABL of K562 cells after 48 hours of treatment. (B) PTK activity of K562 cells and primary BMMNCs from CML-AP patients. Analysis was performed after treatment with As4S4 and/or imatinib for 24 hours and 48 hours (n = 3, mean ± SD). Statistical analysis using a one-side paired t test (*, P < .05 versus control; ▴, P < .01 versus control; ☆, P < .05 versus imatinib, As4S4 group, and control; ⋆, P < .01 versus imatinib, As4S4 group, and control; #, P < .05 versus imatinib, As4S4 group, and P < .01 versus control). CON indicates control; I 0.2, 0.2 μM imatinib; I 0.25, 0.25 μM imatinib; A2, 2 μM As4S4; A2.5, 2.5 μM As4S4; I 0.2A2, 0.2 μM imatinib and 2 μM As4S4; and I 0.25A2.5, 0.25 μM imatinib and 2.5 μM As4S4.
Effects of imatinib and/or As4S4 on BCR-ABL oncoprotein and PTK activity in K562 cells and primary CML cells. (A) Western blot analysis of BCR-ABL of K562 cells after 48 hours of treatment. (B) PTK activity of K562 cells and primary BMMNCs from CML-AP patients. Analysis was performed after treatment with As4S4 and/or imatinib for 24 hours and 48 hours (n = 3, mean ± SD). Statistical analysis using a one-side paired t test (*, P < .05 versus control; ▴, P < .01 versus control; ☆, P < .05 versus imatinib, As4S4 group, and control; ⋆, P < .01 versus imatinib, As4S4 group, and control; #, P < .05 versus imatinib, As4S4 group, and P < .01 versus control). CON indicates control; I 0.2, 0.2 μM imatinib; I 0.25, 0.25 μM imatinib; A2, 2 μM As4S4; A2.5, 2.5 μM As4S4; I 0.2A2, 0.2 μM imatinib and 2 μM As4S4; and I 0.25A2.5, 0.25 μM imatinib and 2.5 μM As4S4.
Discussion
To our knowledge, the present work is the first to address the combined effect of imatinib and As4S4 on CML cells. Imatinib inhibits the activity of ABL tyrosine kinase in a highly specific way, whereas As4S4 may have a wide range of cellular targets by analogy to the related compound arsenic trioxide (As2O3).29 In view of the distinct target profiles of the 2 agents, we selected the CI equation for mutually nonexclusive drugs (with independent modes of action) to study their combined use. Importantly, in both the short-term culture system (within 48 hours) or the relatively long CFU assay (14 days), there was a wide range of synergy between different concentrations of As4S4 and imatinib on CD34+ cells from 22 patients with CML. Overall, this synergy displayed a dose- and time-dependent feature. In our experiment, a high concentration of imatinib (at 2 μM) could mildly inhibit colony forming of the normal control (35.14% total CFUs, 32.36% CFU-GMs, and 36.6% on average, respectively). Similar results have been reported previously.30 The underlying mechanism could be that high-concentration imatinib inhibits a number of wild-type tyrosine kinases activities (such as C-ABL, C-KIT, and platelet-derived growth factor receptor) that are essential for the normal CD34+ cell colony formation. When CFU assays were performed under the 2 μM As4S4 and 0.2 μM imatinib combination, the same level of inhibition could be reached in both CML-CP and -AP groups. However, the clonogenic capacity of normal CD34+ cells seemed much less sensitive to the same conditions. Thus, 2 μMAs4S4 and 0.2 μM imatinib could constitute an ideal combination in achieving therapeutic effect with limited side effects on the normal hematopoiesis. Previous pharmacokinetic studies on patients with APL showed that a standard dose of As4S4 achieved a plasma level of 1 μMto2 μM.23 Under a standard dose of imatinib (300 mg/d to 600 mg/d), on the other hand, the plasma concentrations of the drug fluctuated in the range of 1 μM to 2 μM.8 Therefore, the effective in vitro concentrations of both drugs on CML CD34+ cells established in this study should also be achievable under an in vivo situation.
The synergistic effect of the imatinib and As4S4 combination in terms of antiproliferation might be connected with the distinct but complementary roles of the drugs in interfering with cell cycle progression. Our data showed that As4S4 induced G2/M arrest of K562 cells, whereas imatinib induced G1 arrest. When these 2 drugs were used simultaneously, the K562 cells were arrested at G2/M phase at first and then underwent G1 arrest together with apoptosis. A previous study showed that As2O3 disturbs the assembly of spindle microtubules and induces mitotic arrest in a variety of human cancer cell lines.31 A similar situation may also exist for As4S4. With regard to the cell cycle machinery, it is well known that the phosphorylation of Rb and dephosphorylation of CDK1 play important roles in the transition of G1/S and G2/M phases, respectively.32,33 Rb is a negative regulator of cell proliferation and is inactivated by phosphorylation. Rb could be phosphorylated by RAS/MAPK/ERK–dependent cyclins, CDKs of mitogenic signals, and Fas/p38 kinase of apoptotic signals, leading to dissociation of E2F and increased transcriptional activity.32,34 It has been reported that phospho-Rb regulation of E2F activity affects cellular potential for G1/S phase transition in part via its effect on c-myc transcription.35 On the other hand, phospho-CDK1 represents a repressed form of the protein while the dephosphorylation at Thr14/Tyr15 of CDK1 allows its activation and G2/M progression. Our data demonstrated that imatinib induces significant down-regulation of phosphorylated Rb and CDK1, which is in agreement with the G1/S but not G2/M arrest under this drug. However, As4S4 shows no obvious effect on these proteins in spite of a visible effect on G2/M block, suggesting the involvement of other mechanisms such as cytoskeleton regulation.
The cellular mechanisms of As4S4 and imatinib have been associated with the induction of apoptosis. It is well known that ΔΨm collapse plays an essential role in mediating apoptosis through the release of apoptotic mediators (eg, cytochrome c/Apaf1/caspase-9 and apoptosis-inducing factor) into cytoplasm, a pathway known as intrinsic apoptotic signaling.36 Ligation of the cell-surface Fas molecule by its ligand, on the other hand, also known as the extrinsic pathway, results in the cleavage and activation of the cysteine protease procaspase-8.37,38 The activation of procaspase-3 following caspase-8 or caspase-9 activation is considered to play a crucial role in apoptosis.39,40 It was reported that As2O3-induced apoptosis coincided with ΔΨm collapse and activation of caspase-3 in APL cell line NB4 and primary APL cells.31 Here we showed that either As4S4 or imatinib alone could achieve similar results in CML cell lines and fresh cells. However, the combination of the 2 agents yielded much more significant reduction of ΔΨm and activation of caspase-3, followed by cleavage of PARP and expression of phosphatidyl serine on the cellular surface membrane (detected as annexin V), then displayed morphogenesis features of apoptosis. In addition, the protein level of Fas-L seems to be up-regulated by As4S4 and combination treatment, which may cause an autocrine mechanism and lead to an enhancement of the extrinsic pathway of apoptosis.
The molecular basis of CML is the BCR-ABL fusion protein. BCR-ABL induces deregulation of cell proliferation, inhibition of apoptosis, and adhesion abnormality to marrow stroma. Moreover, some important downstream pathways have been significantly altered.25 Therefore, the application of imatinib in CML has been considered as a molecular triumph of cancer research in the genomic era. On the other hand, resistance to imatinib in a number of patients after long-time use has been ascribed to an elevated expression the BCR-ABL gene or to the mutation at key positions of the BCR-ABL coding sequence around the ATP-binding domain.16 Interestingly, our study showed that one of the targets of As4S4 is also BCR-ABL protein. Unlike imatinib, which inhibits the tyrosine kinase activity of BCR-ABL but apparently does not affect the turnover of the protein, As4S4 triggers the degradation of BCR-ABL, accompanied by a reduction of the tyrosine kinase activity. Although it is still unknown whether As4S4 influences the stability of BCR-ABL in a direct or indirect way, its mode of action seems to be quite different, but highly complementary, to that of imatinib. To this end, combination of imatinib and As4S4 represents a new model of synergistic targeting at the molecular level and might be a promising approach to improve response rates and prevent resistance. As a matter of fact, a clinical trial using imatinib and As4S4 has recently been launched at the Shanghai Institute of Hematology, to evaluate the potential benefit of the combination of these drugs to patients with CML.
In conclusion, our study suggests As4S4 and imatinib have synergistic effects in inhibiting proliferation, inducing apoptosis of cells, and reducing the tyrosine kinase activity of BCR-ABL. Whether this in vitro synergistic activity will translate into better response and survival rates of patients with CML needs to be proven in clinical trials.
Prepublished online as Blood First Edition Paper, August 31, 2004; DOI 10.1182/blood-2004-04-1433.
Supported in part by grants from the Chinese High Tech Program (863), the National Natural Science Foundation of China (90 209 007), the National Key Program for Basic Research (2004CB518606 and 2002CB512 805), the Shanghai Municipal Commission for Education, and the Shanghai Municipal Commission for Science and Technology.
T.Y. and Y.-L.W. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are very grateful to all members of the State Key Laboratory of Medical Genomics and the Shanghai Institute of Hematology for their encouragement and support.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal