Abstract
In myelodysplastic syndromes (MDS), anemia responds to recombinant human erythropoietin (rHuEPO) alone and in combination with recombinant human granulocyte-colony-stimulating factor (rHuGCSF) in 10% to 20% and in 35% to 40% of patients, respectively. We randomly divided 60 patients with low-grade anemic MDS and serum EPO levels lower than 500 IU/L (500 mU/mL) into 2 groups: rHuEPO + rHuG-CSF (arm A) and supportive care (arm B). After 12 weeks, those who had erythroid responses were given rHuEPO alone for 40 additional weeks. They were also given rHuG-CSF if they had relapses. A response was considered major if the hemoglobin (Hb) level was 115 g/L (11.5 g/dL) or higher and minor Hb increase was 15 g/L (1.5 g/dL) or more or if it remained stable without transfusion. Ten of 24 patients responded in arm A, and 0 of 26 responded in arm B (P = .01). Eight patients in arm A continued rHuEPO therapy alone, and 6 had relapses. Responses were always restored when rHuG-CSF was reintroduced. Mean direct costs per patient were 26 723 euros (€ 26 723) (arm A) and € 8746 (arm B). Quality of life was aszsessed with a Functional Assessment of Cancer Therapy-Anemia (FACT-An) scale. Similar percentages of patients from both arms showed significant clinical improvement. rHuEPO plus rHuG-CSF led to responses in 41.7% of MDS patients. This treatment was expensive. No effect on quality of life was demonstrated. (Blood. 2004;104: 321-327)
Introduction
Myelodysplastic syndromes (MDS) are clonal hematopoietic disorders characterized by ineffective hematopoiesis, peripheral cytopenia, and increased risk for acute myelogenous leukemia (AML). Approximately two thirds of patients with MDS have anemia at diagnosis, and it develops in nearly all the rest as the disease progresses. Many MDS patients require frequent transfusions, leading to secondary hemochromatosis and risk for viral infections. In low-risk MDS, anemia is often the major clinical problem. Anemia significantly affects quality of life and causes significant morbidity in older patients. Cardiovascular diseases are often aggravated. Treatment of anemia with recombinant human erythropoietin (rHuEPO) alone is effective only in a small percentage of MDS patients. A meta-analysis of 205 patients with MDS showed that 16% responded to rHuEPO alone.1 Patients who responded to treatment had no or limited transfusion requirements. Granulocyte-colony-stimulating factor (G-CSF) and granulocyte macrophage-colony-stimulating factor (GM-CSF) increase neutrophil counts, do not increase the risk for leukemia, and have no effect on survival.2-4
When combined with myeloid cytokines, rHuEPO can have synergistic effects on erythropoiesis in vitro.5,6 Some clinical studies have shown that the combination of rHuG-CSF and rHuEPO has a better response rate (40%-50%) than rHuEPO alone.7-12 However, none of these clinical trials were randomized, and cost analyses and quality-of-life evaluations are lacking. In one randomized study, the response rate was similar with GM-CSF plus EPO and with placebo4 In these studies, only a small proportion of subjects with baseline serum EPO concentrations exceeding 500 IU/L (500 mU/mL) responded to treatment.9
Therefore, we designed a multicenter randomized trial comparing treatment with rHuEPO plus rHuG-CSF and supportive care in patients with MDS who had serum EPO concentrations lower than or equal to 500 IU/L (500 mU/mL) to determine the effect of this treatment on anemia. Total direct costs of the 2 treatment alternatives and the effect of these treatments on quality of life were also evaluated.
Patients, materials, and methods
Study site and design
Subjects with MDS from 15 French hospitals (including 13 teaching hospitals, 6 of which belong to the Assistance Publique-Hôpitaux de Paris or the Public Hospital Organization of the Paris region) were included between January 1999 and April 2000. An ethics committee from Hôpital Henri Mondor (Créteil, France) approved the protocol, and each subject signed an informed consent form. Subjects were randomly assigned to 1 of 2 treatment groups by means of a central procedure. Treatment allocation was balanced at each center in blocks of 4.
Subjects
Inclusion criteria were (1) age 18 years and older; (2) diagnosis of refractory anemia (RA), RA with ringed sideroblasts (RARS), and RA with excess blasts (RAEB) with less than 10% marrow blasts according to the French-American-British (FAB) classification; (3) hemoglobin (Hb) concentration less than 100 g/L (10 g/dL) for at least 2 months or at least 2 U red blood cells (RBCs) transfused in the previous 2 months; (4) serum EPO concentration 500 IU/L (500 mU/mL) or lower; and (5) written, informed consent. Exclusion criteria were (1) cardiac, pulmonary, neurologic, digestive, or genitourinary disease unrelated to MDS; (2) intensive chemotherapy in the previous 3 months; (3) treatment with rHuEPO or rHuG-CSF in the previous 2 months; (4) Eastern Cooperative Oncology Group (ECOG) score equal to 4; (5) deficit in iron, vitamin B12, or folic acid; (6) hypertension (systolic arterial pressure higher than 160 mm Hg); and (7) life expectancy less than 6 months.
Treatment
Subjects were randomly assigned to 1 of 2 groups. Arm A received rHuEPO (epoetin alfa [Eprex], kindly supplied by Ortho Biotech, Division of Janssen-Cilag, Paris, France) and rHuG-CSF (lenograstim [Granocyte 13], kindly supplied by Laboratoire Aventis and Chugai Pharma France, Paris) for 12 weeks. rHuEPO and rHuG-CSF were subcutaneously (SC) administered (either by the subject or a nurse). Both rHuEPO (20 000 IU) and rHuG-CSF (105 μg) were given 3 times a week. The dose of rHuG-CSF was then adjusted so that the white blood cell (WBC) count remained between 5 × 109/L and 1 × 1010/L, or double the initial number of WBCs. Subjects considered responders after 12 weeks of treatment were given rHuEPO alone for another 40 weeks. rHuG-CSF was reintroduced if anemia recurred.
Subjects in arm B received supportive care for 52 weeks, which included RBCs to maintain the Hb level above 80 g/L (8 g/dL) and normal iron chelation treatment. Subjects were only evaluated if the combination of rHuEPO and rHuG-CSF was given for at least 12 weeks.
Response criteria
A major erythroid response was defined as an increase in Hb concentration to at least 115 g/L (11.5 g/dL). A minor response was defined as an increase in Hb concentration of 15 g/L (1.5 g/dL) or more or as a stable Hb concentration without the need for transfusion.
Cost evaluation
Information on resource use was prospectively collected throughout the clinical trial. Each center investigator interviewed the center's subjects concerning the resources used throughout the study. The costs of these compulsory visits were not included in the cost evaluation because they could not be considered standard care (the number of visits was the same in both groups). Analysis was conducted from the perspective of the French healthcare payer and the hospital. Costs were calculated in euros (€), and the base year for the study was 2000. Because treatment duration lasted 1 year, cost discounting was unnecessary.
Only direct costs were considered in the analysis. We collected data about transfusions, transport, medication (epoetin alfa and lenograstim), nurse visits, other healthcare professional visits, examinations, and hospital stays linked to MDS.
Transfusions were performed on an outpatient basis, and their real cost to the hospitals (materials, transport, medical examinations, doctors' and nurses' wages for the time spent performing transfusions, number of units of RBCs and platelets, iron chelation treatment) was calculated. Costs were based on the activity of Cochin Hospital (the participating center with the most subjects). Wages were based on those of the Assistance Publique-Hôpitaux de Paris (AP-HP). Drug costs were based on the purchase price by AP-HP hospitals (epoetin alfa, lenograstim, and deferiprone [Ferriprox]) or on the recommended retail price (especially deferoxamine [Desferal]). We used official prices for public transport. For ambulance and private car costs, we used the tables published in the Journal Officiel de la République Française.13 Costs of visits from healthcare professionals were based on the “General Nomenclature of Professional Acts.” To calculate costs for hospital stays, we used the diagnosis-related group (DRG) method for each admission. Data were retrieved from the hospitals' information systems, and the cost of an admission was derived from the French Ministry of Health national cost database.14
Quality of life
Quality of life (QOL) was evaluated at baseline and at weeks 12, 28, and 52 using an international validated QOL instrument, the Functional Assessment of Cancer Therapy-Anemia (FACT-An) questionnaire. FACT-An includes the 28-item Functional Assessment of Cancer Therapy-General (FACT-G) questionnaire, which measures the 4 general domains of QOL (physical, social/family, emotional, and functional well-being) and 20 additional questions regarding anemia, called the anemia subscale. Thirteen of these 20 items represent the fatigue component and are called the fatigue subscale, and the other 7 are called the nonfatigue subscale. This instrument has been validated to assess QOL in subjects experiencing fatigue and other anemia-related symptoms, and it has been shown to be reliable.15,16 We used a validated French version of this scale.17
Evaluation criteria
The primary end point was hematopoietic response at 12 weeks, based on the increase of Hb concentration and the modification of transfusion needs as defined in response criteria. Secondary end points were the cost study and the QOL measurement during the 1-year study period.
Statistical analysis
Analyses were made on an intention-to-treat basis. Experimental values are given as mean ± SEM. Proportions of responders in each arm were compared using the χ2 test. As described previously, QOL was measured at baseline and at weeks 12, 28, and 52. We took into account estimates of the minimal clinically importance differences (CIDs) provided by Cella et al.18 The CID in a QOL score is defined as “the smallest difference in score in the domain of interest that patients perceive as beneficial.” Estimates used were 7.0 (total FACT-An score), 3.0 (fatigue subscale), and 6.0 (anemia subscale). We compared percentages of subjects with clinically significant improvement in the 2 groups using the Fisher exact test. Costs are given as mean, standard deviation, median, and range costs per subject.19 We first present costs of treatment for all subjects included in arm A and arm B. We then present costs of treatment for all subjects who completed 1 year of treatment.
Results
Description of subjects
Sixty subjects (30 men and 30 women) were included in the study, with 30 subjects in each arm. Median age was 72 years (range, 43-89 years). Twenty subjects had RA, 26 had RARS, and 14 had RAEB (with less than 10% marrow blasts). Characteristics of the subjects at inclusion are shown in Table 1.
Baseline characteristics of subjects
. | Arm A: rHuEPO + rHuG-CSF . | Arm B: supportive care . |
|---|---|---|
| Median age, y (range) | 73 (43-80) | 71 (48-89) |
| Sex, male/female | 14/16 | 16/14 |
| FAB classification | ||
| RA | 9 | 11 |
| RARS | 15 | 11 |
| RAEB, less than 10% blasts | 6 | 8 |
| IPSS: Low/Int1/Int2/High | 12/10/1/1* | 11/13/1/1† |
| Hemoglobin concentration, g/L, mean ± SEM | 86 ± 12 | 86 ± 11 |
| Prior RBC transfusions, yes/no | 30/0 | 26/4 |
| Serum erythropoietin concentration, IU/L, mean ± SEM | 178.8 ± 124.4 | 138.2 ± 132.2 |
. | Arm A: rHuEPO + rHuG-CSF . | Arm B: supportive care . |
|---|---|---|
| Median age, y (range) | 73 (43-80) | 71 (48-89) |
| Sex, male/female | 14/16 | 16/14 |
| FAB classification | ||
| RA | 9 | 11 |
| RARS | 15 | 11 |
| RAEB, less than 10% blasts | 6 | 8 |
| IPSS: Low/Int1/Int2/High | 12/10/1/1* | 11/13/1/1† |
| Hemoglobin concentration, g/L, mean ± SEM | 86 ± 12 | 86 ± 11 |
| Prior RBC transfusions, yes/no | 30/0 | 26/4 |
| Serum erythropoietin concentration, IU/L, mean ± SEM | 178.8 ± 124.4 | 138.2 ± 132.2 |
For each arm n = 30 subjects. IPSS indicates International Prognostic Scoring System; Low, low risk; Int1, intermediate 1 risk; Int2, intermediate 2 risk; and High, high risk.
Only results concerning 24 subjects could be evaluated.
Only results concerning 26 subjects could be evaluated.
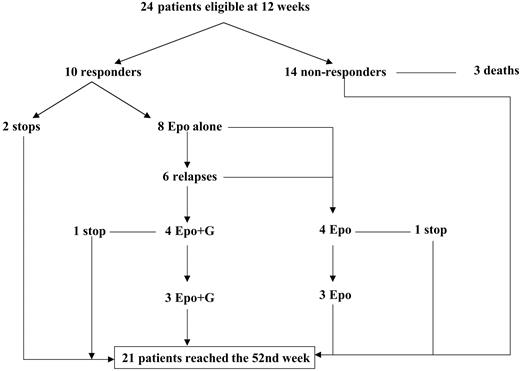
Three subjects from arm A (receiving rHuEPO + rHuG-CSF) withdrew after 1, 8, and 10 weeks, respectively (2 refused further treatment, and 1 acquired acute myeloid leukemia). Three subjects from arm A died of causes unrelated to the study treatment (2 after cardiac failure and 1 after hip fracture) in weeks 5, 8, and 10, respectively. Therefore, the erythroid response was evaluated in only 24 subjects at 12 weeks. All but 3 subjects requested help from a nurse for the injections.
In arm B, 2 subjects died (1 of septic shock and 1 of hemorrhage after a fall) after 8 and 12 weeks, respectively, and 2 were lost to follow-up after 4 and 10 weeks. Thus, 26 subjects reached the 12th week (Figure 1).
After the initial 12 weeks of treatment, 3 other subjects from arm A died (one from cerebral hemorrhage, one from cardiac failure and one from a stroke) as did 2 from arm B (one from acute cardiac failure and one from a stroke). Therefore, 21 and 24 subjects from arms A and B respectively completed the trial.
Treatment results
Evaluation at week 12. Ten (42%) of the 24 subjects from arm A who completed the trial had erythroid responses compared with 0 of 26 in group B (P = .01) (Table 2). Eight subjects showed minor responses (3 subjects had increased Hb concentrations, and 5 subjects became transfusion independent), and 2 had major responses (Hb concentrations: 130 g/L, Δ = 34 g/L [13 g/dL, Δ = 3.4 g/dL]; 123 g/L, Δ = 27 g/L [12.3 g/dL, Δ = 2.7 g/dL]) and were no longer transfusion dependent. The mean Hb concentration at 12 weeks for the 10 responding subjects in arm A was 104 g/L ± 13 g/L (10.4 g/dL ± 1.3 g/dL) compared with 91 g/L ± 18 g/L (9.1 g/dL ± 1.8 g/dL) at baseline (mean difference, 13.3 g/L [1.33 g/dL] = 15%). Response rates were 50%, 46%, and 20% for RA, RARS, and RAEB subjects, respectively. In arm B, there were no significant changes in transfusion needs or in mean Hb concentrations (86 ± 11 g/L [8.6 ± 1.1 g/dL] at baseline and 88 ± 12 g/L [8.8 ± 1.2 g/dL] after 12 weeks).
Erythroid response to treatment in eligible subjects at week 12
. | Arm A: rHuEPO + rHuG-CSF . | . | . | Arm B: supportive care . | ||
|---|---|---|---|---|---|---|
| . | Responders; n = 10 . | . | . | . | ||
. | Major response; n = 2 . | Minor response; n = 8 . | Nonresponders; n = 14 . | All subjects; n = 26 . | ||
| FAB classification, n | ||||||
| RA | 1 | 2 | 3 | 9 | ||
| RARS | 1 | 5 | 7 | 11 | ||
| RAEB | 0 | 1 | 4 | 6 | ||
| RBC transfusion dependent, n, yes/no | 0/2 | 3/5 | 14/0 | 24/2 | ||
| Hemoglobin concentration, g/L, mean ± SEM | 126 ± 5 | 98 ± 7 | 83 ± 13 | 88 ± 12 | ||
. | Arm A: rHuEPO + rHuG-CSF . | . | . | Arm B: supportive care . | ||
|---|---|---|---|---|---|---|
| . | Responders; n = 10 . | . | . | . | ||
. | Major response; n = 2 . | Minor response; n = 8 . | Nonresponders; n = 14 . | All subjects; n = 26 . | ||
| FAB classification, n | ||||||
| RA | 1 | 2 | 3 | 9 | ||
| RARS | 1 | 5 | 7 | 11 | ||
| RAEB | 0 | 1 | 4 | 6 | ||
| RBC transfusion dependent, n, yes/no | 0/2 | 3/5 | 14/0 | 24/2 | ||
| Hemoglobin concentration, g/L, mean ± SEM | 126 ± 5 | 98 ± 7 | 83 ± 13 | 88 ± 12 | ||
The mean hemoglobin concentration (± SEM) among all responders was 104 ± 13 g/L.
Maintenance phase. Two subjects from arm A declined further treatment for personal reasons, and only 8 subjects continued treatment with rHuEPO alone after 12 weeks. Anemia relapsed in 6 of these 8 subjects. In 4 subjects, rHuG-CSF was reintroduced and anemia was corrected. The 2 other subjects refused treatment with rHuG-CSF and continued treatment with rHuEPO alone. One subject (rHuEPO alone) decided to stop treatment after 28 weeks, and another subject (rHuEPO + rHuG-CSF) stopped treatment after 44 weeks because of progression to AML. Six subjects continued treatment until week 52, including 3 treated with rHuEPO + rHuG-CSF and 3 treated with rHuEPO alone (Figure 2). At the end of the 1-year period, 3 subjects (one treated with rHuEPO alone and 2 treated with rHuEPO + rHuG-CSF) still had minor responses: they were transfusion independent and had stable Hb concentrations. The 3 other subjects had relapses and received transfusions between weeks 44 and 52.
Adverse effects. No adverse effects were attributable to rHuEPO or rHuG-CSF. One subject from arm A progressed to AML after 10 weeks of treatment, and another from arm B did so 20 weeks after inclusion. None of the deaths were linked to the treatment evaluated in the study.
Cost evaluation. First, we used intention-to-treat analysis to evaluate data concerning all subjects during the 1-year study period—30 subjects in arm A and 29 subjects in arm B (1 subject in arm B was not included because he was lost very early during follow-up, and no cost data were available) (Table 3). Mean costs per subject were € 26 723 (± € 13 109) and € 8 746 (± € 6 731), respectively, in arm A and arm B. This difference was mainly attributed to the cost of drugs (€ 19 121 in arm A compared with € 0 in arm B).
Costs per subject and per total treatment
. | Arm A: rHuEPO + rHuG-CSF . | . | Arm B: supportive care . | . | ||
|---|---|---|---|---|---|---|
| Treatment . | Per subject; n = 30 . | All subjects; n = 30 . | Per subject; n = 29 . | All subjects; n = 29 . | ||
| Transfusions, mean ± SEM | 7303 ± 6441 | 219 077 | 7886 ± 5616 | 228 695 | ||
| Median | 6200 | — | 7148 | — | ||
| Range | 0-20 672 | — | 0-24 115 | — | ||
| Consumables and staff | 1108 | 33 242 | 1372 | 39 780 | ||
| RBCs* | 3284 | 98 516 | 3975 | 115 288 | ||
| Platelets | 101 | 3052 | 754 | 21 874 | ||
| Iron chelators | 2378 | 71 351 | 1442 | 41 820 | ||
| Transportation charges | 431 | 12 916 | 343 | 9933 | ||
| Medication (rHuEPO + rHuG-CSF), mean ± SEM | 19 121 ± 14 186 | 573 625 | 0 | 0 | ||
| Median | 9797 | — | — | — | ||
| Range | 219-41 366 | — | — | — | ||
| Disease follow-up, mean ± SEM | 299 ± 869 | 8983 | 860 ± 1707 | 24 940 | ||
| Median | 34 | — | 86 | — | ||
| Range | 0-4744 | — | 0-5755 | — | ||
| Only hospital stays, mean ± SEM | 183 ± 870 | 5498† | 690 ± 1634 | 20 007‡ | ||
| Median | 0 | — | 0 | — | ||
| Range | 0-4728 | — | 0-5489 | — | ||
| Total, mean ± SEM | 26 723 ± 13 109 | 801 686 | 8746 ± 6731 | 253 635 | ||
| Median | 27 754 | — | 7 846 | — | ||
| Range | 219-49 018 | — | 53-29 223 | — | ||
. | Arm A: rHuEPO + rHuG-CSF . | . | Arm B: supportive care . | . | ||
|---|---|---|---|---|---|---|
| Treatment . | Per subject; n = 30 . | All subjects; n = 30 . | Per subject; n = 29 . | All subjects; n = 29 . | ||
| Transfusions, mean ± SEM | 7303 ± 6441 | 219 077 | 7886 ± 5616 | 228 695 | ||
| Median | 6200 | — | 7148 | — | ||
| Range | 0-20 672 | — | 0-24 115 | — | ||
| Consumables and staff | 1108 | 33 242 | 1372 | 39 780 | ||
| RBCs* | 3284 | 98 516 | 3975 | 115 288 | ||
| Platelets | 101 | 3052 | 754 | 21 874 | ||
| Iron chelators | 2378 | 71 351 | 1442 | 41 820 | ||
| Transportation charges | 431 | 12 916 | 343 | 9933 | ||
| Medication (rHuEPO + rHuG-CSF), mean ± SEM | 19 121 ± 14 186 | 573 625 | 0 | 0 | ||
| Median | 9797 | — | — | — | ||
| Range | 219-41 366 | — | — | — | ||
| Disease follow-up, mean ± SEM | 299 ± 869 | 8983 | 860 ± 1707 | 24 940 | ||
| Median | 34 | — | 86 | — | ||
| Range | 0-4744 | — | 0-5755 | — | ||
| Only hospital stays, mean ± SEM | 183 ± 870 | 5498† | 690 ± 1634 | 20 007‡ | ||
| Median | 0 | — | 0 | — | ||
| Range | 0-4728 | — | 0-5489 | — | ||
| Total, mean ± SEM | 26 723 ± 13 109 | 801 686 | 8746 ± 6731 | 253 635 | ||
| Median | 27 754 | — | 7 846 | — | ||
| Range | 219-49 018 | — | 53-29 223 | — | ||
Costs are given in euros (€ 1 $1.20).—indicates not applicable.
Based on 2 U RBCs at a cost of € 282 for both units.
Corresponding to 2 hospital stays.
Corresponding to 5 hospital stays.
There was no difference in transfusion costs for arms A and B. In arm A, 28 subjects received transfusions of RBCs at least once, 2 subjects received platelets, and 19 subjects received iron chelators compared with 27, 2, and 14, respectively, in arm B.
We then looked at data concerning only subjects who completed the study (Table 4). The results were similar. However, the mean transfusion cost was much lower for the 6 responders who completed the study than for the other subjects (€ 2085 vs € 7579 for subjects in arm B; Table 4)
Mean cost per subject for 1 year of treatment
. | Arm A: rHuEPO + rHuG-CSF . | . | . | . | ||
|---|---|---|---|---|---|---|
| Treatment . | 52 weeks of follow-up with rHuEPO +/− rHuG-CSF . | 52 weeks of follow-up but stopped rHuEPO + rHuG-CSF after week 12 . | Total subjects who completed 52 weeks of follow-up . | Arm B: supportive care; n = 24 . | ||
| Transfusions, mean ± SEM | 2085 ± 3000 | 9651 ± 5989 | 7489 ± 6295 | 7579 ± 4934 | ||
| Median | 1059 | 7897 | 6567 | 7067 | ||
| Range | 0-8024 | 1347-19 520 | 0-19 520 | 0-20 527 | ||
| rHuEPO + rHuG-CSF, mean ± SEM | 35 666 ± 6705 | 19 263 ± 12 942 | 23 949 ± 13 644 | 0 | ||
| Median | 36 877 | 9999 | 23 222 | — | ||
| Range | 23 222-41 366 | 7613-38 794 | 7613-41 366 | — | ||
| Disease follow-up, mean ± SEM | 172 ± 221 | 126 ± 194 | 139 ± 198 | 580 ± 1443 | ||
| Median | 96 | 33 | 34 | 84 | ||
| Range | 0-607 | 0-602 | 0-607 | 0-5755 | ||
| Only hospital stays, mean ± SEM | 0 | 0 | 0 | 419 ± 1425 | ||
| Median | 0 | 0 | 0 | 0 | ||
| Range | 0-0 | 0-0 | 0-0 | 0-5489 | ||
| Total, mean ± SEM | 37 922 ± 8 064 | 29 040 ± 9607 | 31 577 ± 9 888 | 8158 ± 5723 | ||
| Median | 37 447 | 27 832 | 28 256 | 7816 | ||
| Range | 25 194-49 018 | 14 887-41 197 | 14 887-49 018 | 53-26 282 | ||
. | Arm A: rHuEPO + rHuG-CSF . | . | . | . | ||
|---|---|---|---|---|---|---|
| Treatment . | 52 weeks of follow-up with rHuEPO +/− rHuG-CSF . | 52 weeks of follow-up but stopped rHuEPO + rHuG-CSF after week 12 . | Total subjects who completed 52 weeks of follow-up . | Arm B: supportive care; n = 24 . | ||
| Transfusions, mean ± SEM | 2085 ± 3000 | 9651 ± 5989 | 7489 ± 6295 | 7579 ± 4934 | ||
| Median | 1059 | 7897 | 6567 | 7067 | ||
| Range | 0-8024 | 1347-19 520 | 0-19 520 | 0-20 527 | ||
| rHuEPO + rHuG-CSF, mean ± SEM | 35 666 ± 6705 | 19 263 ± 12 942 | 23 949 ± 13 644 | 0 | ||
| Median | 36 877 | 9999 | 23 222 | — | ||
| Range | 23 222-41 366 | 7613-38 794 | 7613-41 366 | — | ||
| Disease follow-up, mean ± SEM | 172 ± 221 | 126 ± 194 | 139 ± 198 | 580 ± 1443 | ||
| Median | 96 | 33 | 34 | 84 | ||
| Range | 0-607 | 0-602 | 0-607 | 0-5755 | ||
| Only hospital stays, mean ± SEM | 0 | 0 | 0 | 419 ± 1425 | ||
| Median | 0 | 0 | 0 | 0 | ||
| Range | 0-0 | 0-0 | 0-0 | 0-5489 | ||
| Total, mean ± SEM | 37 922 ± 8 064 | 29 040 ± 9607 | 31 577 ± 9 888 | 8158 ± 5723 | ||
| Median | 37 447 | 27 832 | 28 256 | 7816 | ||
| Range | 25 194-49 018 | 14 887-41 197 | 14 887-49 018 | 53-26 282 | ||
Costs are given in euros (€1 = $1.20). Patient populations are as follows: 52 weeks of follow-up with rHuEPO +/− rHuG-CSF, n = 6; 52 weeks of follow-up with rHuEOP + rHuG-CSF stopped after week 12, n = 15; and total subjects who completed 52 weeks of follow-up, n = 21.—indicates not applicable.
Quality of life
Twenty-nine subjects (96.7%) in arm A and 28 subjects (93.3%) in arm B completed the first questionnaire. These percentages decreased with time: 79.2% and 85.2%, respectively, at week 12; 68.2% and 72.0%, respectively, at week 28; and 50.0% and 66.7%, respectively, at week 52. Baseline total FACT-An, anemia, and fatigue subscale scores were similar for the 2 treatment groups. The total FACT-An scores of the 2 groups remained constant throughout the study. Similar percentages of subjects in arms A and B showed significant clinical improvement (Table 5). Similar results were obtained with each of the 3 scales analyzed (total FACT-An, fatigue, and anemia subscales). Finally, similar percentages of responders and nonresponders from arm A showed a significant clinical improvement.
FACT-An scale scores at baseline and during follow-up
. | Week 0 . | . | Week 12 . | . | Week 28 . | . | Week 52 . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | A . | B . | A . | B . | A . | B . | A . | B . | ||||
| FACT-An total score, mean | 115.6 | 112.5 | 111.8 | 114.4 | 123.1 | 118.3 | 107.1 | 105.4 | ||||
| Total anemia subscale score, mean | 46.4 | 46.1 | 50.7 | 48.3 | 55.5 | 47.1 | 45.6 | 42.1 | ||||
| Total fatigue subscale score, mean | 29 | 28.3 | 31.7 | 29.2 | 36.9 | 28.3 | 28.4 | 23.6 | ||||
| Subjects with clinical improvement in Fact-An total score, n (%) | — | — | 6 (33) | 10 (43)* | 7 (46.7) | 12 (62)* | 3 (25) | 3 (17)* | ||||
| Subjects with clinical improvement on anemia subscale, n (%) | — | — | 5 (28) | 7 (30)* | 8 (53) | 6 (32)* | 3 (25) | 2 (11)* | ||||
| Subjects with clinical improvement on fatigue subscale, n (%) | — | — | 6 (33) | 9 (39)* | 8 (53) | 5 (26)* | 3 (25) | 2 (11)* | ||||
| Responses, n completion rate (%) | 29 (97) | 28 (93) | 19 (79) | 23 (85) | 15 (68) | 18 (72) | 10 (50) | 16 (67) | ||||
. | Week 0 . | . | Week 12 . | . | Week 28 . | . | Week 52 . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | A . | B . | A . | B . | A . | B . | A . | B . | ||||
| FACT-An total score, mean | 115.6 | 112.5 | 111.8 | 114.4 | 123.1 | 118.3 | 107.1 | 105.4 | ||||
| Total anemia subscale score, mean | 46.4 | 46.1 | 50.7 | 48.3 | 55.5 | 47.1 | 45.6 | 42.1 | ||||
| Total fatigue subscale score, mean | 29 | 28.3 | 31.7 | 29.2 | 36.9 | 28.3 | 28.4 | 23.6 | ||||
| Subjects with clinical improvement in Fact-An total score, n (%) | — | — | 6 (33) | 10 (43)* | 7 (46.7) | 12 (62)* | 3 (25) | 3 (17)* | ||||
| Subjects with clinical improvement on anemia subscale, n (%) | — | — | 5 (28) | 7 (30)* | 8 (53) | 6 (32)* | 3 (25) | 2 (11)* | ||||
| Subjects with clinical improvement on fatigue subscale, n (%) | — | — | 6 (33) | 9 (39)* | 8 (53) | 5 (26)* | 3 (25) | 2 (11)* | ||||
| Responses, n completion rate (%) | 29 (97) | 28 (93) | 19 (79) | 23 (85) | 15 (68) | 18 (72) | 10 (50) | 16 (67) | ||||
—indicates not applicable.
P > .2 (Fisher exact test).
Discussion
This is the first randomized trial to compare treatment with rHuEPO plus rHuG-CSF and supportive care. We confirmed that rHuEPO plus rHuG-CSF can correct anemia in patients with MDS and that synergy exists between rHuEPO and rHuG-CSF. However, this combination is expensive, and QOL did not improve significantly.
The overall response rate after 12 weeks of treatment was 42%. This rate was higher for subjects with RA (50%) and lower for those with RAEB (20%), confirming published results.6-9
Response criteria used in this study were different from and more stringent than those recently published by the World Health Organization International Working Group (IWG).20 If we had applied the IWG criteria, 15 subjects would have been considered responders at 12 weeks (62.5% of the eligible subjects, and 50% of the initial group)—7 minor responders (2 based only on transfusion needs, 1 only on Hb concentration, and 4 on transfusion needs and Hb concentration) and 8 major responders (5 based only on transfusion needs and 3 only on Hb concentration).
rHuG-CSF was discontinued at 12 weeks in 8 responders, 6 of whom had relapses. The fact that anemia was reversed in 4 of these 6 subjects when rHuG-CSF was reintroduced is consistent with findings from a previous study.7 This demonstrates the in vivo synergy between rHuG-CSF and rHuEPO for RBC production in patients with MDS.
Combined treatment was generally well tolerated, as previously reported.6-11 Two subjects progressed to AML. As previously reported,8 there was no indication that treatment with rHuG-CSF and rHuEPO increased the number of bone marrow blasts or caused progression to acute leukemia.
The rHuG-CSF and rHuEPO treatment was 3- to 4-fold more expensive than supportive care, primarily because of medication costs. On the contrary, transfusion costs were lower for the 6 subjects in arm A who completed the study than for the other subjects. Costs for disease follow-up were also lower for arm A than for arm B. This difference was primarily because 2 arm A subjects were hospitalized compared with 5 arm B subjects.
Cost and clinical studies were carried out simultaneously. The cost evaluation was based on all the real costs incurred during the trial. This method reinforces our results.21,22 We did not take into account indirect costs. A recent study performed in patients with ovarian cancer and anemia showed that indirect costs represent 34% to 86% of the total treatment cost.23 However, most patients in that study were retired, making it difficult to evaluate the indirect costs.
These results have important implications on health policy decision-making (ie, reimbursing a new technology). Cost analysis is becoming increasingly important for decision-making in health care. However, cost assessments based on information obtained in one country may not be relevant in another country. A study comparing costs of ovarian cancer treatment showed that anemia management following a specific treatment differs in the United States and Europe: patients in the United States primarily received EPO, whereas those in Europe primarily underwent transfusions.24
Thus, our results may not be applicable in another health care system with different practices and costs. For example, our results were highly dependent on the retail prices of drugs.
The QOL values observed were similar to those obtained in previous studies using the FACT-An questionnaire performed in samples with the same hemoglobin concentration.15,16 No difference in QOL was revealed by the percentages of subjects showing significant clinical improvement in the different groups. These results do not confirm the results of a recent publication25 that showed a significant improvement of QOL among similar patients. However, this study used a different QOL scale (QLQ-C30; European Organization for Research on the Treatment of Cancer [EORTC]) and was not randomized.
A larger study on QOL in patients with myelodysplasia would be useful. Our results were limited by the number of subjects in our study and by the amount of missing data (50%-66% at the end of the study). In almost all trials with QOL end points, the proportion of subjects completing questionnaires decreases over time.26 In our study, the percentages of subjects who answered the QOL questionnaires are similar to those of other studies. In a recent study on MDS, less than 70% answered the first QOL questionnaire.25 Even though we observed no difference in age and hemoglobin concentration between subjects who answered the QOL questionnaire and those who did not, this low return rate might have influenced our results.
Increased hemoglobin concentrations have been associated with improvements in QOL, but it is possible that the increase in hemoglobin concentration observed in our study was too small to affect QOL. It has been suggested that QOL improvements are maximal when Hb concentrations exceed 110 g/L (11 g/dL).27,28
Appendix
The members of the Groupe Français des Myélodysplasies (classified by institution) are: Hôpital Cochin, Paris: François Dreyfus, Françoise Picard, Michaela Fontenay-Roupie, Marie Catherine Quarré, Didier Bouscary, and Dominique Vassilieff. Hôpital Avicenne, Bobigny: Pierre Fenaux, Florence Cymbalista, Claude Gardin, Jean Jacques Kiladjian, and Virginie Eclache. Hôpital Mondor, Créteil: Stephane Giraudier, Michel Tulliez, and Eric Lepage. Centre Hospitalier Régional Universitaire (CHRU) de Lille: Stéphane De Botton, Bruno Quesnel, and Pascale Lepelley. Hôpital Saint Vincent, Lille: Christian Rose. Hôpital Necker, Paris: Bruno Varet and Gandhi Damaj. Hôpitaux de Brabois, Nancy: Agnès Guerci. Centre Hospitalier Universitaire (CHU) Purpan, Toulouse: Guy Laurent. Hôpital St Louis, Paris: Hervé Dombret and Christine Chomienne. Centre Henri-Becquerel, Rouen: Aspasia Stamatoullas. Hôpital Edouard Herriot, Lyon: Eric Wattel and Xavier Thomas. Hôtel Dieu, Paris: Jean Pierre Marie, Nicole Casadevall, Anne Vekhoff, and Stéphanie Dubois. Hôpital de Hautepierre, Strasbourg: Patrick Lutz and Frédéric Maloisel. Hôtel Dieu, Nantes: Béatrice Mahe. Institut Gustave Roussy, Villejuif: Vincent Ribrag. Hôpital Saint Antoine, Paris: Françoise Isnard and Monique Lagrange. CHU Dupuytren, Limoges: Jean Feuillard and Dominique Bordessoule. Hôpital Archet 1, Nice: Laurence Legros. Hôpital Bichat, Paris: Marie-José Grange. CHU Clemenceau, Caen: Stéphane Chèze. Institut Paoli Calmettes, Marseille: Jean Albert Gastaut and Norbert Vey. Centre Hospitalier Angers: Norbert Ifrah. Hôpital Jean Minjoz, Besançon: Jean Yves Cahn. CHU Bretonneau, Tours: Philippe Colombat. Hôpital Morvan-CHU, Brest: Christian Berthou. Hôpital A. Michallon-CHRU, Grenoble: Jean Jacques Sotto. CHU Dijon: Eric Solary and Elisabeth Berger. Centre Hospitalier Meaux: Christian Allard. Centre René Huguenin, Saint Cloud: Maud Janvier. Centre Hospitalier St Jean, Perpignan: Laurence Sanhes. CHRU Angers: Martine Gardembas. CHU La Miletrie, Poitiers: François Guilhot. Hôpital d'Instruction des Armées Percy, Clamart: Thierry De Revel. Hôpital du Haut LévèquePessac: Jean Michel Boiron. Hôpital Sud-Rennes: Jean Goasguen and Bernard Grosbois. Siege AP-HP (Assistance Publique Hôpitaux de Paris): Henri Rochant.
Prepublished online as Blood First Edition Paper, March 30, 2004; DOI 10.1182/blood-2003-07-2252.
Supported by The Fédération Nationale des Centres de Lutte Contre le Cancer, Ortho Biotech (Division of Janssen-Cilag), Chugai Pharma France, and Laboratoire Aventis.
F.D. and P.F. contributed equally to this work.
A complete list of the members of the Groupe Français des Myélodysplasies appears in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank LC2 (Brindas, France) for logistical support and all the investigators for their valuable input and enrollment of subjects. We also thank all the participating cytologists.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal