Recent articles by Wagner et al1 and Hochegger et al2 reported the expression of perforin and granzymes A and B in human polymorphonuclear cells (PMNs; neutrophils) using flow cytometry assays. Both groups used an intracellular staining technique, including the use of secondary antibodies to detect granzymes A and B. Since previous studies have suggested that granzyme expression is restricted to cytotoxic lymphocytes (ie, natural killer [NK] cells and cytotoxic T cells),3 we also analyzed purified PMNs for granzyme expression using an intracellular flow cytometry assay with primary-conjugated granzyme monoclonal antibodies that were proven to be specific, using competitive recombinant granzymes and knockout mice (W.J.G., James W. Verbsky, Benjamin L. Tollefson, Claudia Kemper, John P. Atkinson, T.J.L., manuscript submitted, 2004).
Peripheral blood mononuclear cells (PBMCs) and PMNs were isolated by density gradient and red blood cell lysis similar to Hochegger et al.2 This isolation technique resulted in more than 95% purity for both PBMCs (lymphocytes and monocytes) and PMNs, as determined by lineage-specific markers and microscopic evaluation (Figure 1A-C). Samples were labeled with surface antibodies (CD15, Leinco Technologies, St Louis, MO; CD8, Becton Dickinson–Pharmingen, Mountain View, CA), fixed, permeabilized (Cytofix/Cytoperm; Pharmingen, San Diego, CA), and stained with primary-conjugated anti–granzyme A antibody (CB9; Becton Dickinson–Pharmingen) diluted at 1:100 in staining buffer (phosphate-buffered saline [PBS], 1% human albumin, 0.2 μg/μL human immunoglobulin) and/or primary-conjugated anti–granzyme B antibody (GB12; Caltag, Burlingame, CA) diluted at 1:400 in staining buffer.
Purified resting human PMNs do not express either granzyme A or B. (A) Fluorescence-activated cell sorter (FACS) blots (forward scatter [FSC] vs side scatter [SSC]) and cytospin analyses of purified human PBMCs and PMNs. Each fraction was determined to be more than 95% purified as determined by FACS analysis using lineage-specific markers and by microscopic evaluation of cytospins (May-Grünwald/Giemsa; original magnification, ×200). Lymphocyte (lymphs), monocyte (monos), and PMN populations based upon forward and light scatter are indicated. The images were taken using a Nikon Microphot-SA microscope; 20× bright-field objective lens, original magnification ×200; Cytoseal XYL mounting media (Richard-Allan Scientific, Kalamazoo, MI); and analysis camera (×10 magnification) and acquisition software from Soft Imaging System (Lakewood, CA). (B) FACS analysis of purified PBMCs and PMNs for granzyme A and B expression against lineage-specific surface markers. Gates used in FACS analysis for lymphocytes and PMNs are indicated in panel A. (C) FACS analysis of purified PMNs for expression of neutrophil elastase (NE) and cathepsin G (CG). Data shown is representative of 4 independent donor samples. Percentages of total cells for each quadrant are indicated in the upper right corner of each subpanel.
Purified resting human PMNs do not express either granzyme A or B. (A) Fluorescence-activated cell sorter (FACS) blots (forward scatter [FSC] vs side scatter [SSC]) and cytospin analyses of purified human PBMCs and PMNs. Each fraction was determined to be more than 95% purified as determined by FACS analysis using lineage-specific markers and by microscopic evaluation of cytospins (May-Grünwald/Giemsa; original magnification, ×200). Lymphocyte (lymphs), monocyte (monos), and PMN populations based upon forward and light scatter are indicated. The images were taken using a Nikon Microphot-SA microscope; 20× bright-field objective lens, original magnification ×200; Cytoseal XYL mounting media (Richard-Allan Scientific, Kalamazoo, MI); and analysis camera (×10 magnification) and acquisition software from Soft Imaging System (Lakewood, CA). (B) FACS analysis of purified PBMCs and PMNs for granzyme A and B expression against lineage-specific surface markers. Gates used in FACS analysis for lymphocytes and PMNs are indicated in panel A. (C) FACS analysis of purified PMNs for expression of neutrophil elastase (NE) and cathepsin G (CG). Data shown is representative of 4 independent donor samples. Percentages of total cells for each quadrant are indicated in the upper right corner of each subpanel.
We detected no granzyme A or B staining in purified PMNs using the assay described above (Figure 1B). As a positive control for granzyme expression, circulating CD8+ T lymphocytes were stained for granzyme A and B expression from each donor in parallel with PMN staining (Figure 1B). Lack of granzyme expression in our purified PMN cells was not due to degranulation during isolation, since we were able to detect intracellular neutrophil elastase and cathepsin G in nearly 100% of PMNs (Figure 1C).
A possible explanation for the reported discrepancies may involve differences in blocking strategies against nonspecific Fc receptor binding. Numerous studies have shown nonspecific binding of antibodies and certain fluorochromes (eg, phycoerythrin [PE], PE–cyanin 5 [Cy5]) to the Fc receptors of murine and human macrophages, B cells, and granulocytes.4-6 Preincubation and coincubation with antibodies directed against Fc receptors or nonlabeled immunoglobulins can reduce the nonspecific binding in most cases. During all steps of our staining, permeabilization, and washing, human immunoglobulins (0.1-0.2 μg/μL final concentration; Gammagaurd, Baxter Healthcare, Miami, FL) and human albumin (1%; Aventis Behring, King of Prussia, PA) were used to block nonspecific Fc receptor binding. In addition to blocking with human immunoglobulins, we analyzed our staining by gating specifically on either lymphocyte or PMN populations based upon forward and light scatter, as well as lineage-specific markers, to overcome the limitation of nonspecific Fc receptor binding. We have shown that certain cell types (eg, human monocytes) cannot be prevented from binding certain antibodies nonspecifically despite using Fc receptor blocking reagents (data not shown). In such circumstances, specific cell population gating, based on forward scatter and light scatter, is required. Finally, we have also shown that secondary detection antibodies substantially increase nonspecific background staining (data not shown).
Polymorphonuclear neutrophils express granzyme A, granzyme B, and perforin: no doubt about it
Metkar and Froelich and Grossman and Ley question the findings by Wagner et al1 and Hochegger et al2 on the constitutive expression of perforin, granzyme A (GzmA), and GzmB in human polymorphonuclear neutrophils (PMNs). Because they were not able to detect perforin and the granzymes using flow cytometry (Grossman and Ley) or by cytofluorometry, Western blot, and enzyme-linked immunosorbent assay (ELISA; Metkar and Froelich), they concluded that the failure to adequately block the Fcγ receptors on PMN, as well as “uncontrollable cellular contamination,” may have contributed to false-positive results reported by us. We absolutely disagree with the conclusion reached by Metkar and Froelich and Grossman and Ley and we reject their conjecture that our conclusions are based on inadequate controls and contaminating cells, with the following arguments.
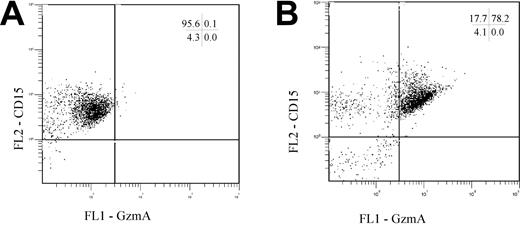
(1) To control for nonspecific, Fc receptor–mediated binding in both studies, isotype controls were used. Also following blockade of the Fcγ receptors (according to the protocol of Metkar and Froelich) and double staining for granzyme A and CD15, a double-positive population was found (Figure 1). A possible explanation for the contrary findings could be differences in the isolation procedures (including isolation at room temperature, or hypotonic lysis with Aqua bidestillata or ammonium chloride lysis buffer), because different protocols can markedly influence the activation status of PMNs (eg, the up-regulation of the high-affinity receptor for immunoglobulin G [IgG], CD64) and also degranulation of preformed proteins.3-5
PMNs were isolated, blocked for Fcγ receptors, stained for CD15, fixed, and intracellularly stained for GzmA. Representative dot plots of ungated cells stained for CD15 and either the respective GzmA isotype control (A) or GzmA (B) are shown. The numbers indicate the percentage of positive cells in each quadrant.
PMNs were isolated, blocked for Fcγ receptors, stained for CD15, fixed, and intracellularly stained for GzmA. Representative dot plots of ungated cells stained for CD15 and either the respective GzmA isotype control (A) or GzmA (B) are shown. The numbers indicate the percentage of positive cells in each quadrant.
(2) The group of Wagner et al1 isolated PMNs using PolymorphPrep (Nycomed, Oslo, Norway) followed by positive selection by anti-CD15–coated magnetic beads; this method yields a purity of higher than 99% PMNs, with only minor activation of the cells. Using these highly purified PMNs by different methods (including confocal microscopy, immunoprecipitation, or Western blotting, a trapping ELISA, and double-staining cytofluorometry), granzyme B and perforin were found (in line with the findings of Hochegger et al2 ). Furthermore, Hochegger et al2 demonstrated constitutive GzmA expression in peripheral blood mononuclear cells (PBMCs), which served as a positive control in the Western blot experiments.
(3) The data provided by Metkar and Froelich showed that only 1 in 4 donors had PBMCs positive for GzmA. In light of previous publications (Spaeny-Dekking et al6 and reviews in Lieberman7 ), it seems very unlikely that GzmA expression occurs in only 25% of healthy donors. A more likely explanation is that the GzmA expression varies among the donors (as it does in PMNs) and that the Western blot is only sensitive enough to detect highly expressed GzmA. Basically, the same arguments apply for the Western blots for perforin and GzmB.
(4) Metkar and Froelich reported that they were not able to detect the granzymes with a “sensitive EIA.” With regard to granzyme A, they report a positive GzmA signal for the PBMCs; and for PMNs, a lower signal amounting to 20% of that of PBMCs. Assuming a contamination of the PMNs with PBMCs of 5% (as Metkar and Froelich do for their PMN preparation), the question arises, how can 5% of the cell population account for 20% of the signal? The data for GzmB were positive (with a weak signal) in only 2 of 4 donors, again leading to the question of whether the EIA used is sensitive enough to detect GzmB in PMNs, because Wagner et al1 (using highly purified, positively selected PMNs) found that, based on the cell number, PMNs contained less GzmB than T cells (approximately 20%).
(5) In the studies by Wagner et al1 and Hochegger et al,2 transcripts for the granzymes and perforin were detected. This confirms a previous study by Kobayashi et al,8 who described phagocytosis-induced up-regulation of GzmA in PMNs after 90 minutes of incubation (using microarray technology; see Figure 6 in Kobayashi et al8 ). It is not possible for natural killer (NK) cells or cytotoxic T cells (CTLs) to reproduce granzymes that quickly,9 demonstrating that GzmA has to be derived from PMNs, and further that GzmA expression is regulated.
In summary, we conclude that the conclusion reached by Metkar and Froelich and Grossman and Ley is not correct, and we stress that from our data there is solid evidence that PMNs do indeed express GzmA, GzmB, and perforin.
Correspondence: G. Maria Hänsch, Institut für Immunologie der Universität Heidelberg, Im Neuenheimer Feld 305, 69120 Heidelberg, Germany; e-mail: n50@ix.urz.uni-heidelberg.de.

![Figure 1. Purified resting human PMNs do not express either granzyme A or B. (A) Fluorescence-activated cell sorter (FACS) blots (forward scatter [FSC] vs side scatter [SSC]) and cytospin analyses of purified human PBMCs and PMNs. Each fraction was determined to be more than 95% purified as determined by FACS analysis using lineage-specific markers and by microscopic evaluation of cytospins (May-Grünwald/Giemsa; original magnification, ×200). Lymphocyte (lymphs), monocyte (monos), and PMN populations based upon forward and light scatter are indicated. The images were taken using a Nikon Microphot-SA microscope; 20× bright-field objective lens, original magnification ×200; Cytoseal XYL mounting media (Richard-Allan Scientific, Kalamazoo, MI); and analysis camera (×10 magnification) and acquisition software from Soft Imaging System (Lakewood, CA). (B) FACS analysis of purified PBMCs and PMNs for granzyme A and B expression against lineage-specific surface markers. Gates used in FACS analysis for lymphocytes and PMNs are indicated in panel A. (C) FACS analysis of purified PMNs for expression of neutrophil elastase (NE) and cathepsin G (CG). Data shown is representative of 4 independent donor samples. Percentages of total cells for each quadrant are indicated in the upper right corner of each subpanel.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/3/10.1182_blood-2004-03-0858/6/m_zh80150464720001.jpeg?Expires=1766553410&Signature=jLfsvZxb6XINp2s4vYIwN3guK-ZtIo~4aqF2f9s75nW0N53TvnJbP7THC1-0tQG8LqgOo3j9a9e5HWMkPT2SERWRPMEEgEdAUQEWbRd9C~9SML-0YKuQpfVGWzWEDERAvqG89pAAmDfxTNAV~6K7es4pi7RAW6kEqkaveDUyIhlDiHO4EGeBzxGr5W3S4gMIADrFGISux-sHkuA8OfBYUZAB3BKPRkeNbPXiUajitWgMkzR8hOdr2e6B86LSYdjBBn~CPF4C7Qwev0n6ehi3DPM5~SiTALuPfR9Xy5WkKz7sJTuXOHRjFBEJik4HvtocuOXTky88AIjSLIQKpPLYWw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal