Abstract
EphrinB2 and EphB4, its cognate receptor, are important in the vascular development of the mouse embryo. Their roles in human inflammatory angiogenesis, however, are not well understood. By examining hyperinflammatory lesions, we saw that ephrinB2 was predominantly expressed in macrophage-like cells and EphB4 in small venules. Because macrophages usually transmigrate through postcapillary venules during inflammation, we wanted to explore the downstream effects of EphB4 after binding to ephrinB2. By using cDNA microarray technique and following reverse transcriptase–polymerase chain reaction (RT-PCR), we found that syntenin and syndecan-1 were up-regulated in EphB4-positive endothelial cells dose dependently and time dependently after stimulation with preclustered ephrinB2. In vitro, ephrinB2 suppressed the angiogenic effects of basic fibroblast growth factor (bFGF) on EphB4-positive endothelial cells, partially due to syndecan-1's competition with fibroblast growth factor receptor (FGFR) for bFGF. However, ephrinB2 exhibited angiogenic effects in vivo, possibly due to an inflammation-associated enzyme—heparanase. The enzymes could convert the inhibitory effect of ephrinB2 on EphB4-positive endothelial cells to an activating effect by removing poorly sulfated side chains of up-regulated syndecan-1 ectodomain. Depending on the presence of heparanases, the roles of syndecan-1 may be opposite in different physiological settings.
Introduction
EphB4 and ephrinB2 (one of its ligands) belong to the biggest family of receptor tyrosine kinases—the Eph family. One of their characteristics is the ability to transduce bidirectional signals into both cells expressing the receptors and the ligands. Because both Eph receptors and their ligands are cell surface molecules, they can interact with each other only if expressed on adjacent cells. Their major function demonstrated to date is controlling accurate spatial patterning and cell positioning.1,2 In 1998, an interesting study revealed that arterial endothelial cells specifically expressed ephrinB2, and venous endothelial cells expressed its cognate receptor, EphB4, in the mouse embryo.3 The interaction between EphB4 and ephrinB2 was thought to play important roles in vasculogenesis and angiogenesis because both ephrinB2 and EphB4 knock-out mice demonstrated similar fatal vascular defects.3,4 Subsequent studies demonstrated that ephrinB2 persistently identifies arterial endothelial cells as well as smooth muscle cells in adult mice.5,6 A recent study of the human embryo, however, found that EphB4 and ephrinB2 did not distinguish between presumptive venous and arterial endothelium as they did in the mouse.7 Our previous immunohistochemical study in an adult human inflammatory setting indicated that ephrinB2 was predominantly expressed in macrophage-like cells and EphB4 was expressed in venule endothelium. A few arterial endothelium cells were weakly stained for ephrinB2.8 It appears that the interaction of ephrinB2 and EphB4 is important in angiogenesis, but the selective marking of ephrinB2 and EphB4 in arterial and venous endothelial cells may not be consistent in different species. Therefore, we were motivated to clarify the downstream effects of ephrinB2 and EphB4 in human vascular physiology, especially in an inflammatory setting, because macrophages usually transmigrate through postcapillary venules during inflammation. That is, macrophage-expressing ephrinB2 is likely to counteract EphB4-positive endothelium during the inflammatory process. For searching downstream, gene expression in a large-scale, complementary DNA (cDNA) microarray has been proven a useful and powerful tool.9 In the present study, we found that syndecan-1 was up-regulated after stimulating EphB4-positive endothelial cells with preclustered ephrinB2 protein by a similar technique. Syndecan-1 is a cell surface proteoglycan that binds extracellular matrix components and modulates the activity of heparin-binding growth factors, including important angiogenic factors, such as basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF).10 Overproduced syndecan-1 inhibits bFGF-induced cell proliferation.11 Shedding of their extracellular domains (ectodomains) is plausible for the inhibitory effect.12 Interestingly, heparanases, a family of enzymes important in the diapedesis of leukocytes and metastasis of tumor cells, convert the soluble syndecan-1 ectodomain from an inhibitor to a potent activator of bFGF in vitro.13,14 Therefore, we further tested whether heparanases were up-regulated during inflammation and whether they possibly converted the inhibitory effect of ephrinB2 on EphB4-positive endothelial cells via syndecan-1. The results of the present study provided plausible mechanisms for several phenomena in vascular biology—for example, the newly grown blood vessels were predominantly from venules instead of arterioles in inflammation, as observed in previous studies,15,16 and how the interface between ephrinB2-positive and EphB4-positive endothelium remains homeostatic when not inflamed.
Materials and methods
Cell culture and human specimens
Four different kinds of human primary endothelial cells, including human umbilical vein endothelial cells (HUVECs), human umbilical artery endothelial cells (HUAECs), human iliac artery endothelial cells (HIAECs), and human saphenous vein endothelial cells (HSVECs) were purchased from a biotechnology company (Cambrex, Walkersville, MD) and maintained in endothelium growth medium (EGM) (Cambrex) at 37° C in a humidified atmosphere of 95% air and 5% CO2. Cells between passages 4 and 6 were used for this study. U937 was a monoblastoid cell line originally purchased from American Type Culture Collection ([ATCC] Manassas, VA). The cells were maintained in RPMI 1640 medium (Gibco, Grand Island, NY) with 10% fetal calf serum (FCS). Primary human gingival fibroblasts from gingival tissue taken during periodontal surgery in our dental clinic were maintained in the same medium. The human specimens used in this study were from patients with a hyperreactive inflammatory lesion, pyogenic granuloma, an excellent model to investigate inflammatory angiogenesis because it contained both a proliferation stage and a regressive stage of angiogenesis. Ten specimens from 10 gravidas with pyogenic granuloma were used. Five of them were excised during the second trimester of pregnancy (proliferative stage), and the others were obtained after parturition (regressive stage). The details have been described elsewhere.17,18
Antibodies and reagents
Polyclonal rabbit antiheparanase-1 was a generous gift from Dr Kazuha Hashizume of Japan. Polyclonal rabbit antisyntenin was purchased from a biotechnology company (Synaptic Systems, Göttingen, Germany). Polyclonal goat anti-EphB4, rabbit antifibroblast growth factor receptor-1 (anti-FGFR1), rabbit antiephrinB2, goat antisyndecan-1, goat antiheparanase-2, and monoclonal mouse anti-FGFR1 from the same commercial resource (Santa Cruz Biotechnology, Santa Cruz, CA) were used in the Western blot, immunohistochemistry, and immunoprecipitation (IP) analyses. Polyclonal goat anti-VEGF and monoclonal mouse antiectodomain of syndecan-1 and -4 (Santa Cruz Biotechnology) were used in the dot blot analysis. Polyclonal goat anti–N-terminal of EphB4 was used as a neutralizing antibody to block the interaction between ephrinB2 and EphB4 in the cell culture study. It is widely accepted that only preclustered ephrin can initiate functional signal transduction; therefore, 2 μg rabbit antihuman Fc of immunoglobulin G (IgG) (Pierce, Rockford, IL) was mixed with 200 ng ephrinB2-Fc (R&D Systems, Minneapolis, MN) to prepare the preclustered form, while antihuman Fc alone was used as a control in all the assays examining the interaction of ephrinB2 and EphB4. Recombinant human interleukin-1 beta (IL-1β), tumor necrosis factor-α (TNF-α), bFGF, and ephrinB2-Fc chimera protein were purchased from R&D Systems. Phorbol 12-myristate 13-acetate (PMA) and ionomycin were purchased from Sigma Chemical (St Louis, MO) to activate U937 cells. Heparitinase (heparinase III; Flavobacterium heparinum [EC 4.2.2.8], Sigma), a bacterial enzyme functionally equivalent to human heparanase, was used in the last part of the experiment.
Immunohistochemistry, double staining, and Western blot
To understand the spatial relationship between ephrinB2 and EphB4 in pyogenic granuloma, we performed a double staining to detect the expression of the ligands and receptors on the same tissue specimens. The kit (Histostain-DS, Zymed Laboratories, San Francisco, CA) was used according to the manufacturer's instructions. The images were captured by a Nikon Eclipse E600 microscope (Tokyo, Japan), acquired by a Photometrics CoolSNAP charge-coupled device (CCD) camera (Roper Scientific, Duluth, GA), and imported into CoolSNAP version 1.1 software. Numerical apertures for objective lenses were 0.30 for × 10 and 0.75 for × 40.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) of immunoblots was carried out on gels of 7.5% acrylamide. Protein samples of cell lysates (80 μg protein for each lane) and 10 μL molecular weight standards (New England Biolabs, Beverly, MA) were applied to the gel. After electrophoresis, transferring, and blocking, the primary antibody was incubated with the membranes for 1 hour at 37° C at a concentration of 0.1 μg/mL. Polyclonal rabbit antihuman β-actin (Santa Cruz Biotechnology, Santa Cruz, CA) was used as an internal control for equal loading. After the membranes were washed, secondary antibody with horseradish peroxidase was incubated with the membrane for 1 hour at 37° C at a concentration of 0.05 μg/mL. Following a final wash with phosphate-buffered saline with Tween 20 (PBST), the membranes were developed with an enhanced chemiluminescence kit (Amersham Biosciences, Piscataway, NJ).
cDNA microarray
To compare the gene expression patterns regulated by ephrin B2 on EphB4-positive endothelial cells, messenger RNAs (mRNAs) from HUAECs with or without 200 ng/mL ephrinB2 treatment for 4 hours were extracted using oligo(dT) cellulose (MessageMaker, Gibco, Rockville, MD). Messenger RNA samples of 0.5 μg were labeled with biotin for color detection. The labeling reactions were done during reverse transcription as previously described.9 A cDNA microarray system and membrane (Boehringer Mannheim, Mannheim, Germany) were then prepared. The cDNA microarray membrane contained 141 expressed sequence tag (EST) clones with putative gene names selected from the IMAGE (Integrated Molecular Analysis of Genomes and their Expression) consortium human cDNA libraries on the basis of the insert length and sequence of the EST clones in the Unigene database. These 141 angiogenesis-associated EST clones served as the hybridization target. The membrane carrying the double-stranded cDNA targets was prehybridized in 1 mL hybridization buffer (5 × saline sodium citrate [SSC], 0.1% N-lauroylsarcosine, 0.1% SDS, 1% blocking reagent mixture [Boehringer Mannheim], and 50 μg/mL salmon sperm DNA) at 68° C for 1 hour before hybridization. After hybridization, the membrane was blocked by 1 mL of 1% blocking reagent (Boehringer Mannheim) containing 2% dextran sulfate at room temperature for 1 hour and then rinsed with 1 × Tris (tris(hydroxymethyl)aminomethane)–buffered saline (TBS) buffer solution (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, and 0.3% bovine serum albumin [BSA]). The membrane was incubated for 2 hours with a 1 mL mixture containing 700 × diluted STREP-GAL (1.38 U/mL, enzyme activity) (Gibco, Rockville, MD), 4% polyethylene glycol 8000 (Sigma), and 0.3% BSA in 1 × TBS buffer. The membrane was then washed with 1 × TBS buffer 3 times for 5 minutes each. The color development reactions were then stopped by 1 × phosphate-buffered saline (PBS) containing 20 mM ethylenediaminetetraacetic acid (EDTA).
Reverse transcriptase–polymerase chain reaction (RT-PCR)
One day before the addition of tested substance, the medium was changed from EGM to M199 medium (Gibco, Rockville, MD). After 2 PBS washes, the cells were incubated for 6 hours in M199 containing 5% FCS and varying concentrations of tested recombinant proteins for an indicated time. With an RNA extraction kit (RNA Extraction Kit, Viogene, Taipei, Taiwan), we collected the total RNA, of which 100 ng was reverse transcribed with a reverse transcriptase–polymerase chain reaction (RT-PCR) kit (Advantage RT-for-PCR Kit, Clontech, Palo Alto, CA). The primer sets and PCR conditions for target genes were performed according to previously published studies,19-34 while those of glyceraldehyde-3-phosphate dehydrogenase (G3PDH) were provided in the RT-PCR kit and used as an internal control.
Immunoprecipitation (IP) and dot blot analysis
HUAECs were incubated in M199 medium with 5% FCS overnight before exposure to varying concentrations of preclustered ephrinB2 in M199 medium. After 12 hours of incubation with ephrinB2 or clustering antibody (Ab) alone, cells were collected and lysed with 150 μL protein lysis buffer (10 mM Tris-HCl, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA [ethylene glycol tetraacetic acid], 1 mM NaF, 20 mM Na4P2O7, 2 mM Na3VO4, 1%Triton X-100, 10% glycerol, 0.1% SDS, 0.5% deoxycholate, 2 mM dithiothreitol, 0.5 mM PMSF [phenylmethylsulfonyl fluoride], pH 7.4). For IP, 500 μg protein from cell lysates was precleared with 5 μL normal mouse agarose conjugate (Santa Cruz Biotechnology) for 1 hour and then incubated with 5 μL primary Ab (monoclonal mouse anti-FGFR1) overnight at 4° C. Then, 20 μL Protein A/G Plus–Agarose bead (Santa Cruz Biotechnology) was added for 1 hour. This complex was washed 4 times with PBS, and 15 μL of sample buffer was added to each. These samples were then analyzed by Western immunoblot assays as indicated. The supernatant before the first PBS wash was also collected for Western blot to detect residual protein after IP.
The dot blot analysis was modified from another study.12 In brief, the conditioned medium of the HUAEC culture with treatments of varying concentrations of ephrinB2 was collected and concentrated by centrifuging (Centricon, Millipore, Bedford, MA). The concentration was adjusted to 2 mg/mL for the following assay. Two microliters of each concentrate was applied to methanol-moistened polyvinylidene fluoride (PVDF) membrane (Millipore) under a mild vacuum in an immunoblot apparatus (V&P Scientific, San Diego, CA). The membranes were washed twice with PBS and then blocked by nonfat milk for 1 hour at 37° C. The following procedures were the same as Western blot. The primary monoclonal antibody (Santa Cruz Biotechnology) is specific for the ectodomain of syndecan-1 with a 2000-fold dilution.
Proliferation assay and migration assay
Subconfluent cultures of HUAECs were used to establish cell proliferation bioassays. Cells at a density of 5 × 103 cells per well per 500 μL were plated into 24-well culture plates in the EGM medium and left for 24 hours to attach to the well surface. The cell media were then replaced with M199 medium containing 5% fetal calf serum (FCS), 1% (vol/vol) penicillin-streptomycin solution (5000 U/mL penicillin and 50 μg/mL streptomycin), and test substances. The cultures were then incubated for 3 days at 37° C under 5% CO2. Cell proliferation was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide (MTT) assays according to the manufacturer's instructions (Promega, Madison, WI). To test whether heparitinase (functionally similar to heparanase) could affect the proliferation of EphB4-positive cells after stimulation of ephrinB2, 5 mIU/mL heparitinase (heparinase III; Flavobacterium heparinum [EC 4.2.2.8]) was added to the medium of ephrinB2-treated or untreated HUAEC cell cultures and incubated at 37° C for 45 minutes. The other subgroup received no heparitinase. The media were collected and centrifuged to discard the pellets and then heated to 50° C for 10 minutes to eliminate residual enzymatic activity. After returning to room temperature, the media were added to 24-well HUAEC culture plates (5 × 103 cells per well per 500 μL). The cultures were incubated for 3 days and then assessed by MTT assays. The samples of each tested group were in sextuplicate, and the experiment was done twice.
A migration assay in HUAECs was performed with a modified Boyden chamber (Neuroprobe, Gaithersburg, MD) according to the manufacturer's instructions. The lower chambers were filled with serum-free medium containing 200 ng/mL preclustered ephrinB2, 2 μg/mL clustering Ab alone, 10 ng/mL bFGF, or a combination (n = 6 for each group). Polycarbonate filters with 8 μm pores were used as the membranes separating upper and lower chambers. Each upper chamber was plated with 1 × 104 cells in 50 μL serum-free medium, and the whole Boyden chamber was incubated at 37° C in a humidified 5% CO2 atmosphere. After 6 hours, cells on the upper surface of the membrane were removed with a moistened cotton swab. The membrane was then fixed with methanol for 8 minutes and stained with Giemsa stain (Sigma, St Louis, MO) for 1 hour. The cells on the lower surface were counted under a light microscope at high-power magnification (× 100). Three fields were counted in each well, and the average number was obtained. Each group was tested in sextuplicate, and the test was done twice.
In vivo wound healing model
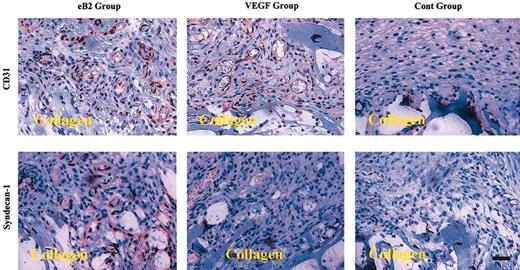
To understand whether ephrinB2 stimulated or inhibited angiogenesis in vivo, we modified the animal wound healing model from another study.15 Briefly, collagen sponges soaked with either 2 μg preclustered ephrinB2 or 0.2 μg VEGF or clustering Ab alone (control) were implanted subcutaneously in BALB/c mice for 7 days (n = 5 for each group). The collagen sponges with surrounding tissue were excised and embedded in paraffin and then processed for immunostaining of CD31 and syndecan-1.
Binding assay of bFGF-FITC
The recombinant protein of bFGF was conjugated with fluorescein isothiocyanate (FITC) by gel filtration chromatography (EZ-Label FITC protein labeling kit, Pierce). A pilot experiment was performed to determine the saturated dosage of bFGF-FITC binding to 1 × 105 cells per well of HUAECs. The amount of bFGF-FITC binding to the HUAECs appeared to be saturated if it was more than 0.1 nM (data not shown). HUAECs were seeded on 12-well culture dishes in a density of 1 × 105 cells per well 24 hours before the binding assay. After 2 washes of binding buffer (M199 medium, 1 mg/mL BSA, 50 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.4), 20 ng bFGF-FITC combined with 1 mL conditioned media with or without pretreatment of ephrinB2 and heparitinase as in the previous experiment were added to each well and then incubated at 4° C for 1 hour. Each well was washed twice with binding buffer, and then 1 mL of 2 M NaCl in 20 mM sodium acetate (pH 4.0) was used to dissociate the bound bFGF-FITC. A total of 150 μL of the supernatant in each well was transferred for measurement of the emitted fluorescence level at 535 nm by a fluorescence enzyme-linked immunosorbent assay (ELISA) reader (PerkinElmer, Wellesley, MA). The samples of each tested group were in sextuplicate, and the experiment was done twice. Each datum was normalized with the average value of the negative control group.
Data analyses
Data are expressed as mean ± SD. Statistical significance was tested using 1-way analysis of variance (ANOVA) followed by Student t test. Statistical significance was set at P values less than .05.
Results
The expression of human ephrinB2 and EphB4 in vitro and in inflammatory lesions
The double staining of human pyogenic granuloma revealed that ephrinB2 was predominantly expressed on macrophage-like cells and that EphB4 was expressed on the endothelial cells of small venules. They were usually in close proximity (Figure 1A). Some arterioles were also positive for ephrinB2, but the staining was weak. We further performed double staining for CD14 (a macrophage cell marker) and ephrinB2 on the same specimens. The results confirmed most of the ephrinB2-positive cells were macrophages (data not shown). The Western blot for ephrinB2 and EphB4 in endothelial cell cultures indicated that only HUAECs expressed the protein of EphB4 alone, while HUVECs, HIAECs, and HSVECs expressed both ephrinB2 and EphB4. EphrinB2 can be detected in PMA-treated U937 cells. Neither EphB4 nor ephrinB2 was detectable in human gingival fibroblasts (Figure 1B). Therefore, in the following experiments, we used HUAECs as the cell representative to examine the effects of ephrinB2 on EphB4-positive endothelial cells.
The expression of ephrinB2 and EphB4 proteins in pyogenic granuloma and cell cultures. (A) A representative double staining from specimens of pyogenic granuloma, which is a hyperreactive inflammatory lesion. EphB4 was detected with peroxidase-AEC (3-amino-9-ethylcarbazole) and appeared red (red arrow). Positive staining for ephrinB2 was dark blue, which was detected by alkaline phosphatase–NBT/BCIP (nitroblue tetrazolium/5-bromo-4-chloro-3-indoyl phosphate p-toluidine salt). Most of the ephrinB2-positive cells were macrophage-like cells (white arrowhead), while EphB4-positive cells were in small venules. Original magnification × 400. Scale bar equals 20 μm. (B) Western blots for ephrinB2 and EphB4 in 5 human primary cell cultures, including 4 endothelial cells (HUAECs, HUVECs, HIAECs, HSVECs) and 1 fibroblast (human gingival fibroblast [HGF]), and 1 monoblastoid cell line (U937) activated with 20 ng/mL PMA. [Beta]-Actin was an internal control.
The expression of ephrinB2 and EphB4 proteins in pyogenic granuloma and cell cultures. (A) A representative double staining from specimens of pyogenic granuloma, which is a hyperreactive inflammatory lesion. EphB4 was detected with peroxidase-AEC (3-amino-9-ethylcarbazole) and appeared red (red arrow). Positive staining for ephrinB2 was dark blue, which was detected by alkaline phosphatase–NBT/BCIP (nitroblue tetrazolium/5-bromo-4-chloro-3-indoyl phosphate p-toluidine salt). Most of the ephrinB2-positive cells were macrophage-like cells (white arrowhead), while EphB4-positive cells were in small venules. Original magnification × 400. Scale bar equals 20 μm. (B) Western blots for ephrinB2 and EphB4 in 5 human primary cell cultures, including 4 endothelial cells (HUAECs, HUVECs, HIAECs, HSVECs) and 1 fibroblast (human gingival fibroblast [HGF]), and 1 monoblastoid cell line (U937) activated with 20 ng/mL PMA. [Beta]-Actin was an internal control.
Syntenin and syndecan-1 were up-regulated in EphB4-positive endothelial cells following treatment with ephrinB2
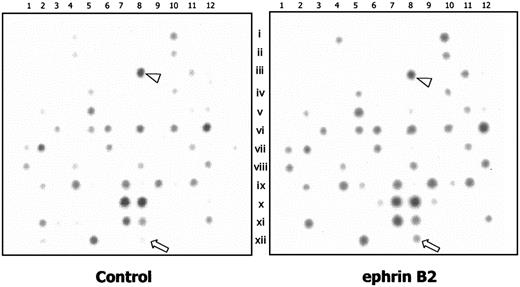
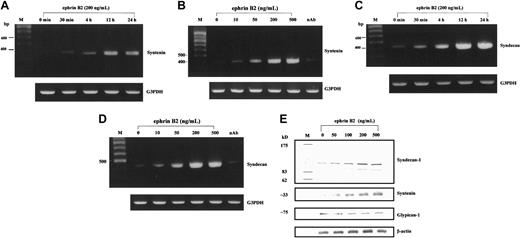
The results of a cDNA microarray in HUAECs indicated that after treatment with 200 ng/mL ephrinB2 for 4 hours, the transcription of 13 genes was enhanced, judging by the larger number of clearly stained spots of cDNA and the readings of the densitometer (Figure 2). The genes are listed in Table 1. We then used RT-PCR to confirm the enhancing effect of ephrinB2 on those 13 genes. One of the 13 genes was syntenin, whose major characteristic was binding with a family of cell surface proteoglycans: syndecans.19 Syndecan-1, -2, and -4 are the family members that can be detected in endothelial cells and that play important roles in modulating the binding of angiogenic factors.35 Therefore, we also included syndecan-1, -2, and -4 in the RT-PCR assays. The results of RT-PCR demonstrated that, after treatment with ephrinB2, only the transcriptions of syntenin and syndecan-1 were up-regulated in HUAECs in dose- and time-dependent manners (Figure 3A-D). The remaining tested targets showed no significant differences (data not shown). Neutralizing antibody for EphB4 nullified the enhancing effect. This indicated that the enhancement of syntenin and syndecan-1 expressions was from the interaction of ephrinB2 and EphB4, even though other members of the EphB family were detected on the endothelial cells.36 Western blots for syntenin and syndecan-1 further confirmed that the translation levels were also up-regulated by ephrinB2. The maximal effective dose seemed to be 200 ng/mL (Figure 3E).
Comparison of the transcriptions of 141 angiogenesis-associated genes between HUAECs with and without treatment of ephrinB2 by cDNA microarray. The cDNA microarray membrane contains 141 gene clones. A total of 2.5 μg mRNA from HUAECs treated with 200 ng/mL preclustered ephrinB2 or 2 μg/mL clustering Ab alone for 4 hours was labeled with biotin-16–deoxyuridine triphosphate (biotin-16-dUTP) by reverse transcription and hybridized to the cDNA membrane, followed by color development. The genes that appeared to be upregulated are listed in Table 1. The arrowhead indicates β-actin (array no. C08), and the arrow indicates syntenin (array no. L08).
Comparison of the transcriptions of 141 angiogenesis-associated genes between HUAECs with and without treatment of ephrinB2 by cDNA microarray. The cDNA microarray membrane contains 141 gene clones. A total of 2.5 μg mRNA from HUAECs treated with 200 ng/mL preclustered ephrinB2 or 2 μg/mL clustering Ab alone for 4 hours was labeled with biotin-16–deoxyuridine triphosphate (biotin-16-dUTP) by reverse transcription and hybridized to the cDNA membrane, followed by color development. The genes that appeared to be upregulated are listed in Table 1. The arrowhead indicates β-actin (array no. C08), and the arrow indicates syntenin (array no. L08).
Putative genes that are enhanced after stimulation with ephrinB2 in HUAECs
Array no. . | Gene name . | GenBank accession no. . | Ratio . |
|---|---|---|---|
| A04 | Interleukin enhancer binding factor-3 | H52498 | 2.1 |
| A10 | Matrix metalloprotease-14 | AI376244 | 2.3 |
| C11 | Interleukin-1β | W46101 | 2.4 |
| D05 | Interleukin-16 | AA286934 | 2.3 |
| D10 | Colony-stimulating factor-3 | AI074784 | 2.0 |
| E11 | Leptin receptor | AI056820 | 2.0 |
| F05 | Hepatocyte growth factor | H13714 | 2.3 |
| H01 | Thrombospondin-4 | H27673 | 2.3 |
| H08 | Tie-1 | R40609 | 2.3 |
| H12 | Integrin α5 | AI500496 | 2.4 |
| I02 | Pigment epithelium-derived factor | H69024 | 2.0 |
| K02 | Vascular endothelial growth factor c | H07899 | 2.6 |
| L08 | Syndecan binding protein (syntenin) | R44784 | 2.1 |
Array no. . | Gene name . | GenBank accession no. . | Ratio . |
|---|---|---|---|
| A04 | Interleukin enhancer binding factor-3 | H52498 | 2.1 |
| A10 | Matrix metalloprotease-14 | AI376244 | 2.3 |
| C11 | Interleukin-1β | W46101 | 2.4 |
| D05 | Interleukin-16 | AA286934 | 2.3 |
| D10 | Colony-stimulating factor-3 | AI074784 | 2.0 |
| E11 | Leptin receptor | AI056820 | 2.0 |
| F05 | Hepatocyte growth factor | H13714 | 2.3 |
| H01 | Thrombospondin-4 | H27673 | 2.3 |
| H08 | Tie-1 | R40609 | 2.3 |
| H12 | Integrin α5 | AI500496 | 2.4 |
| I02 | Pigment epithelium-derived factor | H69024 | 2.0 |
| K02 | Vascular endothelial growth factor c | H07899 | 2.6 |
| L08 | Syndecan binding protein (syntenin) | R44784 | 2.1 |
The temporal and dose effects of ephrinB2 on expressions of syntenin and syndecan-1 in HUAECs by RT-PCR and Western blot. (A-D) To confirm the results of differentially expressed genes obtained by the cDNA microarray technique, each candidate gene was further analyzed with RT-PCR to test dose dependence and time dependence. Syntenin and syndecan-1 were the only 2 genes up-regulated in dose- and time-dependent manners. Neutralizing antibody (10 μg/mL) against the N-terminal of EphB4 receptor suppressed the up-regulation of syntenin and syndecan-1 by ephrinB2 (B,D). G3PDH was used as an internal control. The sizes of the PCR products were 388, 416, and 983 bp for syntenin, syndecan-1, and G3PDH, respectively. (E) Western blot of syntenin and syndecan-1 in HUAECs treated with different doses of ephrinB2. HUAECs were treated with preclustered ephrinB2 (0 to 500 ng/mL) for 24 hours. The cell lysates were then collected and analyzed with Western blot for syntenin and syndecan-1. The molecular weight for syndecan-1 is about 110 kDa for the major band and about 90 kDa for the minor band. The detected band for syntenin is about 33 kDa. Glypican-1 (another cell membrane proteoglycan on endothelial cells) and β-actin were used as controls.
The temporal and dose effects of ephrinB2 on expressions of syntenin and syndecan-1 in HUAECs by RT-PCR and Western blot. (A-D) To confirm the results of differentially expressed genes obtained by the cDNA microarray technique, each candidate gene was further analyzed with RT-PCR to test dose dependence and time dependence. Syntenin and syndecan-1 were the only 2 genes up-regulated in dose- and time-dependent manners. Neutralizing antibody (10 μg/mL) against the N-terminal of EphB4 receptor suppressed the up-regulation of syntenin and syndecan-1 by ephrinB2 (B,D). G3PDH was used as an internal control. The sizes of the PCR products were 388, 416, and 983 bp for syntenin, syndecan-1, and G3PDH, respectively. (E) Western blot of syntenin and syndecan-1 in HUAECs treated with different doses of ephrinB2. HUAECs were treated with preclustered ephrinB2 (0 to 500 ng/mL) for 24 hours. The cell lysates were then collected and analyzed with Western blot for syntenin and syndecan-1. The molecular weight for syndecan-1 is about 110 kDa for the major band and about 90 kDa for the minor band. The detected band for syntenin is about 33 kDa. Glypican-1 (another cell membrane proteoglycan on endothelial cells) and β-actin were used as controls.
EphrinB2 inhibited the bFGF-induced proliferation effect on EphB4-positive endothelial cells in vitro but enhanced angiogenesis in vivo
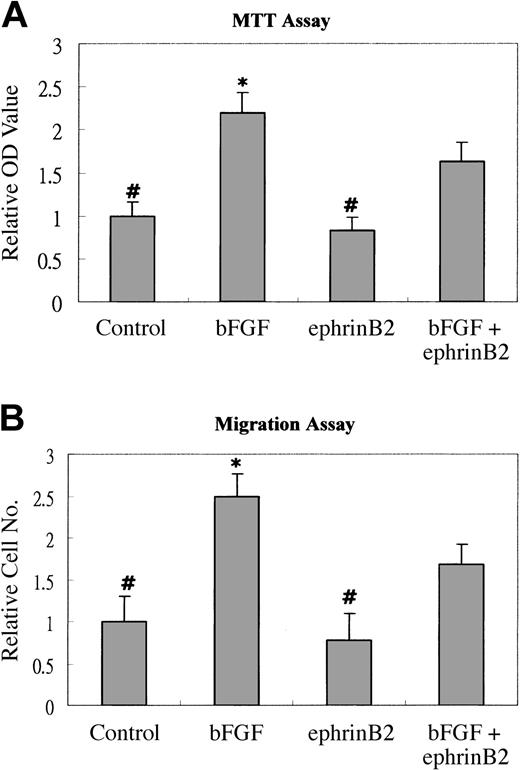
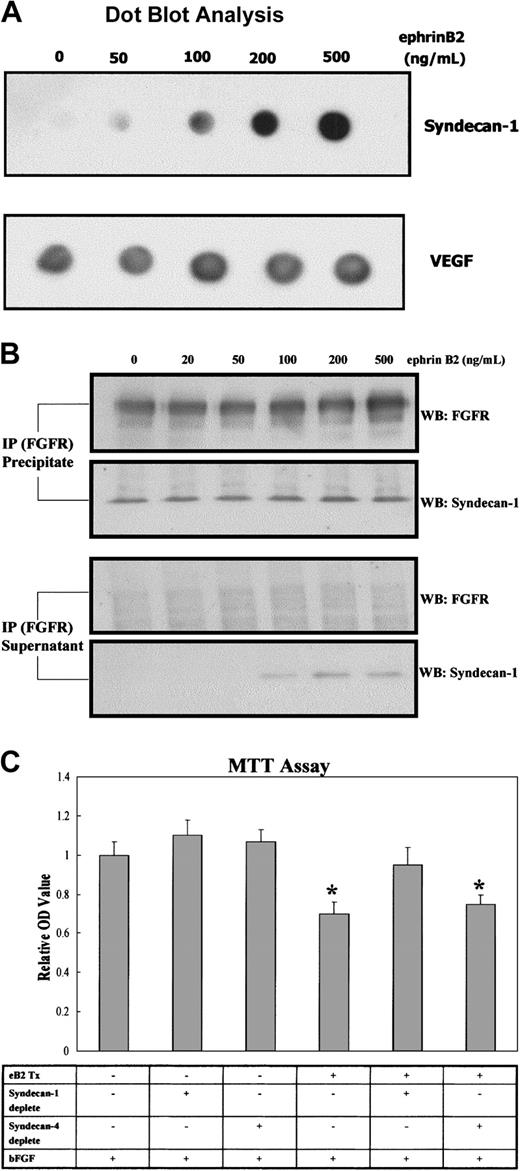
Because it was reported that syndecan-1 could associate with FGFR and modulated the activity of bFGF,10 we performed MTT and migration assays to test whether ephrinB2 could modulate the angiogenic effects of bFGF on EphB4-positive cells via syndecan-1. The results showed that 200 ng/mL ephrinB2 significantly inhibited the proliferative and chemotactic effects of bFGF on EphB4-positive endothelial cells (Figure 4A-B). We also tested the effects of lower doses of ephrinB2 and found negative effects to lesser extents (data not shown). To understand whether syndecan-1 played a role in the inhibition, a dot blot assay and an IP analysis were conducted to determine whether syndecan-1 ectodomain shedding was increased and surplus syndecan-1 protein was not always associated with FGFR1, respectively. The dot blot assay showed that the amount of syndecan-1 ectodomain increased in a dose-dependent manner after the addition of ephrinB2; the amount of VEGF, another secreted peptide in the media, remained unchanged (Figure 5A). The IP analysis showed that syndecan-1 co-immunoprecipitated with FGFR1 and that the quantity of associated syndecan-1 with FGFR1 in EphB4-positive endothelial cells was not significantly changed by the addition of ephrinB2. However, the amount of syndecan-1 not associated with FGFR1 increased and could be detected after the addition of more than 100 ng/mL ephrinB2 (Figure 5B). This result indicated that the ephrinB2-induced increase in syndecan-1 might not always be in close proximity to FGFR1 and that it assisted the binding of their ligands. To understand the role of syndecan-1 ectodomain on the suppressive effect of ephrinB2, we used an immunoprecipitation protocol to deplete syndecan ectodomains from the media of ephrinB2-treated HUAEC cultures. The results demonstrated that either depletion of syndecan-4 ectodomain or sham depletion did not alter the inhibitory effect of ephrinB2 on bFGF-induced HUAEC proliferation. On the contrary, ephrinB2-treated media depleted of syndecan-1 ectodomain lost their inhibitory effect (Figure 5C). The results provided evidence that increased syndecan-1 ectodomain was at least partially responsible for the in vitro suppressive effect of ephrinB2 on EphB4-positive endothelial cells. However, 2 μg ephrinB2 exhibited angiogenic effect comparable to 0.2 μg VEGF in the murine wound healing model. Syndecan-1 was up-regulated in the ephrinB2-treated group but remained similar between VEGF and control groups (Figure 6). The results implied the underlying angiogenic mechanisms of ephrinB2 in vivo are different from those of VEGF.
EphrinB2 inhibits bFGF-induced proliferation and migration of HUAECs. (A) Subconfluent HUAECs at a density of 5 × 103 cells per well per 500 μL were plated into 24-well culture plates in the M199 medium containing 5% fetal calf serum (FCS) and 1 of 4 different regimens: clustering Ab alone (Control), 10 ng/mL bFGF, 200 ng/mL preclustered ephrinB2, or a combination of ephrinB2 and bFGF. The cultures were incubated for 3 days and then assessed by MTT assays. The samples of each tested group were in sextuplicate, and the experiment was performed in triplicate. Each data set was normalized with the average value of the negative control group. Bars represent the mean ± SD from 6 data of 1 representative experiment. (B) Quantification of migrated HUAECs. HUAECs were incubated with the indicated reagents for 6 hours in a modified Boyden chamber. After incubation, migrated cells were fixed and then stained with Giemsa solution. The migrated cells were counted at × 100 magnification. Three fields were counted in each well, and the average number was obtained. Each group was tested in sextuplicate, and tests were performed in triplicate. Each data set was normalized with the average value of the negative control group. Bars represent the mean ± SD from 6 results of 1 representative experiment. *P < .05 significantly greater than bFGF plus ephrinB2 group. #P < .05 significantly smaller than bFGF plus ephrinB2 group.
EphrinB2 inhibits bFGF-induced proliferation and migration of HUAECs. (A) Subconfluent HUAECs at a density of 5 × 103 cells per well per 500 μL were plated into 24-well culture plates in the M199 medium containing 5% fetal calf serum (FCS) and 1 of 4 different regimens: clustering Ab alone (Control), 10 ng/mL bFGF, 200 ng/mL preclustered ephrinB2, or a combination of ephrinB2 and bFGF. The cultures were incubated for 3 days and then assessed by MTT assays. The samples of each tested group were in sextuplicate, and the experiment was performed in triplicate. Each data set was normalized with the average value of the negative control group. Bars represent the mean ± SD from 6 data of 1 representative experiment. (B) Quantification of migrated HUAECs. HUAECs were incubated with the indicated reagents for 6 hours in a modified Boyden chamber. After incubation, migrated cells were fixed and then stained with Giemsa solution. The migrated cells were counted at × 100 magnification. Three fields were counted in each well, and the average number was obtained. Each group was tested in sextuplicate, and tests were performed in triplicate. Each data set was normalized with the average value of the negative control group. Bars represent the mean ± SD from 6 results of 1 representative experiment. *P < .05 significantly greater than bFGF plus ephrinB2 group. #P < .05 significantly smaller than bFGF plus ephrinB2 group.
EphrinB2 can suppress the proliferative effect of bFGF on HUAECs via the syndecan-1 ectodomain. (A) To understand whether shedding of the syndecan-1 ectodomain in HUAECs was increased after the addition of ephrinB2, HUAEC cultures were treated with preclustered ephrinB2 (0 to 500 ng/mL) for 24 hours. The conditioned media were collected and concentrated with Centricon according to the manufacturer's instruction. Under gentle vacuuming, 1.5 μL of each concentrate was applied to the methanol-moistened PVDF membrane. After blocking, monoclonal antiectodomain of syndecan-1 was added and incubated as the protocol of Western blot. The membrane was finally developed with enhanced chemiluminescence (ECL). Another secreted peptide of endothelial cells, VEGF, was used as an internal control because its expression was not regulated by ephrinB2 based on our cDNA microarray and RT-PCR. (B) To test whether overproduced syndecan-1 was consistently associated with FGFR1, a co-IP assay was employed. HUAEC cultures were treated with varying concentrations of preclustered ephrinB2 (0 to 500 ng/mL) for 24 hours. Membrane portions of cell lysates were isolated and processed for IP with monoclonal mouse anti-FGFR1 antibody.After centrifugation, the supernatant and pellet were separately collected and processed for Western blotting with polyclonal rabbit anti-FGFR1 or goat antisyndecan-1 antibodies. (C) To examine whether dispersed syndecan-1 ectodomain was responsible for inhibiting the bFGF-induced proliferative effect, the conditioned media of HUAEC cultures with or without pretreatment of ephrinB2 were collected and concentrated to 2 mg/mL. The methodology of immunoprecipitation was used to deplete either syndecan-1 or syndecan-4 ectodomain from the media. The control samples received protein A/G Plus–Agarose beads alone. A dot blot assay was used to confirm the depletion of syndecan-1 or -4 in the leftover fluid. Then the fluid was added, in a protein concentration of 500 μg/mL, to fresh M199 medium with 10 ng/mL bFGF to undergo MTT proliferation assay as previously described. Each group was tested in sextuplicate, and tests were performed in triplicate. Each data set was normalized with the average value of the control group. Bars represent the mean ± SD from 6 data sets of 1 representative experiment. eB2 indicates ephrinB2. *P < .05 compared with bFGF alone group (first bar from the left).
EphrinB2 can suppress the proliferative effect of bFGF on HUAECs via the syndecan-1 ectodomain. (A) To understand whether shedding of the syndecan-1 ectodomain in HUAECs was increased after the addition of ephrinB2, HUAEC cultures were treated with preclustered ephrinB2 (0 to 500 ng/mL) for 24 hours. The conditioned media were collected and concentrated with Centricon according to the manufacturer's instruction. Under gentle vacuuming, 1.5 μL of each concentrate was applied to the methanol-moistened PVDF membrane. After blocking, monoclonal antiectodomain of syndecan-1 was added and incubated as the protocol of Western blot. The membrane was finally developed with enhanced chemiluminescence (ECL). Another secreted peptide of endothelial cells, VEGF, was used as an internal control because its expression was not regulated by ephrinB2 based on our cDNA microarray and RT-PCR. (B) To test whether overproduced syndecan-1 was consistently associated with FGFR1, a co-IP assay was employed. HUAEC cultures were treated with varying concentrations of preclustered ephrinB2 (0 to 500 ng/mL) for 24 hours. Membrane portions of cell lysates were isolated and processed for IP with monoclonal mouse anti-FGFR1 antibody.After centrifugation, the supernatant and pellet were separately collected and processed for Western blotting with polyclonal rabbit anti-FGFR1 or goat antisyndecan-1 antibodies. (C) To examine whether dispersed syndecan-1 ectodomain was responsible for inhibiting the bFGF-induced proliferative effect, the conditioned media of HUAEC cultures with or without pretreatment of ephrinB2 were collected and concentrated to 2 mg/mL. The methodology of immunoprecipitation was used to deplete either syndecan-1 or syndecan-4 ectodomain from the media. The control samples received protein A/G Plus–Agarose beads alone. A dot blot assay was used to confirm the depletion of syndecan-1 or -4 in the leftover fluid. Then the fluid was added, in a protein concentration of 500 μg/mL, to fresh M199 medium with 10 ng/mL bFGF to undergo MTT proliferation assay as previously described. Each group was tested in sextuplicate, and tests were performed in triplicate. Each data set was normalized with the average value of the control group. Bars represent the mean ± SD from 6 data sets of 1 representative experiment. eB2 indicates ephrinB2. *P < .05 compared with bFGF alone group (first bar from the left).
EphrinB2 enhances angiogenesis and expression of syndecan-1 in the murine wound healing model. Collagen sponges containing either 2 μg preclustered ephrinB2 or 0.2 μg VEGF or clustering Ab alone (control) were implanted subcutaneously in mice for 7 days (n = 5 for each group). The collagen sponges with surrounding tissue were excised and embedded in paraffin and then processed for immunostaining of CD31 and syndecan-1. The positive reaction appeared red from peroxidase-AEC. eB2 indicates ephrinB2; cont, control. Original magnification × 100. Scale bar equals 75 μm.
EphrinB2 enhances angiogenesis and expression of syndecan-1 in the murine wound healing model. Collagen sponges containing either 2 μg preclustered ephrinB2 or 0.2 μg VEGF or clustering Ab alone (control) were implanted subcutaneously in mice for 7 days (n = 5 for each group). The collagen sponges with surrounding tissue were excised and embedded in paraffin and then processed for immunostaining of CD31 and syndecan-1. The positive reaction appeared red from peroxidase-AEC. eB2 indicates ephrinB2; cont, control. Original magnification × 100. Scale bar equals 75 μm.
Heparanase was up-regulated in inflammation and might convert the inhibitory effects of ephrinB2 on EphB4-positive endothelial cells by modifying syndecan-1 ectodomain
Because it has been reported that heparanase-1 converts soluble syndecan-1 ectodomain from an inhibitor into a potent activator of bFGF in vitro,13 we tested whether heparanase was up-regulated during inflammation and could modulate the negative effect of ephrinB2 on bFGF-induced proliferation of EphB4-positive endothelial cells. Western blot assays demonstrated that HUAECs expressed heparanase-1 and -2 after stimulation with 2 inflammatory cytokines (IL-1β and TNF-α). The U937 cells did not express heparanases even after stimulation with IL-1β and TNF-α (data not shown); they did, however, express heparanase-1 (but not heparanase-2) following activation of PMA and ionomycin (Figure 7A). The MTT proliferation assay on HUAECs with or without pretreatment of ephrinB2 demonstrated that bFGF-induced proliferation was significantly more enhanced in conditioned medium with heparitinase treatment than in medium without heparitinase treatment. The enhancing effect was significantly greater in medium from cells pretreated with ephrinB2 than in those not pretreated. On the contrary, without the treatment of heparitinase, the ephrinB2 group exhibited significantly less bFGF-induced proliferation than the control group did (Figure 7B). This indicated that certain molecules regulated by ephrinB2 in HUAECs were converted by heparitinase from inhibitors to activators of bFGF-induced proliferation. To understand whether the conversion of biologic effect was due to the altered binding ability of FGFR for bFGF, we performed binding assays with bFGF-FITC and compared the differences between HUAECs with or without pretreatments of ephrinB2 and heparitinase. The results of the binding assay corresponded well with those of the proliferative assay. Without the influence of heparitinase, ephrinB2 treatment reduced the amount of bound bFGF on HUAECs. Heparitinase treatment, however, dramatically reversed the inhibitory effect of ephrinB2 on the binding of bFGF (Figure 7C). Because the substrates for heparitinase and heparanase are proteoglycans, and syndecan-1 is the only cell surface proteoglycan regulated by ephrinB2 in EphB4-positive endothelial cells in our tests, syndecan-1 was the molecule most likely responsible for the above-mentioned observations (Figure 7B,C).
The expression of heparanases is modulated by proinflammatory cytokines, and the enzymes can convert the bFGF binding and proliferation suppressive effects of ephrinB2. (A) Western blot for Hpa-1 and -2 in HUAECs and U937 cells. After stimulation of the above-mentioned substance for 24 hours, the cell lysates were obtained and underwent immunoblot assay for Hpa-1 and -2. β-Actin was used as an internal control. Two bands, about 35 kDa and about 175 kDa, could be detected in HUAECs stimulated with 20 ng/mL IL-1β or TNF-α on the immunoblot of Hpa-2 while 1 band of about 70 kDa was detected in HUAECs treated with IL-1β or TNF-α as well as U937 cells treated with ionomycin and PMA. (B) HUAEC cultures were either pretreated with 200 ng/mL preclustered ephrinB2 or clustering Ab alone in M199 media with 1% FCS for 12 hours. Then each group was divided into 2 subgroups. One subgroup received heparitinase (heparinase III; Flavobacterium heparinum [EC 4.2.2.8]) in a final concentration of 5 mIU/mL and was incubated at 37° C for 45 minutes. The other subgroup received no treatment of heparitinase. The media were then collected and centrifuged to discard the pellets and then heated to 50° C for 10 minutes to eliminate residual enzymatic activity. After returning to room temperature, the media were further divided into 2 different regimens: they either did or did not receive 10 ng/mL bFGF and then were added to 24-well HUAEC culture plates (5 × 103 cells per well per 500 μL). The cultures were incubated for 3 days and then assessed by MTT assays. The samples of each tested group were in sextuplicate, and the experiment was performed in triplicate. Each data set was normalized with the average value of the negative control group. Bars represent the mean ± SD from 6 data sets of 1 representative experiment. eB2 indicates ephrinB2. *P < .05, significantly greater than the bFGF alone group (fourth bar from the left). #P < .05, significantly smaller than the bFGF alone group. (C) HUAECs were seeded on 12-well culture dishes in a density of 1 × 105 cells per well 24 hours before the binding assay. After 2 washes with binding buffer, 1 mL conditioned media with or without pretreatment of ephrinB2 and heparitinase as in a previous experiment, combined with 20 ng bFGF-FITC, was added to each well and then incubated at 4° C for 1 hour. Each well was washed twice with binding buffer, and then 1 mL of 2 M NaCl in 20 mM sodium acetate (pH 4.0) was used to dissociate the bound bFGF-FITC. To determine the fluorescence level, 150 μL of the supernatant in each well was transferred to an ELISA plate. The samples of each tested group were in sextuplicate, and the experiment was repeated twice. Each data set was normalized with the average value of the negative control group. Bars represent the mean ± SD from 6 data sets of 1 representative experiment. eB2 indicates ephrinB2; Hpa, heparitinase. *P < .05, significantly greater than the negative control group. #P < .05, significantly smaller than the negative control group.
The expression of heparanases is modulated by proinflammatory cytokines, and the enzymes can convert the bFGF binding and proliferation suppressive effects of ephrinB2. (A) Western blot for Hpa-1 and -2 in HUAECs and U937 cells. After stimulation of the above-mentioned substance for 24 hours, the cell lysates were obtained and underwent immunoblot assay for Hpa-1 and -2. β-Actin was used as an internal control. Two bands, about 35 kDa and about 175 kDa, could be detected in HUAECs stimulated with 20 ng/mL IL-1β or TNF-α on the immunoblot of Hpa-2 while 1 band of about 70 kDa was detected in HUAECs treated with IL-1β or TNF-α as well as U937 cells treated with ionomycin and PMA. (B) HUAEC cultures were either pretreated with 200 ng/mL preclustered ephrinB2 or clustering Ab alone in M199 media with 1% FCS for 12 hours. Then each group was divided into 2 subgroups. One subgroup received heparitinase (heparinase III; Flavobacterium heparinum [EC 4.2.2.8]) in a final concentration of 5 mIU/mL and was incubated at 37° C for 45 minutes. The other subgroup received no treatment of heparitinase. The media were then collected and centrifuged to discard the pellets and then heated to 50° C for 10 minutes to eliminate residual enzymatic activity. After returning to room temperature, the media were further divided into 2 different regimens: they either did or did not receive 10 ng/mL bFGF and then were added to 24-well HUAEC culture plates (5 × 103 cells per well per 500 μL). The cultures were incubated for 3 days and then assessed by MTT assays. The samples of each tested group were in sextuplicate, and the experiment was performed in triplicate. Each data set was normalized with the average value of the negative control group. Bars represent the mean ± SD from 6 data sets of 1 representative experiment. eB2 indicates ephrinB2. *P < .05, significantly greater than the bFGF alone group (fourth bar from the left). #P < .05, significantly smaller than the bFGF alone group. (C) HUAECs were seeded on 12-well culture dishes in a density of 1 × 105 cells per well 24 hours before the binding assay. After 2 washes with binding buffer, 1 mL conditioned media with or without pretreatment of ephrinB2 and heparitinase as in a previous experiment, combined with 20 ng bFGF-FITC, was added to each well and then incubated at 4° C for 1 hour. Each well was washed twice with binding buffer, and then 1 mL of 2 M NaCl in 20 mM sodium acetate (pH 4.0) was used to dissociate the bound bFGF-FITC. To determine the fluorescence level, 150 μL of the supernatant in each well was transferred to an ELISA plate. The samples of each tested group were in sextuplicate, and the experiment was repeated twice. Each data set was normalized with the average value of the negative control group. Bars represent the mean ± SD from 6 data sets of 1 representative experiment. eB2 indicates ephrinB2; Hpa, heparitinase. *P < .05, significantly greater than the negative control group. #P < .05, significantly smaller than the negative control group.
Discussion
The results of our cDNA microarray and RT-PCR assays indicated that syntenin and syndecan-1 in EphB4-positive endothelial cells were up-regulated after stimulation with functional ephrinB2. Syntenin is a cytoplasmic adapter protein that reportedly binds to syndecans and the Eph family through its PDZ (postsynaptic density-95/discs large/zona occludens-1 [PSD-95/Dlg/ZO-1]) domains.37,38 It has also been reported that syndecans can form a ternary complex at the cell surface with FGFs and FGFRs and that they can modulate the signaling of FGFRs.10 Although the function of syntenin is still unclear, it is tempting to speculate that syntenin might join a large supramolecular complex comprising the Eph family, syndecans, and FGFRs to perform important physiological functions.
Recently, several studies have tried to elucidate the functions of ephrinB2 and EphB4 in human vascular endothelial cells by in vivo and in vitro tests. Human endothelial cells, however, do not specifically express either ephrinB2 or EphB4 as do mouse-embryo endothelial cells.7,39,40 The expression of ephrinB2 is dependent on local cues that may be derived from adjacent smooth muscle cells.41 Loss of the interaction between endothelial and smooth muscle cells, as in the in vitro system, may lead to loss of expression of ephrinB2 and inconsistent findings. Furthermore, 2 studies indicated that ephrinB2 suppressed proliferation and migration of EphB4-positive endothelial cells, while another study demonstrated the opposite results.39-41 The suppressive effect was inferred to explain why ephrinB2-positive endothelial cells face EphB4-positive endothelial cells at the boundary of capillaries and remain quiescent under physiologic homeostasis.39,42 In our experiments, we also demonstrated that ephrinB2 attenuated the bFGF-induced proliferative and chemotactic effects on EphB4-positive endothelial cells. Another study demonstrated that EphB kinases acted as negative regulators of the Ras/MAPK signaling and exhibited antimitogenic and antimigratory effects on endothelial cells.39 Our study suggested that surplus syndecan-1 provided additional mechanisms for the suppressive effect.
Syndecan-1 belongs to a family of transmembrane proteoglycans, which can modulate the interaction of heparin-binding growth factors (eg, VEGF, bFGF) with their corresponding high-affinity receptors.43 The syndecan family is composed of 4 closely related proteins (syndecan-1, -2, -3, and -4) coded by 4 different genes. Syndecan-1 not only can behave as a coreceptor and attract bFGF but also can bind to many extracellular matrix components (eg, fibronectin, fibrillar collagens, thrombospondin, tenascin) via its heparan sulfate side chains.44 Syndecan-like heparan sulfate proteoglycans also play a role in regulating leukocyte endothelium interactions.45 It has been recently demonstrated that overexpression of syndecan-1 can disturb the proliferative effect of bFGF and aFGF on 3T3 fibroblasts.11 Although a minimal amount of cell surface heparan sulfate is required for FGF to interact with FGFR and activate downstream intracellular signaling, it is now recognized that at heparan sulfate levels lower or higher than optimal, the effective ligand concentration for engaging the receptor will fall.10 Of all the plausible mechanisms, shedding of the extracellular domains (ectodomains) of syndecan-1 by an unidentified membrane to form protease was regarded as playing an important role in the inhibitory effects.46 Inhibition of cell proliferation by shedding syndecan-1 ectodomains has also been observed with several carcinoma cell lines, including myeloma and mammary tumor cell lines.47,48 Our study found that not only shedding of syndecan-1 ectodomain by EphB4-positive endothelial cells increased after stimulation with ephrinB2 but also that the excessive syndecan-1 was not always associated with FGFR1 on the cell surface. This result suggested that surplus syndecan-1 up-regulated by ephrinB2 was not spatially beneficial in FGFR signaling in vitro.
Questions remained about whether overexpressed syndecan-1 was really an inhibitor for angiogenesis in vivo despite the in vitro findings. In vivo, syndecan-1 was up-regulated during wound healing, and the up-regulation was restricted to the newly grown endothelial cells of the granulation tissue.49 In our immunohistochemical staining on the superreactive inflammatory lesions, pyogenic granuloma demonstrated similar results. Therefore, it seems unlikely that syndecan-1 plays a negative role in inflammatory angiogenesis. Interestingly, heparanase, an invasion-associated enzyme, reportedly could convert the soluble syndecan-1 ectodomain from an inhibitor to a potent activator of bFGF. Our study using heparitinase (functionally similar to heparanase) also demonstrated that heparitinase switched the inhibitory effect of ephrinB2 on EphB4-positive endothelial cells to an enhancing effect on bFGF-induced proliferation. Syndecan-1 was most plausible in this process, because it was the only cell surface proteoglycan that exhibited a quantitative difference in our tests.
Our results showed heparanase-1 and -2, 2 members of the mammalian heparanase family, were positively associated with inflammation. Heparanase-1 was previously identified in highly invasive normal and malignant cells, including cytotrophoblasts, activated cells of the immune system, platelets, lymphoma, melanoma, and carcinoma cells.50 Heparanase-2 has recently been cloned, but its biologic function and cell distribution are not known.51 We have performed immunohistochemistry for heparanase-1 and -2 on human inflammatory lesions and observed that endothelial cells were strongly stained for heparanase-1 and -2 (data not shown). We also found that the character of conditioned media of ephrinB2-treated cell culture could be altered by the addition of heparitinase and that the medium was switched from an inhibitor to an enhancer in bFGF binding to FGFR and the following signaling. The underlying mechanism might be due to the substrate specificity of heparitinase and heparanase, both of which preferably degraded heparan sulfate domains without sulfation rather than those with sulfation.13,52 Guimond et al had demonstrated that desulfated heparin (at certain sites) competed with native heparin for binding to bFGF and blocked the ability of native heparin to promote the mitogenic activity of bFGF.53 Therefore, removing the competitive desulfated domains by heparanase or heparitinase seemed to be plausible for the extinction of inhibitory effect. In addition to the different sulfation state on heparan sulfate chains, other mechanisms may also be involved in the versatility of syndecan ectodomains. One study54 reported that the affinity of bFGF for an immobilized FGFR1 ectodomain was increased when the syndecan-4 ectodomain was coimmobilized with the receptor. On the contrary, free soluble syndecan-4 ectodomain had no effect on the binding. These results implied that soluble syndecan ectodomain might have a different conformation than it has on the cell surface. The conformational change may also contribute to the role conversion of syndecan ectodomains in FGFR signaling. In summary, with the abundant amount of heparanase in inflammation, the up-regulated syndecan-1 from EphB4-positive endothelial cells may act more as an enhancer than suppressor for FGFR signaling after poorly sulfated chains are removed and the conformation changes.
From our findings in inflammatory lesions, macrophages and small venules expressed ephrinB2 and EphB4, respectively. Most of the endothelial cells in inflammation significantly expressed heparanase. We hypothesize a model that can be used to explain 2 important physiological phenomena. First, in undisturbed and uninflamed conditions, the syndecan-1 regulated by ephrinB2-positive arterial endothelial cells and EphB4-positive venous endothelial cells acts as a negative regulator for FGF functioning. Therefore, the interface between arterial and venous endothelial cells responds poorly to heparin-binding angiogenic factors and becomes quiescent and stable. Second, and conversely, under inflammation, ephrinB2-positive macrophages transmigrate through EphB4-positive postcapillary venules and up-regulate the expression of syndecan-1 in venule endothelium. Simultaneously, heparanases convert the function of syndecan-1 from inhibitor to activator. In addition to other angiogenic factors secreted by macrophage, new vessels arise overwhelmingly from the venous side of circulation, as observed in previous studies.15,16 Inflammation-associated heparanases may play the pivotal role in determining the various functions of syndecan-1 in different situations of vascular physiology.
Prepublished online as Blood First Edition Paper, May 4, 2004; DOI 10.1182/blood-2003-09-3334.
Supported in part by grants NSC 89-2314-B-006-042 (K.Y.) and NSC 91-3112-B-006-014 (M.T.L.) from the National Science Council of the Republic of China, EX91-9027SP (M.T.L.) from the National Health Research Institute of Taiwan, and NCKU 91-069 (K.Y.) from National Cheng Kung University Hospital.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Drs Yin-Tai Jin and Dar-Bing Shieh for their help with the pathological processing and diagnosis and to Dr Kazuha Hashizume for providing us the antibody of heparanase-1. We thank Bill Franke for editing our English manuscript.

![Figure 1. The expression of ephrinB2 and EphB4 proteins in pyogenic granuloma and cell cultures. (A) A representative double staining from specimens of pyogenic granuloma, which is a hyperreactive inflammatory lesion. EphB4 was detected with peroxidase-AEC (3-amino-9-ethylcarbazole) and appeared red (red arrow). Positive staining for ephrinB2 was dark blue, which was detected by alkaline phosphatase–NBT/BCIP (nitroblue tetrazolium/5-bromo-4-chloro-3-indoyl phosphate p-toluidine salt). Most of the ephrinB2-positive cells were macrophage-like cells (white arrowhead), while EphB4-positive cells were in small venules. Original magnification × 400. Scale bar equals 20 μm. (B) Western blots for ephrinB2 and EphB4 in 5 human primary cell cultures, including 4 endothelial cells (HUAECs, HUVECs, HIAECs, HSVECs) and 1 fibroblast (human gingival fibroblast [HGF]), and 1 monoblastoid cell line (U937) activated with 20 ng/mL PMA. [Beta]-Actin was an internal control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/4/10.1182_blood-2003-09-3334/6/m_zh80160465120001.jpeg?Expires=1769250787&Signature=nAtrcoIKlrJOJhGtcuL6sg7PWh3buCHS9qEIK5Q16d~Se4dGWV7YquxLKxWxiGjY8srUhLyJ617QmeoTKBaxO-ml4yLkQZ9Txg7Vw88IAX48AuwexApyTaGgnKll-RSfBLAbAM6q-Mz9uDH-ySI0DUkUnQZqIidXM990iPDgptEwVRpUskel~MLwo4rLn7RzzFxiqUC98ExnelaQy22k42CoP-G0MvCN~9ThoiG7leOvPC4jyJClkt1LIjAx~Lxqm8HJ3O9B943l1RRZ-1yPZG9tF2tIHDyRlysx2J6pT2JSl6vd~0hVksn9Ve6E-NOUhbXGMdXVB7JKkxk9nMW~Mg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. The expression of heparanases is modulated by proinflammatory cytokines, and the enzymes can convert the bFGF binding and proliferation suppressive effects of ephrinB2. (A) Western blot for Hpa-1 and -2 in HUAECs and U937 cells. After stimulation of the above-mentioned substance for 24 hours, the cell lysates were obtained and underwent immunoblot assay for Hpa-1 and -2. β-Actin was used as an internal control. Two bands, about 35 kDa and about 175 kDa, could be detected in HUAECs stimulated with 20 ng/mL IL-1β or TNF-α on the immunoblot of Hpa-2 while 1 band of about 70 kDa was detected in HUAECs treated with IL-1β or TNF-α as well as U937 cells treated with ionomycin and PMA. (B) HUAEC cultures were either pretreated with 200 ng/mL preclustered ephrinB2 or clustering Ab alone in M199 media with 1% FCS for 12 hours. Then each group was divided into 2 subgroups. One subgroup received heparitinase (heparinase III; Flavobacterium heparinum [EC 4.2.2.8]) in a final concentration of 5 mIU/mL and was incubated at 37° C for 45 minutes. The other subgroup received no treatment of heparitinase. The media were then collected and centrifuged to discard the pellets and then heated to 50° C for 10 minutes to eliminate residual enzymatic activity. After returning to room temperature, the media were further divided into 2 different regimens: they either did or did not receive 10 ng/mL bFGF and then were added to 24-well HUAEC culture plates (5 × 103 cells per well per 500 μL). The cultures were incubated for 3 days and then assessed by MTT assays. The samples of each tested group were in sextuplicate, and the experiment was performed in triplicate. Each data set was normalized with the average value of the negative control group. Bars represent the mean ± SD from 6 data sets of 1 representative experiment. eB2 indicates ephrinB2. *P < .05, significantly greater than the bFGF alone group (fourth bar from the left). #P < .05, significantly smaller than the bFGF alone group. (C) HUAECs were seeded on 12-well culture dishes in a density of 1 × 105 cells per well 24 hours before the binding assay. After 2 washes with binding buffer, 1 mL conditioned media with or without pretreatment of ephrinB2 and heparitinase as in a previous experiment, combined with 20 ng bFGF-FITC, was added to each well and then incubated at 4° C for 1 hour. Each well was washed twice with binding buffer, and then 1 mL of 2 M NaCl in 20 mM sodium acetate (pH 4.0) was used to dissociate the bound bFGF-FITC. To determine the fluorescence level, 150 μL of the supernatant in each well was transferred to an ELISA plate. The samples of each tested group were in sextuplicate, and the experiment was repeated twice. Each data set was normalized with the average value of the negative control group. Bars represent the mean ± SD from 6 data sets of 1 representative experiment. eB2 indicates ephrinB2; Hpa, heparitinase. *P < .05, significantly greater than the negative control group. #P < .05, significantly smaller than the negative control group.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/4/10.1182_blood-2003-09-3334/6/m_zh80160465120007.jpeg?Expires=1769250787&Signature=jX1W5PDvz-yrERqG0YWaTvrurPO7z7~gjkY1ZcO0K4i-KfrnWKsjwRUIucOigifMNGRdG7yOOKaKTd5AHMhd6xYUqUq4~i8FdfyNCzMsibAu-5EUw~NLaW17Uj0NTmQLJhfXQVvMmwoxs6k3RNFTib89Bz9o0ANN-~DsapfjgvFL1VgslFm62zaVHTQzRnsR~TawY8tfFLZ2h1BIx8t418oR1zszhMW3fDLfMQZZRMpOK98WEmpRWLWvLJiznlAu7BtNcNAA-qPaxq0znhzLG0WO7ciD~NLu3s0JXqdVG1tVz9F8qTQTuMoaOp2nreicgcKGudRtYSgz3ETG-3SROg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal