Abstract
The triggering receptors expressed on myeloid cells (TREMs) have drawn considerable attention due to their ability to activate multiple cell types within the innate immune system, including neutrophils, monocyte/macrophages, and dendritic cells, via their association with DAP12. TLT-1 (TREM-like transcript-1) lies within the TREM gene cluster and contains the characteristic single V-set immunoglobulin (Ig) domain of the family, but its longer cytoplasmic tail is composed of both a proline-rich region and an immune receptor tyrosine-based inhibitory motif, the latter known to be used for interactions with protein tyrosine phosphatases. Here we report that TLT-1 is expressed exclusively in platelets and megakaryocytes (MKs) and that TLT-1 expression is up-regulated dramatically upon platelet activation. Consistent with this observation, confocal microscopy demonstrates that TLT-1 is prepackaged, along with CD62P, into both MK and platelet α-granules. Differences in thrombin-induced redistribution of CD62P and TLT-1 indicate that TLT-1 is not simply cargo of α-granules but may instead regulate granule construction or dispersal. Together these data show that that TLT-1 does not function to inhibit members of the TREM family but instead may play a role in maintaining vascular hemostasis and regulating coagulation and inflammation at sites of injury.
Introduction
The triggering receptors expressed on myeloid cells (TREMs) are involved in the activation of various cell types of the innate immune system, including monocytes, macrophages, microglia, and neutrophils.1-6 The family is characterized by a single V-set immunoglobulin (Ig) domain, a short cytoplasmic tail, and a charged residue in the transmembrane domain, enabling them to bind the DAP12 signaling chain.2,3 To date, 3 activating TREMs have been characterized forming a loose cluster occupying a 150 kb region of human chromosome 6 and murine chromosome 17.3,5,7-9
TREMs play important roles in the regulation of both innate and adaptive immunity. TREM-1 is broadly expressed on neutrophils and monocytes, is up-regulated during bacterial infection, and administration of TREM-1/Ig–Fc fusion protein significantly decreases mortality in mice challenged with lipopolysaccharide (LPS).2,3,10 TREM-2 is expressed on monocytes, macrophages, and osteoclasts, and its expression is up-regulated during dendritic cell development in vitro.1,11 In fact, engagement of TREM-2 on immature dendritic cells (DCs) leads to DC maturation, identifying TREMs as important proteins in the transition from an innate to an adaptive immune response.1 In addition, TREM-2 is associated with polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy (PLOSL, also known as Nasu-Hakola disease [NHD]).11-14 NHD patients harbor mutations in either TREM-2 or its signaling chain, DAP12, and suffer from systemic bone cysts and psychotic symptoms that rapidly progress to presenile dementia in the third or fourth decade of life. Patients die from NHD usually in their fifties, often due to chest infections. Recently, TREM-3 was characterized and shown to activate murine macrophages via its association with DAP12.5
Given the well-established role of TREMs in the biology of various myeloid lineages, several laboratories have sought to identify regulatory receptors within the TREM locus.6-8 Our studies identified TREM-like transcript-1 (TLT-1) as a gene within the TREM locus whose extracellular domain has significant homology with those of the TREMs.7 In contrast to the TREMs, however, TLT-1 is not predicted to bind DAP12. Instead, its longer cytoplasmic tail carries a canonical immunoreceptor tyrosine-based inhibitory motif capable of becoming phosphorylated and binding Src homology-containing protein tyrosine phosphatase-1 (SHP-1), identifying TLT-1 as the only putative inhibitory member of the TREM cluster.7 Previous analysis of TLT-1 expression showed overlapping expression with TREM-1, suggesting this receptor as a target of TLT-1–mediated inhibition in monocytes and neutrophils.7
Here we demonstrate that, in fact, murine TLT-1 is expressed exclusively in megakaryocytes (MKs) and peripheral blood platelets whereas TREM-1 is not. Moreover, we show considerable up-regulation of TLT-1 upon platelet activation due to its redistribution from storage in platelet α-granules. These results extend the spectrum of myeloid lineages regulated by TREM and TREM-like receptors and suggest that TLT-1 does not function to inhibit TREM-1 or other members of the TREM family of receptors but, rather, functions in the regulation of vascular homeostasis.
Materials and methods
Cell purifications and stimulation
Mice were bred and maintained under specific pathogen-free conditions at the National Cancer Institute (NCI)–Frederick, MD. Animal care was provided in accordance with the procedures outlined in A Guide for the Care and Use of Laboratory Animals.15 Peripheral blood collection via cardiac puncture and platelet purification from peripheral blood was performed as described.16 Platelets were then washed in modified mouse Tyrode buffer (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] [pH 7.4], 138 mM NaCl, 5.5 mM glucose, 2.9 mM KCl, and 12 mM NaHCO3). Human platelets were isolated by leukopheresis and further purified by centrifugation at 750g for 15 minutes in the presence of 50 ng/mL prostaglandin I2 (Sigma, St Louis, MO). Cells were briefly resuspended in human Tyrode (5 mM HEPES [pH 7.4], 137 mM NaCl, 1% dextrose, 2.7 mM KCl, 11.9 mM NaHCO3, 1 mM MgCl2, and 0.1% bovine serum albumin [BSA]) before lysis. For stimulation cells were resuspended in modified mouse Tyrode at a density of 2 × 108/mL. EGTA (ethylene glycol tetraacetic acid) was added to a final concentration of 5 mM to prevent clumping, and cells were stimulated with 2 to 5 U/mL purified human thrombin (Calbiochem, La Jolla, CA) at 37° C for 10 minutes. Stimulation was stopped by centrifugation onto poly-l-lysine–coated slides. Murine bone marrow MKs were enriched through culture of unfractionated bone marrow with recombinant murine thrombopoietin (Calbiochem, San Diego, CA) (3 ng/mL) in RPMI 1640 supplemented with 2 mM glutamine, 10% fetal bovine serum, and antibiotics for 7 to 10 days. After culture, cells were collected, washed, and cytospun onto poly-l-lysine–coated slides. Murine dendritic cells were generated through culture of unfractionated bone marrow in RPMI 1640 supplemented with 2 mM glutamine, 10% fetal bovine serum, antibiotics, recombinant interleukin-4 (IL-4), and granulocyte-macrophage colony-stimulating factor (GM-CSF) (Peprotech, Rocky Hill, NJ) for 5 to 7 days. Phenotype was confirmed by the expression of I-Ab, CD11c, and Gr-1 as detected by fluorescence-activated cell sorting (FACS). Human peripheral blood monocytes and platelets were isolated by elutriation from the blood of healthy donors at the National Institutes of Health (Bethesda, MD) as described.17
Cell culture and transfection
Human embryonic kidney (HEK)293T cells were maintained and transfected as described. Expression plasmids for TLT-1 have been described.7 The murine TLT-1–Fc fusion protein was produced by using polymerase chain reaction (PCR) to amplify the extracellular domain of TLT-1 from the TLT-1 cDNA using the following primers: forward (fwd) 5′-cctagctagctgacagtcatcccgagg-3′ and reverse 5′-cgcggatcctcacgacgtctgaactcgtg-3′. The PCR product and the CD5 vector, described by Winter and Long,18 were then digested with BamHI and NheI, gel purified, and ligated to form CD5-TLT-1–Fc. The completed CD5-TLT-1–Fc cDNA was then used to create TLT-1–Fc TOPO by amplification of the coding region with the primers fwd 5′-accggaagcttctagagatc-3′ and reverse 5′-agatacattgatgagtttggac and, subsequently, TOPO cloning the PCR product into pEF6/V5-HisTOPO (Invitrogen, Carlsbad, CA) according to manufacturer specifications. The BamHI and NheI sites in the primers are bolded. The sequence of the resulting TLT-1 domain was confirmed by DNA sequencing. Fusion protein was produced and purified as described.18 The mouse activating receptor 1 (MAR-1) expression plasmid was a gift from Dr John Ortaldo (NCI-Frederick).
Antibodies
Biotin-conjugated anti-CD11c, biotin-conjugated anti-CD62P, fluorescein isothiocyanate (FITC)–conjugated anti–I-Ab, allophycocyanin (APC)–conjugated streptavidin, and appropriate controls were from PharMingen (San Diego, CA). Antimurine CD62P for immunofluorescence was from R&D Systems (Minneapolis, MN). Antimurine TLT-1 was generated by immunizing rabbits with a fusion protein composed of amino acids 21 to 167 of the TLT-1 Ig domain tagged with polyhistidine for purification (Fusion Antibodies, Belfast, Northern Ireland). Our antihuman TLT-1 was generated by immunizing rabbits with a peptide derived from the membrane proximal region of human TLT-1 (amino acids 228 to 241) (AnaSpec, San Jose, CA). For FACS, anti–TLT-1 was detected using FITC-conjugated goat antirabbit IgG (Jackson Immunoresearch Labs, West Grove, PA). Control antibodies were hyperimmune rabbit antisera that recognize irrelevant proteins.
Microscopy
Cytospun platelets or bone marrow samples were fixed using BD Cytofix/Cytoperm and then blocked in 1 × BD Perm/Wash (BD Biosciences, San Diego, CA). Primary antibody was diluted in Perm/Wash. After antibody incubations, slides were washed in Perm/Wash. Antimurine TLT-1 and antimurine CD62P were visualized without bleed-through by using Alexa Fluor 488 goat antirabbit IgG and Alexa Fluor 633 donkey antigoat IgG (Molecular Probes, Eugene OR), respectively. Controls included slides stained with either primary antibody alone and counterstained with both secondary antibodies, demonstrating that the secondary reagents do not react with one another. Additional controls included slides stained with secondaries without primaries and slides stained with irrelevant hyperimmune rabbit primary antibodies (data not shown). Cells were examined using a Zeiss LSM 510 NLO Inverted Confocal Laser Scanning Microscope equipped with a Plan-Neofluar 100 ×/1.3 oil objective (Carl Zeiss, Jena, Germany). Images were viewed and overlaid using the Ziess LSM Image Browser software. Light microscopy of immunohistochemistry was performed using an Olympus BX60 microscope (Olympus Optical, Tokyo, Japan) equipped with a × 100/1.35 oil immersion lens. Images were obtained using an RT Color Camera (Diagnostic Instruments, Sterling Heights, MI) and analyzed using SPOT Image Acquisition Software (Diagnostic Instruments).
Results
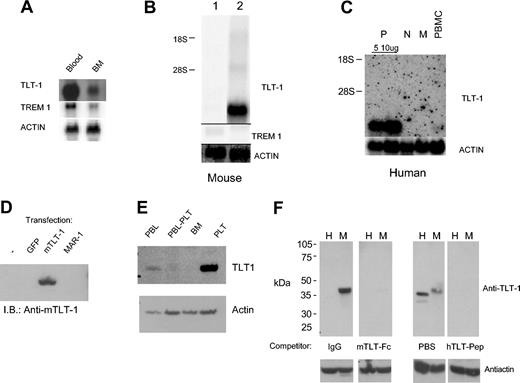
Previous analysis of unfractionated bone marrow by Northern blotting showed mRNA coexpression of TLT-1 and TREM-1.7 To identify TLT-1–expressing leukocytes in the periphery, we extracted RNA from whole blood. This analysis demonstrated an extraordinary level of TLT-1 message relative to bone marrow (Figure 1A). The high levels of TLT-1 mRNA in peripheral blood, together with the lack of TLT-1 mRNA in thymus (and lymph node we reported earlier), suggested TLT-1 might be expressed only within peripheral blood platelets and their bone marrow precursors. We first tested this possibility by comparing TLT-1 and TREM-1 mRNA levels in murine bone marrow–derived dendritic cells and purified platelets. TLT-1 was highly expressed in platelets whereas TREM-1 was only weakly detectable in the dendritic cell cultures (Figure 1B). This strikingly limited expression pattern of TLT-1 was further confirmed by comparison of human peripheral blood mononuclear cells (PBMCs), purified monocytes, purified polymorphonuclear neutrophils (PMNs), and platelets by Northern analysis (Figure 1C). TLT-1 mRNA was detectable only in platelets. This is inconsistent with the apparent high levels of TLT-1 mRNA detected in blood and purified platelets. Therefore, a polyclonal anti–TLT-1 antibody was generated against the extracellular domain of murine TLT-1. This antibody reacts well with TLT-1 expressed in HEK293T cells but not in transfection controls or cells expressing MAR-1, a receptor with homology to TREMs and TLT-1 (Figure 1D). Western analysis of TLT-1 in blood leukocytes (Figure 1E) demonstrated a low level of TLT-1 (Figure 1E, lane 1) with an apparent mass of 38 to 40 kDa that was eliminated when platelets were removed by slow-speed centrifugation (Figure 1E, lane 2). Unfractionated bone marrow cells (Figure 1E, lane 3) also showed no detectable TLT-1 (Figure 1E, lane 3), but lysate derived from enriched platelets showed readily detectable levels of TLT-1 (Figure 1E, lane 4). Probing this filter with antiactin confirmed near equal protein loading (Figure 1E, lower panel). Immunohistochemistry of peripheral blood leukocytes confirmed TLT-1 expression only in platelets (data not shown).
Expression of TLT-1 in platelets. (A) Northern analysis of mRNA isolated from mouse peripheral blood (lane 1) or bone marrow leukocytes (lane 2). Probes are as indicated. (B) Northern analysis of mRNA from mouse dendritic cell cultures (lane 1) and platelets (lane 2). (C) Northern analysis of mRNA from human blood platelets (P, either 5 or 10 μg total RNA loaded), PMNs (N), monocytes (M), or unfractionated PBMCs as indicated. Probes were as indicated. (D) Western blot analysis of lysates from HEK293T cells transfected as indicated immunoblotted with anti–TLT-1. (E) Whole cell lysates from murine peripheral blood leukocytes (PBL), PBLs cleared of platelets (PBL-PLT), bone marrow leukocytes (BM), or enriched platelets (PLT) were immunoblotted with anti–TLT-1 (top) followed by antiactin (bottom). (F) A total of 30 μg whole cell lysate from human (H) or murine (M) platelets was resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Quadruplicate filters were probed with either antimurine TLT-1 (left panels) or antihuman TLT-1 (right panels). Antimurine TLT-1 was competed with either human IgG or a fusion protein composed of the extracellular domain of TLT-1 fused to the Fc portion of human IgG. The antihuman immunoblot was competed with PBS or the peptide immunogen. All 4 filters with then washed and bound antibody detected using goat antirabbit IgG coupled to horseradish peroxidase (HRP). The same filters were then stripped and reprobed with antiactin to show loading (bottom panels).
Expression of TLT-1 in platelets. (A) Northern analysis of mRNA isolated from mouse peripheral blood (lane 1) or bone marrow leukocytes (lane 2). Probes are as indicated. (B) Northern analysis of mRNA from mouse dendritic cell cultures (lane 1) and platelets (lane 2). (C) Northern analysis of mRNA from human blood platelets (P, either 5 or 10 μg total RNA loaded), PMNs (N), monocytes (M), or unfractionated PBMCs as indicated. Probes were as indicated. (D) Western blot analysis of lysates from HEK293T cells transfected as indicated immunoblotted with anti–TLT-1. (E) Whole cell lysates from murine peripheral blood leukocytes (PBL), PBLs cleared of platelets (PBL-PLT), bone marrow leukocytes (BM), or enriched platelets (PLT) were immunoblotted with anti–TLT-1 (top) followed by antiactin (bottom). (F) A total of 30 μg whole cell lysate from human (H) or murine (M) platelets was resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Quadruplicate filters were probed with either antimurine TLT-1 (left panels) or antihuman TLT-1 (right panels). Antimurine TLT-1 was competed with either human IgG or a fusion protein composed of the extracellular domain of TLT-1 fused to the Fc portion of human IgG. The antihuman immunoblot was competed with PBS or the peptide immunogen. All 4 filters with then washed and bound antibody detected using goat antirabbit IgG coupled to horseradish peroxidase (HRP). The same filters were then stripped and reprobed with antiactin to show loading (bottom panels).
Because of the extraordinary expression pattern of TLT-1, we further confirmed the specificity of our finding by examining TLT-1 expression in human and murine platelets in parallel. Quadruplicate filters were probed with either antimurine TLT-1 or antihuman TLT-1 in the presence or absence of competitors for the antisera. Antimurine TLT-1 was competed with either phosphate-buffered saline (PBS) containing human IgG or PBS containing a fusion protein composed of the extracellular domain of TLT-1 fused to the Fc portion of human IgG. The antihuman immunoblot was competed with PBS or the peptide immunogen. Because the antimurine TLT-1 was generated against the extracellular domain of TLT-1, it does not cross-react with human TLT-1. In contrast, the antihuman TLT-1 was generated against a peptide that differs from the murine sequence by only 3 amino acids. Therefore, the antihuman antibody detects murine TLT-1 but to lesser extent than it detects human TLT-1. The smaller apparent molecular weight of human TLT-1 is highly consistent but at present unexplained.
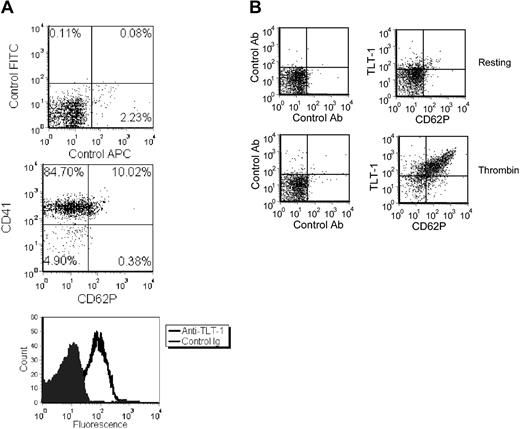
The potential for TLT-1 expression on the surface of freshly isolated murine peripheral blood platelets was next determined by flow cytometry. Platelets were identified by their light scatter properties and their expression of CD41. Their resting phenotype was confirmed by the relative lack of CD62P expression.19,20 Staining with TLT-1 revealed only a modest shift, suggesting all platelets have a low level of surface TLT-1 (Figure 2A). Control sera showed no staining. Similarly, flow cytometric analysis of bone marrow leukocytes detected no appreciable surface TLT-1 (data not shown). Together these data define TLT-1 as the first putative inhibitory receptor to be expressed exclusively in platelets.
Regulation of surface TLT-1 by thrombin. (A) Mouse platelets were stained with anti-CD41 or anti-CD62P as indicated and analyzed by FACS. Percentage of events in each quadrant is indicated. (B) Resting (left panels) or thrombin-stimulated (right panels) platelets were stained with anti-CD62P and anti–TLT-1 as indicated and analyzed by FACS.
Regulation of surface TLT-1 by thrombin. (A) Mouse platelets were stained with anti-CD41 or anti-CD62P as indicated and analyzed by FACS. Percentage of events in each quadrant is indicated. (B) Resting (left panels) or thrombin-stimulated (right panels) platelets were stained with anti-CD62P and anti–TLT-1 as indicated and analyzed by FACS.
Therefore, we asked whether activation of platelets with thrombin would result in heightened TLT-1 surface expression. Figure 2B shows a massive increase in TLT-1 on the platelet surface after 10 minutes of stimulation with thrombin. Similar results were found following stimulation with the platelet agonists collagen or, to a lesser extent, adenosine diphosphate (ADP) (data not shown). Moreover, the increase in TLT-1 appeared to parallel the surface expression of CD62P over a variety of stimulation dosages and times (data not shown). Together, these data suggest that TLT-1, similar to CD62P, might be sequestered within platelet granules. Detection of an agonist by the platelet would then result in rapid translocation of both TLT-1 and CD62P to the platelet surface.
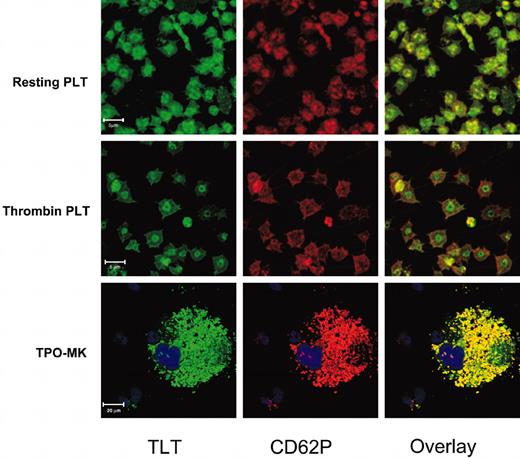
The apparent concurrent translocation of TLT-1 and CD62P suggested that α-granules would be the most likely candidates for the intracellular pools of TLT-1. To address if TLT-1 was stored in the α-granules, resting platelets, or those first stimulated with thrombin, were fixed, permeabilized, and stained with combinations of anti-TLT and anti-CD62P and analyzed using confocal microscopy. Figure 3 shows representative confocal images of resting and thrombin-activated platelets stained with anti–TLT-1 (green) and anti-CD62P (red). Both CD62P and TLT-1 staining exhibit relatively low background fluorescence with strong granular staining. Overlay of the images shows a high degree of colocalization, suggesting that TLT-1 is localized within the α-granule along with CD62P. The identity of TLT-1–containing granules as α-granules was further confirmed by staining platelets from ruby-eye mice. These have defective platelet-dense granules due to defects in granule packaging and/or production machinery.21 There was no appreciable effect of this mutation of TLT-1 distribution (data not shown). Our studies on human platelets show similar results to the murine platelets, confirming TLT-1's expression in human platelets. Upon stimulation, most CD62P quickly moves to the periphery of the cell, consistent with the dramatic increase in CD62P staining by FACS demonstrated in Figure 2B. In contrast, although the granular staining of TLT-1 is clearly gone, TLT-1 protein is often retained in the central portion of the platelets as if demarcated by the contracted marginal band (Harrison and Cramer22 and references therein). The degree to which TLT-1 is retained in this fashion is variable and may reflect the intensity of the activation signal and/or other parameters of the stimulation.
Localization of TLT-1 to platelet and MK α-granules. Resting (top row) or thrombin-stimulated (middle row) platelets or in vitro–derived MKs (bottom row) were permeabilized and then stained with anti–TLT-1 (left column, green) and anti-CD62P (middle column, red) followed by a combination of Alexa 488–conjugated antirabbit and Alexa 633–conjugated antigoat antibodies. The right column is an overlay of the left and middle images.
Localization of TLT-1 to platelet and MK α-granules. Resting (top row) or thrombin-stimulated (middle row) platelets or in vitro–derived MKs (bottom row) were permeabilized and then stained with anti–TLT-1 (left column, green) and anti-CD62P (middle column, red) followed by a combination of Alexa 488–conjugated antirabbit and Alexa 633–conjugated antigoat antibodies. The right column is an overlay of the left and middle images.
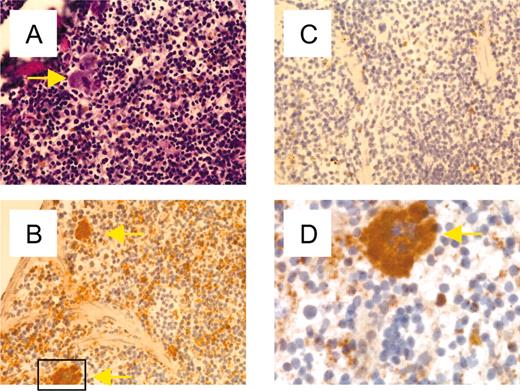
Platelets are derived from MKs; however, platelet granular content can be derived either from plasma pools via pinocytosis or endocytosis or by production and granule packaging by either the MK or platelet.22 Immunohistochemisty of mouse spleen (Figure 4) confirmed the lineage specificity of TLT-1 and showed high levels of TLT-1 only in splenic MKs and platelets of the red pulp. Together with the levels of TLT-1 message detectable in bone marrow, this finding suggested MKs as the source of platelet TLT-1. We confirmed this hypothesis by performing confocal microscopy to localize TLT-1 and CD62P in primary bone marrow–derived MKs and MKs derived via in vitro culture with thrombopoietin (TPO). Culture of unfractionated bone marrow in TPO for 7 to 10 days results in the appearance of large immature MKs. These cells are 50 to 75 μm in diameter and contain abundant α-granules and large multilobular nuclei but do not produce proplatelets under these culture conditions.23 The results shown in Figure 3 demonstrate that these early MKs also exhibit significant colocalization of TLT-1 and CD62P, suggesting that TLT-1 has already been packaged into the α-granules at this stage of thrombopoeisis. Staining of primary bone marrow with anti–TLT-1 demonstrated large clouds of prepackaged, TLT-1–positive granules surrounding a large multilobular nucleus, further supporting a model where TLT-1 is produced and packaged within MKs.
Expression of TLT-1 in splenic MKs and platelets. Frozen serial sections of murine spleen were fixed and stained with hematoxylin and eosin (A) or anti–TLT-1 followed by horseradish peroxidase–conjugated antirabbit antibody (B) or secondary antibody alone (C). (A-C) Magnification × 200. (D) Enlargement of the approximate area demarcated in panel B. MKs are marked by yellow arrows.
Expression of TLT-1 in splenic MKs and platelets. Frozen serial sections of murine spleen were fixed and stained with hematoxylin and eosin (A) or anti–TLT-1 followed by horseradish peroxidase–conjugated antirabbit antibody (B) or secondary antibody alone (C). (A-C) Magnification × 200. (D) Enlargement of the approximate area demarcated in panel B. MKs are marked by yellow arrows.
Discussion
Given the growing body of evidence suggesting regulation of both the innate and adaptive immune response by members of the TREM family, our finding that TLT-1 is platelet and megakaryocyte specific in both humans and mice is surprising. Our results make TLT-1 the first gene within the TREM cluster to be expressed within a single lineage. In addition, TLT-1 becomes only the second inhibitory receptor to be described in platelets, the other being platelet endothelial cell adhesion molecule (PECAM).24 PECAM, however, is also expressed in endothelial cells. Our analysis of multiple murine endothelial cell lines showed no substantial TLT-1, and immunostaining of primary murine lung endothelial cells yielded cells that were CD62P+ and had clear von Willebrand factor containing Weibel-Palade bodies, but these cultures had no cells exhibiting TLT-1 staining over control values. In addition, our immunohistochemistry did not detect significant levels of TLT-1 in endothelium (data not shown). Taken together, this would make TLT-1 the only platelet-specific inhibitory receptor described to date. Moreover, TLT-1's specificity makes it a good marker for megakaryocytes and platelets.
Platelets are highly reactive cells that carry large quantities of both soluble and cell-bound cargo in 2 principal types of secretory granules, α-granules and dense granules (reviewed by Rendu and Brohard-Bohn25 ). These granules sequester highly reactive compounds and/or receptors that are only made available when the platelet is stimulated with indicators of vascular damage.25 Within seconds of exposure to an agonist such as thrombin or collagen, platelets undergo a dramatic change in cellular morphology, including contraction of the platelet marginal band toward the center of the cell and the formation of adhesive pseudopods (reviewed by Harrison and Cramer22 and George26 ). Simultaneously, there is a release of granule contents resulting in dramatic increases in cell surface expression of several receptor proteins; prominent among these is the platelet selectin, CD62P, and now TLT-1. The high levels of CD62P facilitate the tethered rolling required for additional platelet receptors to further interrogate the vascular wall.25 Our demonstration of cocompartmentalization and display of TLT-1 and CD62P suggests that, like CD62P, TLT-1 may play an important role in the adhesion of activated platelets to endothelium or one another. In addition, this tight regulation of the bioavailability of granule proteins like TLT-1 may explain why our work is the first to demonstrate TLT-1 in platelets despite multiple platelet proteomic studies.27,28 Based on our previous data showing the ability of the cytoplasmic domain of TLT-1 to recruit SHP-1, we suggest that TLT-1 might dampen the platelet aggregation response or perhaps play a role in reversible platelet adhesion.
We demonstrate here that stimulation of platelets with thrombin or collagen causes dramatic redistribution of both TLT-1 and CD62P; however, even when CD62P is seen to fully redistribute, TLT-1 is often retained in the central portion of the cell in a distinct ring or disc pattern. This staining pattern is reminiscent of the pattern seen when activated platelets are stained with phalloidin or tubulin, suggesting that TLT-1 is retained within, or in proximity of, the contracting marginal band.29 In light of this difference in subcellular location after activation, it is interesting that although both TLT-1 and CD62P are both packaged into α-granules by the MK sorting machinery, the cytoplasmic tails of the 2 receptors vary greatly. Whereas the cytoplasmic domain of CD62P is only 35 residues, perhaps just enough to target it to the granule and anchor the receptor for its role as an adhesion molecule, that of TLT-1 is 118 residues long and contains multiple potential protein-protein interaction domains.7,30 Whether the difference in distribution reflects a more prominent role for TLT-1 in the production, movement, or regulation of granule function as opposed to serving strictly as granule cargo, as is the case for CD62P, is currently under investigation. Interestingly, studies utilizing the pearl mutation affecting AP3 function have suggested the existence of myeloid lineage–specific machinery for packaging granule cargo such as CD62P.30 It is tempting, therefore, to speculate that the extensive cytoplasmic tail of TLT-1 may be a reflection of a role for TLT-1 in MK-specific granule formation and sorting.
In addition to packaging proteins produced in the MK or platelet, α-granules contain proteins captured from the plasma via either fluid phase endocytosis (ie, IgG and albumin) or receptor-mediated endocytosis.22,31 Examples of the latter include fibrinogen, endocytosed by platelet glycoprotein IIbIIIa, and clotting factor V (fV), which is endocytosed via an as yet unknown receptor.32,33 Upon platelet activation, a highly reactive platelet surface facilitates thrombin formation by providing lipid cofactors and facilitating high local concentrations of clotting factors including fVIII, fVIIIa, fX, fXa, fV, and fVa.31 These observations suggest the possibility that TLT-1 may serve to capture, package, and/or sequester intermediates of thrombin formation in anticipation of activation. Upon activation, TLT-1 along with its cargo are rapidly made available at the cell surface. Efforts to identify the TLT-1 ligand are underway and may suggest further studies of these possibilities.
Regardless of whether TLT-1 functions to promote or direct granule construction or trafficking, to promote thrombus formation at the surface of activated platelets, or to dampen platelet function via its intracellular immune receptor tyrosine-based inhibitory motif (ITIM), a full elucidation of TLT-1's role in platelet biology should open inroads into our understanding of the maintenance of vascular homeostasis.
Prepublished online as Blood First Edition Paper, April 20, 2004; DOI 10.1182/blood-2004-01-0315.
By acceptance of this article, the publisher or recipient acknowledges the right of the US government to retain a nonexclusive, royalty-free license in and to any copyright of the article. The content of this publication does not necessarily reflect the views or policies of the department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr Rivka Rachel, Dr Nancy Jenkins, and Dr Neil Copland of NCI-Frederick for ruby-eye mice.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal