Abstract
Evidence has been mounting for peripheral functions for tachykinins, a family of neuropeptides including substance P (SP), neurokinin A, and neurokinin B, which are recognized for their roles in the central and peripheral nervous system. The recent discovery of 4 new members of this family, the endokinins (EKA, B, C, and D), which are distributed peripherally, adds support to the notion that tachykinins have physiologic/endocrine roles in the periphery. In the present study we report a fundamental new function for tachykinins in the regulation of platelet function. We show that SP stimulates platelet aggregation, and underlying this is the intracellular mobilization of calcium and degranulation. We demonstrate the presence of the tachykinin receptors NK1 and NK3 in platelets and present evidence for the involvement of NK1 in SP-mediated platelet aggregation. Platelets were found to contain SP-like immunoreactivity that is secreted upon activation implicating SP-like substances in the autocrine/paracrine regulation of these cells. Indeed, NK1-blocking antibodies inhibited aggregation in response to other agonists. Of particular note is the observation that EKA/B cross-react in the SP immunoassay and are also able to stimulate platelet activation. Together our data implicate tachykinins, specifically SP and EKA/B, in the regulation of platelet function.
Introduction
Platelets form a first line of defense upon injury through their critical role in hemostasis. Through interactions with subendothelial extracellular matrix proteins, platelets adhere to damaged tissue and then spread to form a monolayer of cells to cover the area of damage. The initial entrapment of platelets is mediated by an indirect interaction between platelets and exposed collagen, via the plasma protein von Willebrand factor (VWF), that is able, under high shear conditions, to bind to the platelet glycoprotein Ib-V-IX (GPIb-V-IX) complex and collagen. This interaction is transient and is superceded by more stable interaction with the platelet collagen receptors GPVI and integrin α2β1.1,2 The ensuing cell signaling that is stimulated principally by collagen and VWF, and by thrombin, which is generated at the injury site, results in the secretion by platelets of an array of prothrombotic factors that are able to signal to and activate approaching platelets.3-7 These include adenosine diphosphate (ADP) and 5-hydroxytryptamine (5-HT) in addition to the synthesis and release of thromboxane A2.8 Platelet activation also leads to the up-regulation of affinity of the platelet receptor for the plasma protein fibrinogen, integrin αIIbβ3, which results in fibrinogen-mediated platelet aggregation and the development of a stable thrombus. A combination of these positive feedback systems, synergism between agonists, and functional redundancy ensures a rapid and effective response.
Recent reports have identified members of the tachykinin family of neuropeptides to have peripheral functions, most notably in the pathology of pre-eclampsia.9-11 Since a major complication of this disorder is platelet pathology, this led us to examine a potential role for tachykinins in the regulation of platelet function. The tachykinins comprise a family of short peptides that share the C-terminal sequence Phe-X-Gly-Leu-Met-NH2 (where X denotes an aromatic or branched aliphatic amino acid residue) and have established roles as neurotransmitters in the central and peripheral nervous systems. Members of this family include substance P (SP), neurokinin A (NKA), and neurokinin B (NKB). Recently in humans this family has been extended to include 4 new members named the endokinins (EKA, EKB, EKC, and EKD),12 and a C-terminal 11-mer fragment of endokinin A has been independently identified as a hemospecific tachykinin and alternatively named hemokinin-1 (HK-1).11,13 The mRNA for endokinin precursors has been detected in a range of peripheral tissues and cell types.12,13 In particular TAC4 and not TAC1 expression was found in the placenta. The physiologic functions of the endokinins are uncertain, although EKA/B (HK-1) have been shown to cause hemodynamic effects in rats,12 and shown to play an important role in T- and B-cell lymphopoiesis.11,14 Tachykinins are believed to exert most of their actions via the 3 tachykinin receptors that have been cloned and characterized: NK1, NK2, and NK3, which bind preferentially to SP, NKA, and NKB, respectively, although each of these peptides is able to bind each of the receptors albeit at a lower affinity.15 Non-NK receptor–mediated effects of NKB and SP, however, have also been reported.10,16
The biologic effects of tachykinins have been essentially described on smooth muscle and in the nervous system,17 however, additional properties suggest that tachykinins are also involved in the periphery in immunologic and inflammatory processes.18 A possible role has also been suggested in platelet function. Gudat et al19 demonstrated reversible shape change reactions of rabbit platelets caused by SP but not by other basic peptides, suggesting a possible membrane receptor for SP on platelets.
We therefore sought to investigate the effects of tachykinins on human platelet function. In this study we show that SP can fully activate human platelets and that SP-like immunoreactivity is released from platelets following activation. We demonstrate the presence of at least 2 of the known mammalian tachykinin receptors on human platelets and show that NK1 partially mediates the response of platelets to SP. At the outset of this project, SP was the only known tachykinin found to stimulate platelet activation. With the recent characterization of the endokinins, we have begun to investigate the effects of these peptides on platelet regulation. We present data to show that EKA/B may be important players in tachykinin-mediated regulation of these cells. We believe these findings point to a fundamental new peripheral tachykinin-mediated process in the regulation of platelet function.
Materials and methods
Materials
NK receptor antibodies, fluorescein isothiocyanate (FITC)–conjugated secondary antibodies, and phycoerythrin (PE)–conjugated secondary antibodies were obtained from Novus Biologicals (Littleton, CO), Harlan Sera Lab (Loughborough, United Kingdom), and Pharmingen (BD Biosciences, Oxford, United Kingdom), respectively. Horseradish peroxidase–conjugated (HRP) secondary antibodies and enhanced chemiluminescence (ECL) reagents were from Amersham Biosciences (Buckinghamshire, United Kingdom). Tri reagent and Histopaque-1077 were obtained from Sigma (Dorset, United Kingdom). The Access reverse transcription–polymerase chain reaction (RT-PCR) system was supplied by Promega (Southampton, United Kingdom) and agarose, by Gibco (Paisley, United Kingdom). SP was purchased from Neosystems (Strasbourg, France), and the SP Enzyme immunoassay was from Peninsula (St Helens, United Kingdom). EKA/B (GKASQFFGLM-NH2) was synthesized by Dr G. Bloomberg, Department of Biochemistry, Bristol, United Kingdom. Wild-type and NK1 receptor knock-out Sv129 + C57BL/6 mice were obtained from Perlmutter Laboratory (Children's Hospital, Boston, MA) and bred in house.10
Platelet preparation, stimulation, and aggregation assay
Washed platelets were prepared from fresh blood obtained from aspirin-free donors by differential centrifugation as described previously.20 Platelets, resuspended at a density of 4 × 108 cells/mL in modified Tyrode-HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer (134 mM NaCl, 0.34 mM Na2HPO4, 2.9 mM KCl, 12 mM NaHCO3, 20 mM HEPES, 5 mM glucose, 1 mM MgCl2, pH 7.3) were stimulated with agonists in siliconized glass tubes in an optical aggregometer (Chronolog, Havertown, PA). Assays were performed at 37° C and with constant stirring, and aggregation was determined by measurement of optical density of the platelet suspension. Where required, an anti-NK1 antibody was added 2 minutes prior to the addition of agonist, following preincubation for 2 minutes with Fab fragment of monoclonal antibody IV.3 to block the platelet immunoglobulin G (IgG) receptor FcγRIIA.
RNA isolation from platelets
Blood (200 mL) was obtained from drug-free volunteers in the presence of sterile sodium citrate. Acid-citrate-dextrose (ACD, 85 mM Na citrate, 71 mM citric acid, 110 mM glucose) was added to the blood (1:7), which was then decanted into polypropylene tubes and centrifuged at 200g for 20 minutes at room temperature. The upper half of the platelet-rich plasma was aspirated and placed in fresh centrifuge tubes and centrifugation repeated at 200g for 20 minutes. The supernatant was transferred to a fresh centrifuge tube, leaving the pellet and approximately half the supernatant behind. Prostaglandin E2 (50 ng/mL) was added prior to recentrifugation at 1400g for 10 minutes at room temperature. After discarding supernatant, platelet pellet was resuspended in Tri reagent for the extraction of RNA following the manufacturer's instructions.
RNA isolation from leukocytes and HEL cells
Blood was collected as previously described, 15 mL whole blood was layered onto 15 mL Histopaque-1077, and leukocytes were isolated by centrifugation following the manufacturer's instructions. RNA was extracted from washed and pelleted leukocytes and HEL cells using Tri Reagent.
RT-PCR
RT-PCR was performed on RNA preparations using the Access RT-PCR system. RNA was reverse transcribed and subjected to PCR with the control primers β-actin, GPIIb (platelet marker), and HLA-DQB (white blood cell marker) for 40 cycles (94° C for 30 seconds, 55° C for 1 minute, 68° C for 2 minutes) using a thermal cycler. RNA was subjected to PCR with the first set of external primers to NK1, NK2, and NK3 receptors, and subsequent PCR was performed with internal (nested) primers for 30 cycles each (94° C for 30 seconds, 68° C for 1 minute). PCR products were separated on 2% agarose gel and DNA bands visualized under ultraviolet (UV) light. Products were cloned and sequenced to confirm identity. The following primers were used: NK1, 5′ CTGGGCATGGTCTCTGTGGTTGAGTAG; NK1, 3′ GGCTTTACCGCCTAGCTTCGAAATGGA; NK1, 5′ (nested) GGATAACGTCCTCCCGGTGGACTCAGA; NK1, 3′ (nested) CCCTGGGGGAAGGCCAGCAGGAGAGCCAGG; NK2, 5′ATGGGGACCTGTGACATTGTGACTGAAGG; NK2, 3′ CCTGGTCCATGGTGACGGTGGAGTAGA; NK2, 5′ (nested) CTGAGAGCAACACCACGGGCATCACA; NK2, 3′ (nested) GGGGAGGCCAGGGCGAGAGCCACCAGCC; NK3, 5′ GGGACTGCAGACCGGTGGCGATGGCCA; NK3, 3′ CTGTCCACCGCAATGGCCGTCATGGA; NK3, 5′ (nested) CACTCTCCCAGCAGCAGAAACCTGGA; NK3, 3′ (nested) GGCCGTCATGGAGTAGATGCTGGCGAACAC; β-actin, 5′ GGCGTGATGGTGGGC; β-actin, 3′ CGCACGATTTCCCGC; GPIIb, 5′ GCCATCGTGGTGGGC; GPIIb, 3′ CCGCAACTGGAGCC; HLA-DQB, 5′ CCGAGAGGAGTACGC; and HLA-DQB, 3′ GGTGCTCCACGTGGC.
Flow cytometry
Washed platelets (2 × 108 cells/mL; 1 mM EGTA [ethylene glycol-bis(B-aminoethyl ether)-N,N,N′,N′-tetraacetic acid], 10 μM indomethacin) were stimulated for 90 seconds and stopped by adding an equal volume of filtered ice-cold modified Tyrode-HEPES buffer containing 1% (wt/vol) bovine serum albumin (BSA) and 0.02% (wt/vol) sodium azide. Samples were incubated with NK1 or NK3 receptor primary antibody diluted 1:100 on ice for 1 hour, and then incubated with FITC-conjugated antirabbit secondary antibody diluted 1:200. Data were collected using a FACScan flow cytometer (Becton Dickinson, San Jose, CA) and analyzed using CELLQuest software (Becton Dickinson). The platelet IgG receptor FcγRIIA was blocked using a saturating concentration of Fab fragment of monoclonal antibody IV.3, and nonspecific binding was controlled for in replicate samples using an irrelevant IgG in place of the primary antibody. Relative quantification from flow cytometry data was performed using geometric mean data derived from histograms.
Dense granule secretion assay
Platelets were loaded with [3H]5-HT, and [3H]5-HT secretion following stimulation was measured as described previously.20
Measurement of intracellular calcium concentration
Platelet intracellular calcium was measured spectrofluorimetrically in fura2-am–loaded platelets following stimulation as described previously.21
Western blot analysis
Proteins were separated using sodium dodecyl sulfate–polyacrylamide electrophoresis (SDS-PAGE) under reducing conditions and transferred to polyvinylidene difluoride (PVDF) membrane. Membranes were blocked by incubation with 10% (wt/vol) BSA in Tris (tris(hydroxymethyl)aminomethane)–buffered saline–Tween (TBS-T; 20 mM Tris, 137 mM NaCl, 0.1% [vol/vol] Tween 20, pH 7.6) overnight. Membranes were incubated with primary antibody (1:500 dilution) in 2% (wt/vol) BSA in TBS-T for 1 hour at room temperature. After washing 3 × 15 minutes in TBS-T, membranes were incubated with horseradish peroxidase–conjugated anti–rabbit immunoglobulin (1:7500 dilution) in 2% (wt/vol) BSA in TBS-T for 1 hour at room temperature. Following incubation, membranes were washed 3 × 15 minutes in TBS-T. Antibody-antigen complexes were visualized on X-ray film using an enhanced chemiluminescence detection system (ECL).
Mouse platelet preparation and aggregation assay
Blood was collected from NK1-deficient transgenic mice and litter-matched controls (3 months of age; 25 g) under terminal anesthesia by cardiac puncture. Platelets were isolated by differential centrifugation as described previously,22 and the final platelet count was adjusted to 2 × 108/mL using modified Tyrode-HEPES buffer. Platelets (90 μL) were stimulated (10 μL agonist) for 90 seconds, fixed by addition of 100 μL ice-cold 6% (vol/vol) glutaraldehyde, and immediately placed on ice. Single platelet numbers in each sample were determined using a Z2 Coulter particle count and size analyzer (Beckman Coulter, Hialeah, FL) to assess the level of aggregation.
SP enzyme-linked immunoassay
An SP peptide immunoassay kit was used following the manufacturer's instructions. Washed platelets were resuspended to a concentration of 4 × 109 platelets/mL. For detection of total SP levels, 1 mL platelet suspension was snap frozen at –70° C, thawed, and sonicated. For detection of secreted SP, 200 μL platelet suspension was stimulated with convulxin (a GPVI collagen receptor agonist) or thrombin for 90 seconds, platelets were pelleted by centrifugation at 15 000g for 10 minutes at 4° C, and the concentration of SP in the supernatant was measured.
Statistical analysis
Paired t tests, one-way univariate analysis (ANOVA), and 2 factor within subjects ANOVA were performed using SPSS 10.0 for windows after assessing data for normality (SPSS, Chicago, IL).
Results
Substance P stimulates human platelet aggregation
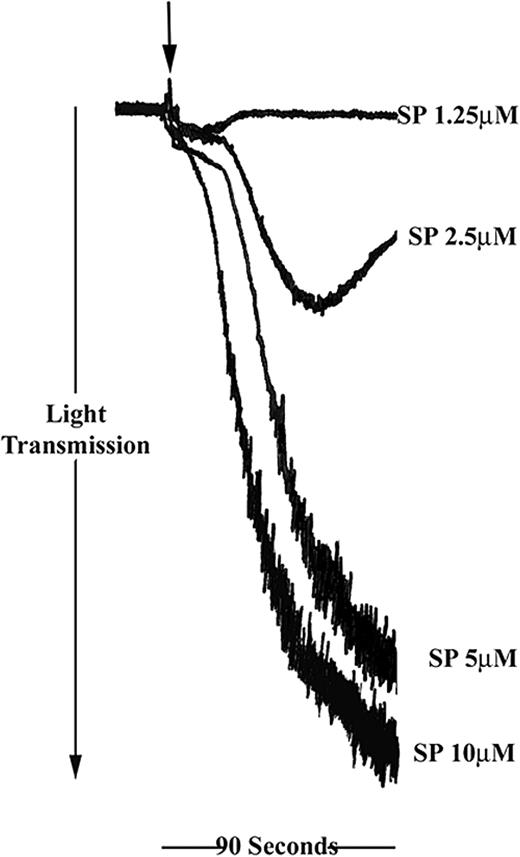
SP was found to induce platelet aggregation in a concentration-dependent manner in a range between 2.5 and 10 μM (Figure 1). Full aggregation, comparable with that stimulated with collagen or thrombin, was achieved between 5 and 10 μM SP. Treatment of platelets with a low concentration of SP (1.25 μM) resulted in a small increase in optical density, which is indicative of platelet shape change. At higher concentrations of SP, shape change responses were not detectable.
Substance P stimulates human platelet aggregation. Washed platelets were stimulated for 90 seconds with SP in the concentration range shown (arrow indicates addition of agonist). Aggregation was monitored by turbidimetric aggregometry at 37° C under stirring conditions. Traces are representative of 5 separate experiments.
Substance P stimulates human platelet aggregation. Washed platelets were stimulated for 90 seconds with SP in the concentration range shown (arrow indicates addition of agonist). Aggregation was monitored by turbidimetric aggregometry at 37° C under stirring conditions. Traces are representative of 5 separate experiments.
Additional markers of platelet activation were assessed to ensure that this phenomenon was not due to agglutination. 5-HT secretion assays were performed to determine if dense granule secretion was stimulated in response to SP. Platelets were preloaded with [3H]-5HT, stimulated with increasing concentrations of SP, and the proportion of total platelet [3H]-5HT secreted into the buffer measured (Figure 2A). Dense granule secretion was stimulated by SP (2.5-20 μM) in a concentration-dependent manner. SP stimulation of platelets was also found to be accompanied by α-granule secretion and increased platelet binding of fibrinogen, determined by flow cytometry using anti–P-selectin (CD62P) antibodies and FITC-labeled fibrinogen, respectively (not shown).
SP stimulates platelet secretion and calcium-dependent signaling. (A) Dense granule secretion was measured using a 5-HT secretion assay at the concentrations of SP shown. Levels of [3H]-5HT secretion are shown as percentage of total cell content following subtraction of release under resting conditions. Values shown are mean percent of total [3H]-5HT released ± SEM (n = 5). *P < .005 with respect to basal; **P < .005 with respect to 2.5 and 5 μM. (B) The mobilization of calcium from intracellular stores was determined at increasing concentrations of SP as described in “Materials and methods.” (C) Platelets loaded with increasing concentrations of BAPTA-am or dimethyl sulfoxide (DMSO) control were stimulated with SP (20 μM) for 90 seconds (arrow indicates addition of agonist). Aggregation was monitored by turbidometric aggregometry at 37° C under stirring conditions. Traces are representative of 3 separate experiments.
SP stimulates platelet secretion and calcium-dependent signaling. (A) Dense granule secretion was measured using a 5-HT secretion assay at the concentrations of SP shown. Levels of [3H]-5HT secretion are shown as percentage of total cell content following subtraction of release under resting conditions. Values shown are mean percent of total [3H]-5HT released ± SEM (n = 5). *P < .005 with respect to basal; **P < .005 with respect to 2.5 and 5 μM. (B) The mobilization of calcium from intracellular stores was determined at increasing concentrations of SP as described in “Materials and methods.” (C) Platelets loaded with increasing concentrations of BAPTA-am or dimethyl sulfoxide (DMSO) control were stimulated with SP (20 μM) for 90 seconds (arrow indicates addition of agonist). Aggregation was monitored by turbidometric aggregometry at 37° C under stirring conditions. Traces are representative of 3 separate experiments.
SP stimulates the intracellular mobilization of calcium in platelets
The existence of tachykinin receptors on human platelets has not been reported, although a number of authors have reported data to suggest that the NK1 receptor may be present on rabbit platelets.17,19,23 The 3 characterized tachykinin receptors, NK1, NK2, and NK3, are members of the G-protein–coupled receptor (GPCR) superfamily and signal via the Gαq/G11 family,15 of which Gαq is known to be present on platelets. Stimulation of these GPCRs activates phospholipase C causing the hydrolysis of phosphatidylinositol-4,5-bisphosphate and generation of the second messengers inositol 1,4,5-trisphosphate (Ins(1,4,5)P3) and diacylglycerol (DAG). Ins(1,4,5)P3 is responsible for the intracellular mobilization of calcium and DAG activates protein kinase C.15,24,25 Experiments were therefore performed using fura2-am–loaded platelets to establish whether SP stimulates the mobilization of calcium from intracellular stores. As shown in Figure 2B, the stimulation of platelets with SP resulted in intracellular calcium mobilization in a concentration-dependent manner. Following an initial apparent dip in Ca2+ level (an optical artifact on addition of agonist in the spectrofluorimeter) calcium levels elevated rapidly, returning to resting levels within 2 minutes. Platelets were loaded with various concentrations of the intracellular calcium chelator, BAPTA-am, and stimulated with SP (20 μM) to determine whether SP-stimulated Ca2+ mobilization is necessary for platelet aggregation. BAPTA-am inhibited aggregation in a concentration-dependent manner, consistent with a role for calcium in SP-stimulated platelet aggregation (Figure 2C). Experiments using platelets loaded with both Fura2-am and BAPTA-am were performed initially to determine concentrations of BAPTA required to sequester calcium in platelets. At the concentration of SP shown (20 μM), inhibition of calcium mobilization correlated closely with the inhibition of aggregation.
At low concentrations SP enhances aggregation responses of platelets to other agonists
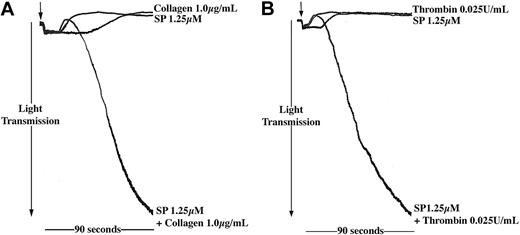
The level of SP to which platelets may be exposed in vivo is uncertain, and this may be lower than the concentrations shown to stimulate aggregation in vitro. We therefore investigated the effects of SP at lower concentrations, which alone were unable to stimulate aggregation, to examine whether SP could act synergistically with other platelet agonists. Preincubation of platelets with subaggregatory concentrations of SP synergized with other agonists such as thrombin and collagen (Figure 3), also present at subthreshold levels, to produce full platelet aggregation responses. Data are presented for platelets treated with collagen (1 μg/mL) and thrombin (0.025 U/mL), and SP at a concentration of 1.25 μM, where a substantial and reproducible synergism was observed. Subthreshold concentrations of collagen, thrombin, and SP are variable between donors and platelet preparations. At concentrations of SP lower than 1.25 μM, synergism was not observed reproducibly.
SP enhances the aggregation response of platelets to other agonists. Washed platelets were stimulated for 90 seconds with SP (1.25 μM) and (A) collagen (1 μg/mL) or (B) thrombin (0.025 U/mL) either alone or in combination (arrow indicates addition of agonist). Aggregation was monitored by turbidometric aggregometry at 37° C under stirring conditions. Traces are representative of 3 separate experiments.
SP enhances the aggregation response of platelets to other agonists. Washed platelets were stimulated for 90 seconds with SP (1.25 μM) and (A) collagen (1 μg/mL) or (B) thrombin (0.025 U/mL) either alone or in combination (arrow indicates addition of agonist). Aggregation was monitored by turbidometric aggregometry at 37° C under stirring conditions. Traces are representative of 3 separate experiments.
Tachykinin receptors are present on human platelets
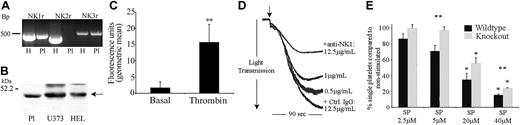
RT-PCR using platelet RNA indicated the presence of NK1 and NK3 mRNA in platelets (Figure 4A). For RT-PCR experiments on platelet RNA, the presence of the platelet-specific gene marker GPIIb and the absence of the leukocyte marker HLA-DQB confirmed that the positive bands obtained using NK receptor oligonucleotide primers were not due to leukocyte contamination (not shown). NK1, NK2, and NK3 receptor mRNA were also detected by RT-PCR on RNA isolated from the megakaryocytic cell line HEL (and shown in Figure 4 as positive controls). Indeed, quantitative RT-PCR indicates that the mRNA for these receptors is present at substantial levels in these cells in comparison with a wide range of other tissues and cell lines (data not shown). The identities of RT-PCR products were confirmed by DNA sequencing.
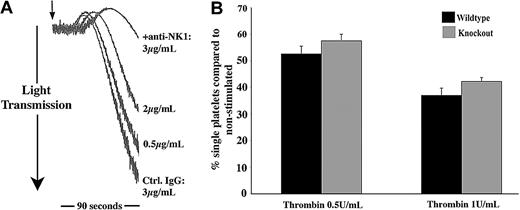
NK1stimulates SP-mediated activation of platelets. (A) RNA isolated from human platelets (Pl; lanes 2, 4, 6) and HEL (H, human erythroleukemia cell line; lanes 1, 3, 5) was subjected to RT-PCR, resulting in the amplification of bands of the sizes 495, 449, and 528 bp representing NK1, NK2, and NK3, respectively. (B) Whole cell lysates from platelets, U373MG, and HEL cells were separated by SDS-PAGE, transferred to PVDF membrane, and immunoblotted to detect NK1. (C) Flow cytometry was used to detect NK1 protein on the surface of unstimulated or thrombin-stimulated (0.1 U/mL) platelets. Relative quantification was calculated using geometric mean data. Values shown are mean ± SEM (n = 3); **P < .02 (t test). (D) Platelets preincubated for 2 minutes with increasing concentrations of anti-NK1 antibody or control antibody (Ctrl) were stimulated with SP (7.5 μM) for 90 seconds (arrow indicates addition of agonist). Aggregation was monitored by turbidometric aggregometry. Traces are representative of 3 separate experiments. (E) Platelets from NK1-deficient mice and litter-matched controls were stimulated for 90 seconds with increasing concentrations of SP. The percentage of single platelets remaining in suspension was determined. Values shown are mean percentage of single platelets compared with basal ± SEM (n = 6). *P < .003 with respect to basal (within); **P < .05 between control and knock out.
NK1stimulates SP-mediated activation of platelets. (A) RNA isolated from human platelets (Pl; lanes 2, 4, 6) and HEL (H, human erythroleukemia cell line; lanes 1, 3, 5) was subjected to RT-PCR, resulting in the amplification of bands of the sizes 495, 449, and 528 bp representing NK1, NK2, and NK3, respectively. (B) Whole cell lysates from platelets, U373MG, and HEL cells were separated by SDS-PAGE, transferred to PVDF membrane, and immunoblotted to detect NK1. (C) Flow cytometry was used to detect NK1 protein on the surface of unstimulated or thrombin-stimulated (0.1 U/mL) platelets. Relative quantification was calculated using geometric mean data. Values shown are mean ± SEM (n = 3); **P < .02 (t test). (D) Platelets preincubated for 2 minutes with increasing concentrations of anti-NK1 antibody or control antibody (Ctrl) were stimulated with SP (7.5 μM) for 90 seconds (arrow indicates addition of agonist). Aggregation was monitored by turbidometric aggregometry. Traces are representative of 3 separate experiments. (E) Platelets from NK1-deficient mice and litter-matched controls were stimulated for 90 seconds with increasing concentrations of SP. The percentage of single platelets remaining in suspension was determined. Values shown are mean percentage of single platelets compared with basal ± SEM (n = 6). *P < .003 with respect to basal (within); **P < .05 between control and knock out.
Western blotting was performed on platelet and HEL cell lysates to confirm the presence of NK1 protein (antibodies specific for NK2 and NK3 for immunoblotting are not presently available), with the human glioblastoma astrocytoma cell line U373 used as a positive control (Figure 4B). NK1 receptor expression on the platelet surface was also confirmed by flow cytometry (Figure 4C). The binding of anti-NK1 receptor antibodies on the surface of resting platelets was detectable at relatively low levels. This binding was, however, increased dramatically (approximately 9-fold, n = 3, P < .02) within 90 seconds following platelet stimulation with an established platelet agonist such as thrombin.
NK1 plays a role in SP-mediated activation of platelets
Of the 3 human tachykinin receptors that have been identified, NK1 is the preferential receptor for SP. Experiments were performed to examine whether the preincubation of platelets with an antibody specific for an external domain of NK1 was able to inhibit platelet aggregation stimulated by SP. Antibody concentration-dependent partial inhibition was observed, where control antibody had no effect, strongly implicating NK1 in the responses of platelets to SP (Figure 4D). To investigate this further, SP responses in mouse platelets that are deficient in NK1 were examined. Platelets from NK1-deficient mice and litter-matched controls were stimulated with increasing concentrations of SP, and the level of aggregation was determined by measuring the number of single platelets remaining in suspension, with a decrease in this number indicating an increase in the level of aggregation. The response of control mouse platelets to SP was found to be very similar to that of human platelets. Platelets from NK1-deficient mice exhibited reduced aggregation in response to SP over a range of concentrations (Figure 4E). As for antibody-mediated inhibition of SP aggregation (Figure 4D), the inhibitory effect was partial (approximately 30%).
Platelets as a store of tachykinins
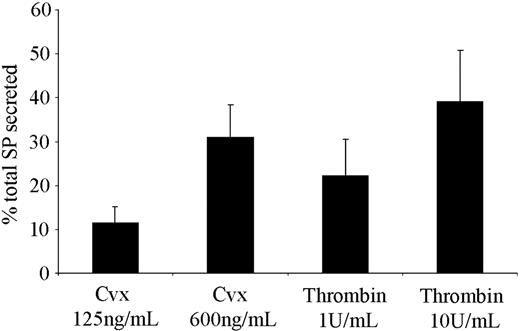
SP is predominantly present in the central and peripheral nervous system, although a number of peripheral sources and functions for this peptide have been proposed.26-28 The observation that platelets respond to SP prompted an investigation of where and how platelets may become exposed to this factor. Using an immunoassay, the presence of SP-like immunoreactivity in human platelets was detected, which is consistent with a report by Nakano et al.29 A degree of variability between platelet donors was observed, with concentrations of 7.08 ± 1.65 ng per 1010 platelets (mean ± SEM, n = 3). Of particular significance is the observation that when stimulated with the GPVI agonist convulxin, or by thrombin, platelets secrete SP-like immunoreactivity in an agonist concentration-dependent manner (Figure 5). The lower agonist concentrations used (convulxin, 125 ng/mL; thrombin, 1 U/mL) produce strong aggregatory responses, although submaximal secretion was observed. Maximal levels of secretion were determined using high concentrations of agonists (convulxin, 600 ng/mL; thrombin, 10 U/mL).
Platelets are able to secrete SP from internal stores following activation. Levels of SP-like immunoreactivity released from platelets were determined following stimulation with the GPVI collagen receptor agonist convulxin (Cvx), and thrombin using an SP immunoassay. Values shown are mean percent of total SP released (7.08 ± 1.65 ng/1010 platelets) ± SEM (n = 3).
Platelets are able to secrete SP from internal stores following activation. Levels of SP-like immunoreactivity released from platelets were determined following stimulation with the GPVI collagen receptor agonist convulxin (Cvx), and thrombin using an SP immunoassay. Values shown are mean percent of total SP released (7.08 ± 1.65 ng/1010 platelets) ± SEM (n = 3).
Tachykinins may function in the positive feedback activation of platelets
The secretion of SP-like immunoreactivity by platelets indicates that SP may contribute to the positive feedback regulation of platelet function. To begin to address this issue, platelets were incubated with NK1-blocking antibodies (as used in Figure 4D) or control antibodies, prior to stimulation of platelet aggregation with other agonists at concentrations that gave relatively weak aggregation responses. NK1-blocking antibodies were found to cause a concentration-dependent inhibition of platelet aggregation of collagen and thrombin (Figure 6A and not shown). With higher concentrations of antibody used, platelet aggregation was inhibited completely. In some experiments, aggregation traces were continued for several minutes to confirm that aggregation was inhibited and not merely delayed (not shown). At higher agonist concentrations, the inhibitory effects of anti-NK1 were overcome. The ability of NK1-deficient mouse platelets to respond to thrombin was also examined. Platelet aggregation responses to a range of thrombin concentrations were moderately diminished (approximately 15%-20%) in comparison with platelets from litter-matched control mice. Data presented in Figure 6B indicate that this is maintained at relatively high thrombin concentrations.
NK1functions in a positive-feedback pathway. (A) Platelets preincubated for 2 minutes with increasing concentrations of anti-NK1 antibody or control antibody were stimulated with collagen (5 μg/mL) for 90 seconds. Aggregation was monitored by turbidometric aggregometry. Traces are representative of 3 separate experiments. (B) Platelets from NK1-deficient mice and litter-matched controls were stimulated for 90 seconds with thrombin at the concentrations shown. The percentage of single platelets remaining in suspension was determined. Values shown are mean percentage of single platelets cf basal ± SEM (n = 6).
NK1functions in a positive-feedback pathway. (A) Platelets preincubated for 2 minutes with increasing concentrations of anti-NK1 antibody or control antibody were stimulated with collagen (5 μg/mL) for 90 seconds. Aggregation was monitored by turbidometric aggregometry. Traces are representative of 3 separate experiments. (B) Platelets from NK1-deficient mice and litter-matched controls were stimulated for 90 seconds with thrombin at the concentrations shown. The percentage of single platelets remaining in suspension was determined. Values shown are mean percentage of single platelets cf basal ± SEM (n = 6).
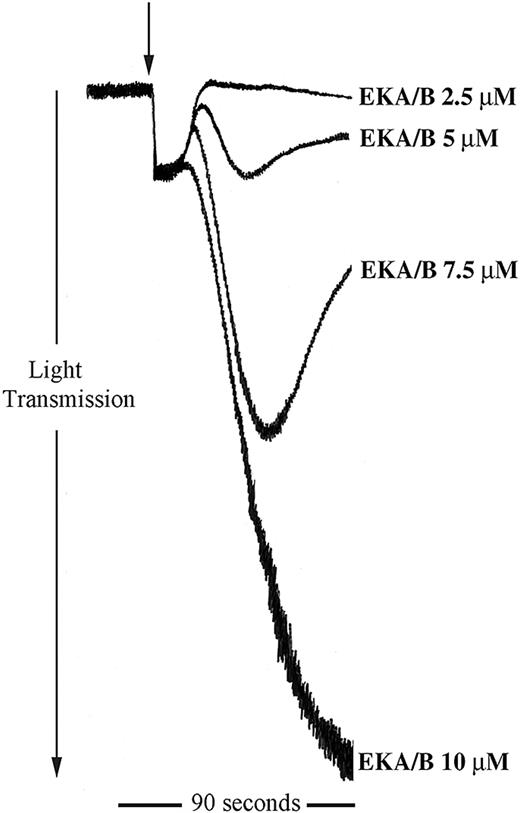
EKA/B stimulates platelet aggregation
The data described clearly show a potential regulatory role for tachykinins in platelet function. Being peripherally distributed tachykinins, the endokinins are more likely candidates for the stimulation of platelet tachykinin receptors in the periphery. We examined the ability of the endokinins to stimulate platelet aggregation. Endokinins A and B are peptides that have the identical C-terminal amidated hexapeptide sequence to SP with a similar potency at NK1 receptors.12 The 10-mer C-terminal EKA/B peptide was also found to stimulate platelet aggregation in a concentration-dependent manner and within a similar concentration range to SP (Figure 7). EKC and EKD, however, which are unable to bind the known tachykinin receptors,12 have no stimulatory effect on human platelets. In addition, EKA/B were found to display approximately 25% cross-reactivity in the immunoassay for SP used in this study (not shown).
EKA/B stimulates platelet aggregation. Washed platelets were stimulated for 90 seconds with EKA/B in the concentration range shown (arrow indicates addition of agonist). Aggregation was monitored by turbidometric aggregometry. Traces are representative of 5 separate experiments.
EKA/B stimulates platelet aggregation. Washed platelets were stimulated for 90 seconds with EKA/B in the concentration range shown (arrow indicates addition of agonist). Aggregation was monitored by turbidometric aggregometry. Traces are representative of 5 separate experiments.
Discussion
Platelet aggregation plays an essential role in hemostasis and thrombosis. Following adhesion to the subendothelium, activation events lead to platelet aggregation and stable thrombus formation. This response is aided through positive feedback mechanisms brought about by the release of secondary mediators from activated platelets. In this study we present data that indicate that tachykinins, namely SP and EKA and B, are platelet agonists that may contribute to the activation of platelets during the process of thrombus formation.
We report that substance P is able to fully activate human platelets in a manner comparable with that seen with other characterized potent platelet agonists such as thrombin and collagen. SP stimulates platelet aggregation, dense- and α-granule secretion, and an increase in integrin αIIbβ3 affinity. Our results are consistent with the observation that tachykinins, most particularly SP, can stimulate shape change in rabbit platelets.17 Gecse et al30,31 reported that SP could decrease the synthesis of thromboxane A2 by platelets at picomolar concentrations. They also reported, however, an increased formation of thromboxane A2 with higher concentrations of SP (nanomolar range). Consistent with this, we have found that when the cyclooxygenase inhibitor indomethacin is included in assays to measure SP-stimulated α-granule secretion, a modest reduction in secretion is observed. The potential effects of tachykinins on human platelets have not, however, been previously examined.
The stimulation of platelets with SP was found to cause concentration-dependent mobilization of calcium from intracellular stores. The preincubation of platelets with the intracellular calcium chelator BAPTA-am, at concentrations found to chelate SP-mediated calcium mobilization, resulted in the inhibition of SP-mediated aggregation, consistent with an important role of calcium mobilization for aggregation in response to a range of agonists. The calcium response to SP was rapid and was similar in profile to that obtained with thrombin (not shown), which signals in part through a Gαq-coupled receptor, protease-activated receptor-1 (PAR1).32 The 3 known mammalian tachykinin receptors have also been shown to couple to Gαq, and their activation leads to increased intracellular calcium concentrations. Secondary platelet activation as a consequence of platelet secretion upon stimulation with SP may contribute to the calcium mobilization response and the effects that are inhibited by BAPTA-am, although this has not been specifically addressed in this study.
In the present study a number of approaches were used to examine whether tachykinin receptors are present in platelets. RT-PCR analysis revealed NK1 and NK3 mRNA to be present in these cells. The presence of mRNA in platelets is unlikely to reflect protein production by platelets but reflects gene expression in the megakaryocyte. All 3 receptor species were found to be present in the megakaryocytic cell line HEL, and this has been confirmed using quantitative RT-PCR. The presence of the NK1 protein was also detected by Western blotting, and flow cytometry analysis confirmed that both NK1 and NK3 are present in human platelets. Low levels are seen on the surface of resting platelets with a rapid and dramatic increase (approximately 9-fold) in surface exposure following activation (Figure 4). This rapid response suggests the presence of intracellular stores of NK receptors, possibly inside α- or dense-granules, that undergo exocytosis upon platelet activation. This is a feature that is shared with a number of platelet receptors such as platelet endothelial cell adhesion molecule 1 (PECAM-1),33 P-selectin,34 and integrin αIIbβ3.
Of the known tachykinin receptors, NK1 has the highest affinity for SP, although SP is able to activate each of the receptors. Experiments were performed to determine whether the effects of SP on platelets are mediated though NK1. The blocking of NK1 on human platelets using a specific antibody resulted in partial, but not complete, inhibition of aggregation. Similarly, aggregation in response to SP was partially reduced in NK1-deficient mouse platelets. Taken together these data indicate that while NK1 is involved in the response of platelets to SP, this receptor is not essential. It is therefore likely that SP mediates its effects through more than one tachykinin receptor. This may also explain why resting platelets that possess relatively low levels of NK1 on the cell surface are able to respond to SP. The most likely candidate second receptor is NK3, which we have also shown to be present in platelets; although we cannot rule out the potential involvement of non-NK receptor–mediated effects of tachykinins on platelets, as has been reported in other cell types and tissues.10,16 Indeed, the stimulation of mast cell degranulation by micromolar concentrations of SP has been reported to involve rapid receptor-independent peptide internalization and direct stimulation of cell signaling.16
At sites of injury, platelets are activated by a number of agonists, some primary and some secondary agonists, that act in concert to ensure a rapid response. The concentrations of SP required to stimulate full platelet aggregation are relatively high, and the levels to which platelets may be exposed in vivo in the vicinity of a developing thrombus are presently unclear. It is probable that this concentration would be substantially higher than levels in systemic plasma samples. It is interesting to note in in vivo and ex vivo models that for the actions of tachykinins, such as microvascular plasma extravasation and T-cell precursor proliferation systems, micromolar concentrations of substance SP are also required.10,14 Furthermore the effects of SP on mast cell degranulation are also seen at concentrations similar to those used in this study.16 Work is in progress to develop immunoassays that distinguish between tachykinins, which will enable us to address this question more fully.
Since we cannot presently be certain of the physiologic concentrations of SP at sites of blood vessel injury, the ability of SP at lower concentrations to act synergistically with other agonists was therefore also investigated. Platelets were preincubated with SP at subthreshold concentrations that did not stimulate platelet aggregation and then stimulated with a range of known agonists. SP was found, down to a concentration of 1.25 μM in this assay system, to enhance activation caused by other platelet agonists and indicated an ability to prime platelets or synergize with other platelet agonists.
In this study we demonstrate that SP-like immunoreactivity is released from platelets upon activation, suggesting an autocrine/paracrine role in platelet regulation. We have begun to examine this notion, and we have shown that NK1-blocking antibodies inhibit platelet aggregation in response to collagen and thrombin, used at concentrations that stimulate weak aggregation responses. Aggregation experiments using NK1-deficient mouse platelets also support this, although in this model less substantial inhibition (approximately 15%-20%) was observed but maintained at relatively high agonist concentrations. More detailed future studies in mouse and human platelets are required to examine this further.
While we, and others,29 have shown that platelets contain SP, at the present time the source of platelet SP is unclear. Platelets possess a mechanism to enable the uptake and storage of 5-HT; although the existence of such a mechanism for tachykinins has not been shown, SP has been reported to be rapidly internalized into mast cells.16 The peripheral production of SP has been proposed in a number of cell types, particularly cells of the immune system such as T lymphocytes,26 monocytes, macrophages,27 and eosinophils.28 The fact that most leukocytes also possess NK1 receptors indicates that SP may be involved in paracrine/autocrine regulation of immune function. Indeed, dendritic cells have recently been shown to enhance T-cell proliferation via endogenously produced and secreted SP.35 The detection of mRNA for TAC1, the precursor for SP, in HEL cells12 may suggest that platelet SP is derived from megakaryocytes. Furthermore, we have repeated the expression survey we reported recently12 and have found that HEL cells contain substantially higher levels (> 10-fold) of TAC4 mRNAs (the endokinin transcripts) than a wide range of other cell types and tissues. This raises the possibility that platelets may also be a source of endokinins.
We therefore examined the effects of the endokinins on human platelets. EKA/B, a 10-mer peptide common to the C-termini of endokinin A and B (and HK-1), stimulated very similar functional effects on platelets to SP, and in the same peptide concentration range. Conversely, endokinins C and D, which have been reported to be unable to bind NK1, NK2, or NK3, do not stimulate platelet activation. The peripheral source of EKA and B that may regulate hemostasis is uncertain. We have, however, determined that EKA/B cross-reacts (approximately 25%) in the SP immunoassay that was used in this study to measure SP secretion from platelets. While this is of concern with regard to the many other studies in which this assay has been used, in the present context it indicates that platelets may also contain EKA and/or B, which is secreted upon activation. This notion is also consistent with the presence of TAC4 mRNA in the megakaryocytic cell line HEL. Work is currently under way to dissect the individual roles of SP and EKA and B in the regulation of platelet function.
Of particular interest is the physiologic role that SP and/or EKA and B may play in hemostasis. The observation that SP and potentially EKA and B are released from platelets following activation, and their potential role in a positive feedback mechanism, indicates that platelet-derived tachykinins may play a role in the regulation of thrombus formation. Future work is required to address the relative contributions of SP/EKA/B in the regulation of platelets to determine the peripheral distributions and concentrations of these tachykinins and to establish the importance of this mechanism for hemostasis.
Prepublished online as Blood First Edition Paper, May 6, 2004; DOI 10.1182/blood-2003-11-3979.
Supported by research grants from the Medical Research Council (G.J.G., J.M.G., P.J.L.), the Biotechnology and Biological Sciences Research Council (J.M.G.), and the British Heart Foundation (J.M.G., J.M.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank Eeva Kuisma, Gary Hubbard, Peter Jordan, Tanya Sage, Natasha Barrett, Sarah Jones, and Philip Strange (University of Reading) for useful discussion and technical assistance; Drs Kalwant Authi and Sheila Hassock (King's College, London) for use of equipment; Dr Dermot Reilly (Royal College of Surgeons in Ireland, Dublin) for techniques to prepare RNA from platelets; and Drs Cassian Bon and Mireille Leduc (Institute Pasteur, Paris) for purified convulxin.

![Figure 2. SP stimulates platelet secretion and calcium-dependent signaling. (A) Dense granule secretion was measured using a 5-HT secretion assay at the concentrations of SP shown. Levels of [3H]-5HT secretion are shown as percentage of total cell content following subtraction of release under resting conditions. Values shown are mean percent of total [3H]-5HT released ± SEM (n = 5). *P < .005 with respect to basal; **P < .005 with respect to 2.5 and 5 μM. (B) The mobilization of calcium from intracellular stores was determined at increasing concentrations of SP as described in “Materials and methods.” (C) Platelets loaded with increasing concentrations of BAPTA-am or dimethyl sulfoxide (DMSO) control were stimulated with SP (20 μM) for 90 seconds (arrow indicates addition of agonist). Aggregation was monitored by turbidometric aggregometry at 37° C under stirring conditions. Traces are representative of 3 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/4/10.1182_blood-2003-11-3979/6/m_zh80160465080002.jpeg?Expires=1769124644&Signature=T9fjR73PP2fUm~kPK~fjrTLfP1j7G8Hd6fKAKV4dSnYXW6Q4CCrNJUdH575ib5wgdSfSqTyozNBjz8Rlzqe8PhdAPPthZvsmiBEW2ZwRpVop3HlgJNpklOtyDClWGcqJdThBFxyFu8rwRhV1iIrOJIw4ZM4lTh6fURhAChDuNbPNnndNqdt6FHrMR~U991PxDfJw6sGR5Rbconsc6eOVTLQm7ERiAGMt2zD96JvHqZq-yQC0VA4UxCRZMFa94vGvFzT1vMdlxfSFOSJ4KdnyJzPUGKMgmVIIQUrXFKOrRRmCYoEl4DJPFbnQkw~BT6IGBCr2grMUC-jypkuRiWNXFQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal