Abstract
Interleukin 7 (IL-7) is critical in maintaining thymic-dependent and thymic-independent pathways of T-cell homeostasis. T-cell receptor (TCR) rearrangement excision circles (TRECs) have been used as markers for recent thymic emigrants (RTEs) in assessing human thymic function. To study the thymic and peripheral effects of IL-7 on RTEs, we measured TREC content and peripheral naive T-cell subsets and turnover in IL-7-treated mice. Short-term administration of IL-7 into thymus-intact mice resulted in increased total TREC numbers, consistent with RTE accumulation. Decreases in TREC frequency were attributable to dilution secondary to increased cell turnover. Significantly, IL-7 administration into thymectomized mice resulted in patterns of decreased TREC frequency and increased total TREC number similar to those in IL-7-treated thymus-intact mice. Distinct patterns of naive cell and RTE distribution among peripheral immune organs and altered expression of CD11a were observed following IL-7 treatment in thymus-intact and thymectomized mice. These results demonstrate (1) that total TREC number and not TREC frequency accurately reflects quantitative changes in RTEs; (2) that short-term IL-7 administration results in preferential accumulations of RTEs among peripheral immune organs, accounting for the increase in TRECs in the total peripheral lymphoid pool; and (3) no evidence for regulation of thymic function by short-term IL-7 administration. (Blood. 2004;104:1110-1119)
Introduction
Identifying factors regulating thymic function is critical in developing strategies to enhance and/or preserve immune function in immunodeficient states associated with HIV infection and hematopoietic stem cell transplantation (HSCT). An important parameter of thymic function is the generation of T cells as recent thymic emigrants (RTEs). In the absence of phenotypic markers distinguishing RTEs, measurement of T-cell receptor (TCR) rearrangement excision circles (TRECs) has been developed for quantifying RTEs in humans.1 TRECs are episomal DNA circles generated during TCR rearrangement. Most human TCRα/β+ T cells contain TRECs formed by the rearrangement between the δ-rec and ψJα loci during TCRδ locus deletion and TCR α-chain gene rearrangement.2 Because episomal DNA does not replicate, TREC frequency (ie, TREC molecules per cell number) decreases with successive cell divisions, permitting its use not only as a marker of thymic function, but also as a quantitative marker for RTEs in the periphery.
Interpretation of human TREC data expressed as a frequency is problematic because this value can be influenced by changes in peripheral cell turnover in the absence of altered RTE generation.3-6 Importantly, sampling of human tissue for TREC analysis is almost exclusively limited to the peripheral blood. Even when quantitatively expressed, TREC numbers in blood cannot detect changes in RTE trafficking among multiple lymphoid compartments and extra-lymphoid tissue, nor does it provide information regarding the maintenance of RTEs in these sites. Thus, changes in TREC frequency in many cases may not reflect thymic function, and measuring TRECs in peripheral blood may not be representative of changes to peripheral RTE numbers as a whole. In this regard, the development of a TREC assay in mice enhances the interpretative value of TREC data because unrestricted tissue sampling makes it possible to determine T-cell population size and turnover and total peripheral TREC number in peripheral lymphoid organs and peripheral blood. The murine homologs to δrec and ψJα have been cloned and sequenced,7 and enumeration of this single murine TREC species has been demonstrated.8-11 However, to date, there have been no studies with murine TRECs that examine the relative contributions of thymic output and peripheral T-cell homeostasis on TREC frequency and number.
Cytokines influence the production and turnover of peripheral T-cell populations and could also lead to alterations in RTE homeostasis. Interleukin 7 (IL-7) is required for T-cell development in the thymus,12,13 and has been shown to enhance thymopoiesis following HSCT.14,15 Additionally, IL-7 has been shown to have potent effects on the proliferation and survival of peripheral T cells.16-18 The importance of IL-7 as a regulator of peripheral T-cell homeostasis is highlighted by the observation that serum levels of IL-7 are elevated in states of peripheral T-cell depletion.19 However, IL-7 effects on RTE homeostasis remain largely unexplored. Here, we demonstrate that exogenous administration of IL-7 into normal thymus-intact mice results in an increase in spleen and lymph node (LN) RTE numbers in the absence of detectable changes to thymic function, and that similar increases in spleen and LN RTE numbers are also observed in thymectomized mice. The changes in total spleen and LN RTEs suggest a potential effect of IL-7 on lymphocyte trafficking, resulting in accumulations of RTEs in peripheral immune organs.
Materials and methods
Animals
Thymus-intact and thymectomized C57Bl/6 mice (8 to 12 weeks old) were purchased from the Animal Production Unit, National Cancer Institute. IL-7 knockout mice were acquired from DNAX Research Institute (Palo Alto, CA). Suction thymectomies were performed at 4 to 6 weeks of age. Completeness of the procedure was confirmed by visual inspection at the time of sacrifice. Studies were carried out under approved animal study protocols.
Administration of murine IL-7
Recombinant murine IL-7 (rmIL-7; Peprotech, Rocky Hill, NJ) was reconstituted according to the manufacturer's instructions and resuspended in 5% sucrose and 0.1% C57Bl/6 normal mouse sera in phosphate-buffered saline (PBS). rmIL-7 was administered by continuous infusion at 5 μg per day via subcutaneous osmotic pumps for 7 days (model no. 1007D) or 14 days (model no. 1002; DURECT, Cupertino, CA). Mice were anesthetized by intraperitoneal injection of xylazine and ketamine. Pumps were implanted according to institutionally approved manufacturer's protocols.
Flow cytometry
Single-cell suspensions were prepared from thymus, spleen, and pooled inguinal,2 axillary,2 cervical,2 and mesenteric3 LNs of mice killed by CO2 asphyxiation. Red blood cells were lysed by treatment with ACK lysing buffer (Quality Biological, Gaithersburg, MD). Peripheral blood lymphocytes (PBLs) were isolated from whole blood after 2 rounds of ACK lysing buffer treatment. Cells were counted and prepared for flow cytometry. Cells (1 × 106) were incubated with 2.4G2 followed by staining with fluorescent antibodies. Intracellular staining of Ki-67 or bromodeoxyuridine (BrdU) was performed using the Cytofix/Cytoperm and BrdU Flow Kits (Pharmingen, San Diego, CA). Directly conjugated and biotinylated monoclonal antibodies (mAbs) used included: CD25, CD69, CD71, CD45RB, CD103, Ki-67, CD3, CD4, CD5, CD8, CD11a, CD44, B220, immunoglobulin M (IgM), heat-stable antigen (HSA), Gr-1, DX5, Mac-1, H57, GL-3, BrdU (all from Pharmingen), and cyanin 5 (Cy5)-conjugated CD4 and CD8 (Caltag, Burlingame, CA). Biotinylated mAbs were developed with Cy5-conjugated streptavidin (Pharmingen). Isotype control mAbs were Leu4 for surface staining, mouse IgG1 for Ki67 staining, and mouse IgG1κ (Pharmingen) for BrdU staining. Three-color flow cytometry was performed using a FACSVantage and was analyzed with Cell Quest software (Becton Dickinson, Franklin Lakes, NJ).
For cell sorting, combined splenocyte and LN CD4+ and CD8+ cells were purified using immunomagnetic beads (Miltenyi Biotec, Auburn, CA). CD4+ cells were sorted into naive (CD45RBhi, CD44lo) and memory (CD45RBlo, CD44hi) subsets; CD8+ cells were sorted into naive (CD103hi, CD44lo) and memory (CD103lo, CD44hi) subsets. Live cells were selected based on light scatter and propidium iodide staining. The purity of all sorted populations was confirmed to be more than 99%.
TREC analysis
Cell lysates were prepared by incubation at 55°C for one hour in 100 μg/mL proteinase K (Boehringer Mannheim, Mannheim, Germany), followed by inactivation at 95°C for 15 minutes. The lysate from 50 000 cells was added to a real-time quantitative polymerase chain reaction (PCR), containing mδRec primer (5′-GGGCACACAGCAGCTGTG), ψJα primer (5′-GCAGGTTTTTGTAAAGGTGCTCA), and mδRec-ψJα fluorescent probe (5′-FAM-CACAAGCACCTGCACCCTGTGCA-TAMRA-3′). Lysates were separately amplified for the single-copy CD8β gene using forward primer (5′-CAGGACCCCAAGGACAAGTACT-3′), reverse primer (5′-CACTTTCACCATACAAAACTCCTTTG-3′), and probe (5′-FAM-TGAGTTCCTGGCCTCCTGGAGTTCTTC-TAMRA-3′). Primers were synthesized by Invitrogen (Carlsbad, CA) and fluorescent probes by Biosource (Camarillo, CA). PCR reactions contained 0.5 μM of each primer, 0.3 μM fluorescent probe, 1× Platinum Quantitative PCR Supermix-UDG (Invitrogen), and Blue-636 reference dye (MegaBases, Evanston, IL). Amplifications were performed in triplicate on an ABI Prism 7700 Sequence Detection system (Perkin-Elmer, Shelton, CT) and analyzed using associated software. PCR conditions were 50°C for 2 minutes, 95°C for 5 minutes, then 40 cycles of 95°C for 15 seconds and 60°C for one minute.
Standard curves for murine TRECs were generated by cloning a δRec-ψJα TREC PCR product into pCR-XL-TOPO (Invitrogen). Stock dilutions of 107, 106, 105, 104, 103, 102, 101.5, and 101.25 plasmids per 5 μL were generated. Standard curves for the CD8β chain gene were similarly prepared. For each real-time PCR experiment, δRec-ψJα TREC and CD8β chain gene standards were run in duplicate per experiment to generate standard curves. TREC frequency, expressed as the number of TREC molecules per 50 000 cells, was determined by normalizing the number of TRECs amplified in the real-time PCR reaction to the number of amplified CD8β molecules. The total number of TRECs in a given cell population was calculated by multiplying the TREC frequency by the number of cells in the population.
BrdU labeling
Mice were given a continuous oral infusion of BrdU (Sigma) in the drinking water at a concentration of 0.8 mg/mL. BrdU-treated water was made fresh and changed daily. Untreated mice were used as a negative control for BrdU staining.
Histology
Paraffin sections (5 μm) of thymus, spleen, and lymph nodes were generated, stained with hematoxylin and eosin, and visualized by light microscopy using a Leitz Diaplan microscope containing a Leitz Fluotar ×10 objective (Wetzlar, Germany). Images were obtained using an Olympus DP12 camera (Melville, NY) and acquisition software.
Results
Exogenous IL-7 expands combined spleen and LN RTE populations in thymus-intact and thymectomized mice
We sought to determine the effect of exogenous IL-7 on peripheral RTE and non-RTE T-cell populations in mice. Subcutaneous pumps were placed into young adult mice to deliver rmIL-7 by continuous infusion. Combined single-cell suspensions from spleen and LNs were analyzed for their overall cellularity and total TREC number. Because RTEs reside within the naive cell compartment, sorted naive CD4+ and CD8+ cells were quantified and analyzed for TREC frequency and total TRECs. Cells with activated/memory phenotype were also collected to confirm that TRECs resided predominantly in the naive compartment (data not shown). Studies in our laboratory (data not shown) and the laboratories of others8,11 have shown that measurable TREC levels persist following thymectomy. Parallel experiments were performed in thymectomized mice to determine the effect of absent thymic function on peripheral naive cell and RTE populations.
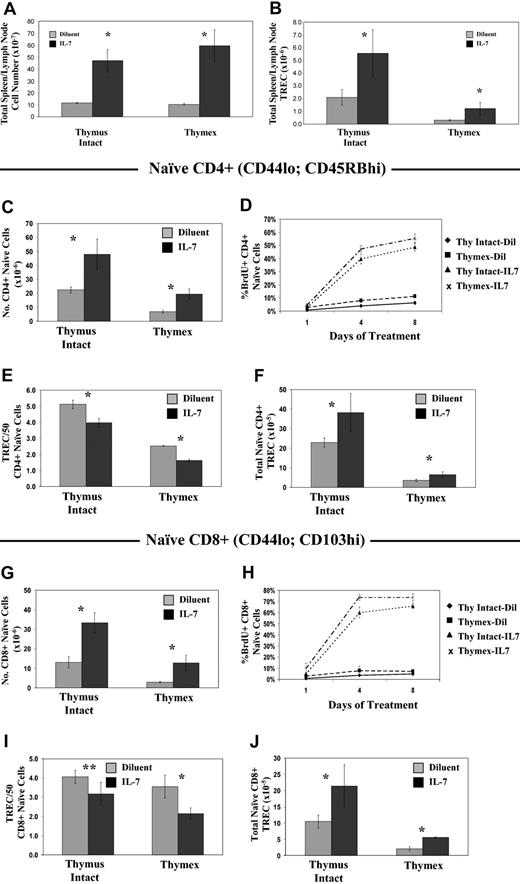
rmIL-7 administration into thymus-intact mice resulted in significant increases in total spleen and LN cell number and TRECs, and in the size of naive CD4+ and CD8+ populations (Figure 1A,C,G). TREC frequencies in both naive CD4+ and CD8+ cells were decreased by 20% with rmIL-7 treatment (Figure 1E,I). Accounting for increases in naive T-cell numbers, the total TREC number was increased by roughly 2-fold (Figure 1F,J). The observed decrease in TREC frequency indicated a dilutional effect as a result of rmIL-7-induced cell proliferation. We therefore measured the rate of BrdU incorporation in naive CD4+ and CD8+ cells. Mice were given BrdU in the drinking water at the start of the rmIL-7 infusion. BrdU incorporation was measured in naive T cells during rmIL-7 and BrdU coadministration. rmIL-7 administration dramatically increased cell division in naive T cells. By day 8 of rmIL-7 infusion, 50% of naive CD4+ cells were BrdU+ in IL-7-treated mice, compared with 6% in diluent-treated mice (Figure 1D). Naive CD8+ cells showed a more pronounced degree of cell proliferation with rmIL-7 treatment in that 70% of naive CD8+ cells were BrdU+ compared with 5% in the control mice (Figure 1H). Additional evidence for increased proliferation in naive T cells was that expression of the cell proliferation marker Ki67 in these populations was increased by 10-fold after 7 days of rmIL-7 treatment (data not shown).
Short-term administration of IL-7 similarly affects cell numbers, cell turnover, and TREC content in thymus-intact and thymectomized mice. Mice were given rmIL-7 by continuous infusion at a dose of 5 μg per day, or diluent only, for 14 days. Total combined spleen and lymph node cell number (A) and TREC number (B), naive CD4+ cell number, defined by CD44lo and CD45RBhi coexpression (C), and CD8+ cells, defined by CD44lo and CD103hi coexpression (G), were determined. TREC frequency (number of TRECs/50 cells) was measured by real-time quantitative PCR (E,I), and the total TREC number in sorted naive CD4+ (F) and naive CD8+ (J) cells was calculated. To assess changes in cell turnover as a result of rmIL-7 administration, rmIL-7- or diluent-treated mice were given BrdU in the drinking water and the percentages of BrdU+ naive (CD44lo) CD4+ (D) and CD8+ (H) cells were measured at the indicated time points. Data in panels A and B are data from a single experiment, representative of 2 experiments carried out, showing the mean plus or minus the standard deviation (SD) of 6 mice per group. Data in panels C through J are representative data from single experiments, representative of 2 experiments carried out, showing the mean plus or minus SD of 3 mice per group. *P < .05 when comparing rmIL-7 treatment to diluent control by the Mann-Whitney U test. **P = .09.
Short-term administration of IL-7 similarly affects cell numbers, cell turnover, and TREC content in thymus-intact and thymectomized mice. Mice were given rmIL-7 by continuous infusion at a dose of 5 μg per day, or diluent only, for 14 days. Total combined spleen and lymph node cell number (A) and TREC number (B), naive CD4+ cell number, defined by CD44lo and CD45RBhi coexpression (C), and CD8+ cells, defined by CD44lo and CD103hi coexpression (G), were determined. TREC frequency (number of TRECs/50 cells) was measured by real-time quantitative PCR (E,I), and the total TREC number in sorted naive CD4+ (F) and naive CD8+ (J) cells was calculated. To assess changes in cell turnover as a result of rmIL-7 administration, rmIL-7- or diluent-treated mice were given BrdU in the drinking water and the percentages of BrdU+ naive (CD44lo) CD4+ (D) and CD8+ (H) cells were measured at the indicated time points. Data in panels A and B are data from a single experiment, representative of 2 experiments carried out, showing the mean plus or minus the standard deviation (SD) of 6 mice per group. Data in panels C through J are representative data from single experiments, representative of 2 experiments carried out, showing the mean plus or minus SD of 3 mice per group. *P < .05 when comparing rmIL-7 treatment to diluent control by the Mann-Whitney U test. **P = .09.
Significantly, when rmIL-7 was administered into age-matched thymectomized mice, similar changes in naive and RTE populations were observed. Compared to thymus-intact mice, total spleen and LN TREC numbers, as well as naive T-cell and TREC numbers, were decreased in thymectomized animals, consistent with the expected loss of RTE production (Figure 1). Similar to observations in thymus-intact mice, administration of rmIL-7 into thymectomized mice resulted in a marked increase in the number of naive T cells (Figure 1C,G). TREC frequencies in naive CD4+ and CD8+ cells from rmIL-7-treated mice were decreased by 40% from those of control diluent-treated mice (Figure 1E,I). That the decrease in TREC frequency was due to the effects of rmIL-7 on cell proliferation was again demonstrated by the increased rate of BrdU uptake in naive cells (Figure 1D,H) corroborated by a 10-fold increase in the levels of Ki67 expression on the seventh day of rmIL-7 infusion (data not shown). Despite the decrease in TREC frequency, total combined spleen and LN and naive CD4+ and CD8+ TREC numbers were increased in rmIL-7-treated mice by a proportion comparable to that observed for thymus-intact animals (Figure 1A,F,J). These results demonstrate that even in the absence of thymic function, exogenous IL-7 resulted in an accumulation of combined spleen and LN RTEs. Furthermore, these results highlight the limitations of interpreting TREC frequency as a measure of thymic output during altered peripheral T-cell homeostasis, and that determination of total TRECs in a broad sampling of peripheral lymphocytes provides a more accurate assessment of the size of the RTE population.
Short-term IL-7 administration has no effect on thymic function
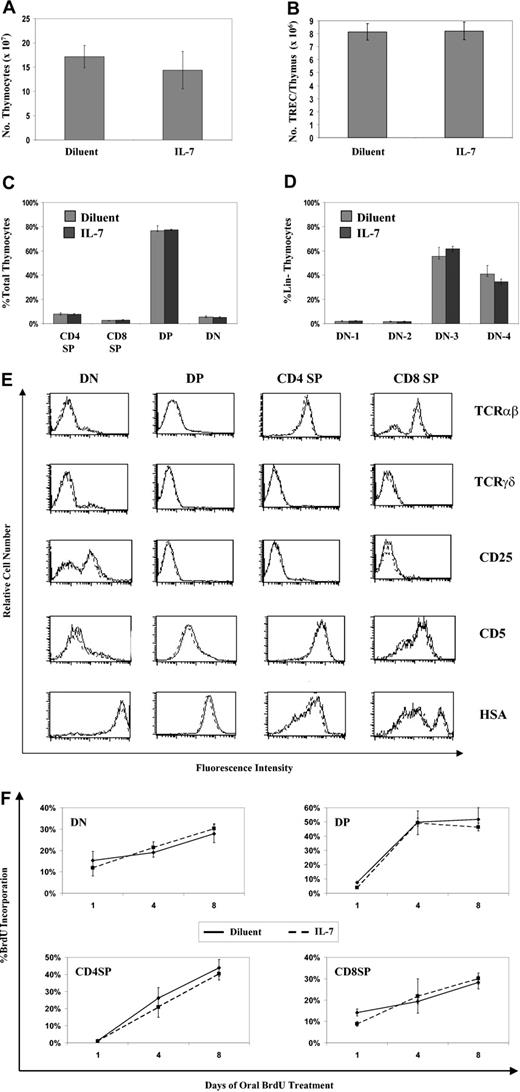
Analysis of the thymuses from rmIL-7-treated mice revealed no evidence of altered thymic function, further supporting the concept of an extrathymic mechanism accounting for the accumulation of combined spleen and LN RTEs in thymectomized mice. There were no differences in the size or cytoarchitecture of thymuses taken from rmIL-7-treated mice. In contrast, their spleens and LNs were noticeably enlarged, with evidence of cell proliferation in T-cell-rich areas consistent with the previously described changes in cell numbers and rates of cell proliferation (Figure 2). Thymocyte number and total intrathymic TREC content in the rmIL-7-treated mice were identical to those of control mice (Figure 3A-B). Proportions of double-negative (DN), double-positive (DP), CD4+ single-positive (CD4 SP), and CD8+ single-positive (CD8 SP) thymocytes were unaltered in IL-7-treated mice (Figure 3C). Because IL-7 has been shown to be critical in the development of DN thymocyte population,20 we examined the distribution of the DN subpopulations in IL-7-treated versus diluent-treated control mice based on dual CD25 and CD44 expression in lineage thymocytes. No differences were seen in the numerical distribution of DN1 through DN4 thymocytes (Figure 3D). Finally, comparing maturation marker expression patterns in DN, DP, CD4 SP, and CD8 SP thymocytes revealed no differences between IL-7-treated versus diluent-treated mice (Figure 3E).
Short-term administration of IL-7 does not affect thymic cytoarchitecture, but greatly affects spleen and lymph node cytoarchitecture. Hematoxylin and eosin-stained sections were prepared of thymus (cortex [C] and medulla [M]), spleen (arrows indicating arterioles within periarteriolar T-cell zones), and mesenteric lymph node (B-cell follicles [B] and T-cell parafollicular zones [T]). Mice treated with rmIL-7 (5 μg per day for 14 days) were compared with diluent controls. Magnification: ×100 in all sections. Sections shown here are representative of 6 mice in each group.
Short-term administration of IL-7 does not affect thymic cytoarchitecture, but greatly affects spleen and lymph node cytoarchitecture. Hematoxylin and eosin-stained sections were prepared of thymus (cortex [C] and medulla [M]), spleen (arrows indicating arterioles within periarteriolar T-cell zones), and mesenteric lymph node (B-cell follicles [B] and T-cell parafollicular zones [T]). Mice treated with rmIL-7 (5 μg per day for 14 days) were compared with diluent controls. Magnification: ×100 in all sections. Sections shown here are representative of 6 mice in each group.
Short-term IL-7 administration does not alter thymic phenotype or function. Mice treated with rmIL-7 (5 μg per day for 14 days) or diluent were analyzed for thymocyte number (A), total intrathymic TRECs (B), and distribution of thymocyte subsets (C). (D) Lin- thymocytes (CD3, CD4, CD8, B220, IgM, Mac-1, pan-NK, and Gr-1 negative) were analyzed for CD44 and CD25 expression that defines the DN1 through DN4 subpopulations. Data shown in graphs A through D are data from a single experiment, representative of 2 experiments carried out, showing the mean plus or minus SD of 3 mice per group. (E) Patterns of thymocyte maturation markers within each thymocyte subset were analyzed in IL-7-treated (dashed line) and diluent-treated (solid line) mice. Shown are representative data from 3 separate experiments. (F) The kinetics of thymocyte development was determined in IL-7- and diluent-treated mice by continuous oral administration of BrdU (“Materials and methods”). At the indicated time points, the percentage of thymocyte subset populations incorporating BrdU was determined. Shown are data from a single experiment, representative of 2 experiments carried out, with the mean plus or minus SD of 3 mice per group.
Short-term IL-7 administration does not alter thymic phenotype or function. Mice treated with rmIL-7 (5 μg per day for 14 days) or diluent were analyzed for thymocyte number (A), total intrathymic TRECs (B), and distribution of thymocyte subsets (C). (D) Lin- thymocytes (CD3, CD4, CD8, B220, IgM, Mac-1, pan-NK, and Gr-1 negative) were analyzed for CD44 and CD25 expression that defines the DN1 through DN4 subpopulations. Data shown in graphs A through D are data from a single experiment, representative of 2 experiments carried out, showing the mean plus or minus SD of 3 mice per group. (E) Patterns of thymocyte maturation markers within each thymocyte subset were analyzed in IL-7-treated (dashed line) and diluent-treated (solid line) mice. Shown are representative data from 3 separate experiments. (F) The kinetics of thymocyte development was determined in IL-7- and diluent-treated mice by continuous oral administration of BrdU (“Materials and methods”). At the indicated time points, the percentage of thymocyte subset populations incorporating BrdU was determined. Shown are data from a single experiment, representative of 2 experiments carried out, with the mean plus or minus SD of 3 mice per group.
To investigate the possibility that exogenous rmIL-7 enhanced thymic function through alterations in thymocyte development kinetics, without alteration of thymic size or cellularity, we measured the rate of BrdU uptake in each thymocyte subset in mice given continuous oral BrdU. Cell division in the thymus predominantly occurs at the late DN stage II,1 and these cells remain BrdU+ during subsequent maturation stages.21,22 The rate of BrdU acquisition in DP thymocytes therefore reflects the rate of DN to DP thymocyte maturation. In the CD4 and CD8 SP populations, BrdU acquisition reflects a combination of 3 events: (1) proliferation of intermediate SP cells that are the developmental precursors to DP thymocytes; (2) maturation of DP into mature SP populations; and (3) proliferation of mature SP thymocytes.23,24 In marked contrast to the rapid rate of BrdU incorporation in peripheral T-cell subsets described previously, the kinetics of BrdU incorporation in the 4 thymocyte subsets, reflected by changes in the percentage of BrdU+ cells (Figure 3F) and the absolute number of BrdU+ cells (data not shown) over time, were similar between the rmIL-7-treated and control diluent-treated mice. The identical rate of BrdU incorporation among DP thymocytes in the 2 groups demonstrated that the kinetics of the DN to DP transition were not affected by rmIL-7 administration. Likewise, given the identical rate of BrdU incorporation in CD4+ and CD8+ SP thymocyte populations between the rmIL-7-treated and control diluent-treated mice, none of the aforementioned events that promote BrdU incorporation in these populations were noticeably affected by rmIL-7 treatment.
A possible explanation for the aforementioned findings is that the dosage and/or route of administration of rmIL-7 used in these experiments were insufficient to effect any phenotypic or functional change in the thymus. Yet, rmIL-7 given in an identical dose schedule to young adult IL-7 knockout mice25 resulted in a 7-fold increase in both thymic cellularity and total intrathymic TRECs (data not shown), demonstrating that the dose and route of administration of rmIL-7 used in these experiments was sufficient to alter thymic phenotype in IL-7-deficient states.
Exogenous IL-7 affects the distribution of RTEs among peripheral immune organs
Possible mechanisms to explain the accumulation of combined spleen and LN TRECs in the absence of changes to thymic function with exogenous rmIL-7 include: (1) TREC duplication; (2) increased extrathymic T-cell development; (3) enhanced survival of RTEs; and (4) preferential accumulation of RTEs in individual lymphoid compartments due to an effect of exogenous IL-7 on RTE trafficking. No evidence supporting the first 3 mechanisms was found in multiple experiments. First, correlates of decreases in TREC frequency resulting from cell division in vitro showed no evidence of TREC duplication, even when rmIL-7 was added to the cultures (data not shown). Second, analysis of T-cell populations in thymectomized mice treated with rmIL-7 suggested that the induction of extrathymic T-cell development was not a major mechanism for accumulation of spleen and LN TRECs, in that (1) aged thymectomized mice treated with rmIL-7, containing negligible TREC levels, exhibited no increases in spleen and LN TRECs; (2) there were no observed indications of increased extrathymic T-cell development with respect to increases in peripheral CD4+/CD8+ DP cells, intraepithelial lymphocyte-derived CD8αα+ cells, or CD3lo-int T cells typically seen in models of extrathymic T-cell development26-28 ; and (3) histologic analysis of the small intestine in rmIL-7-treated mice failed to show expansion of Peyer patches or intraepithelial lymphocyte (IEL)-associated areas in the lamina propria (data not shown). Moreover, while administration of IL-7 into athymic nude mice showed increases in the number of naive cells and TRECs, indicative of IL-7-dependent extrathymic T-cell development, the magnitude of these increases was limited and could not account for the degree of naive T-cell and TREC accumulation observed in identically treated thymectomized mice described earlier (data not shown). Third, whereas a principal mechanism of IL-7 on T cells is enhanced cell survival through bcl-2 up-regulation, administration of exogenous IL-7 into bcl-2 transgenic mice resulted in a similar accumulation of TRECs in combined spleen and LNs (data not shown), indicating that additional mechanisms accounting for the increases in combined spleen and LN TRECs exist. Moreover, kinetic measurements of spleen and LN TREC numbers during rmIL-7 infusion into thymectomized mice showed a progressive increase in the total TREC number over time (data not shown) rather than a persistent maintenance of the TREC number that would be predicted if preferential survival of RTEs was the predominant effect of IL-7.
To provide evidence that exogenous IL-7 can influence the accumulation of naive cells and RTEs in peripheral immune organs, we sought to determine if distributive changes in naive cell number, cell proliferation, and TREC numbers among separate lymphoid compartments occurred with IL-7 treatment. Following 7 or 14 days of continuous rmIL-7 administration, naive T-cell and TREC numbers in each of 3 individual peripheral lymphoid compartments (spleen, LNs, and PBLs) were determined in thymus-intact and thymectomized mice. Cell proliferation was determined by assessing Ki67 expression. Evidence for preferential trafficking of RTEs into an individual lymphoid compartment with IL-7 treatment would comprise a change in the distribution of total organ TREC numbers among the spleen, LNs, and PBLs that are independent of alterations in cell numbers and the degree of cell proliferation. In this regard, organ TREC frequency is useful because it is indicative of the relative contribution of RTE entry versus cell proliferation to total T-naive cell numbers within that organ.
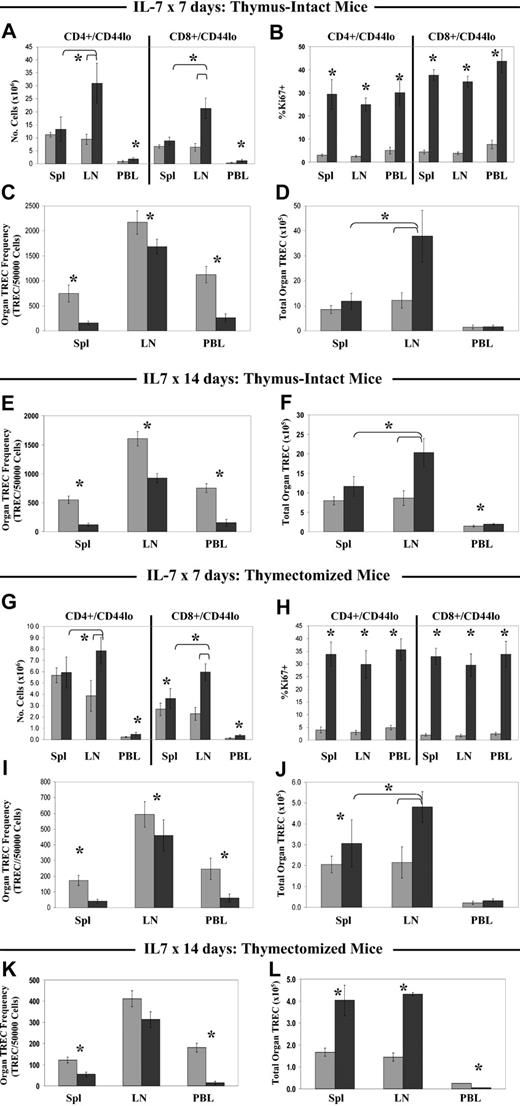
Treatment for 7 days with rmIL-7 resulted in an altered distribution of RTEs among the peripheral immune organs, with patterns that were similar in thymus-intact and thymectomized mice. Expansion of both naive CD4+ and CD8+ populations occurred almost exclusively in the LNs and peripheral blood, with a 3-fold increase in the number of naive CD4+ and CD8+ cells in LNs and a 2-fold increase in PBLs. In contrast, the number of CD4+ naive cells in the spleen was not increased and the number of CD8+ naive cells was only modestly, though significantly, increased (Figure 4A,G) in thymectomized mice. Coincident with the altered distribution pattern of naive cells among the spleen, LNs, and PBLs, proliferation of naive T cells as measured by Ki67 expression was significantly higher in all 3 compartments of IL-7-treated mice compared with diluent-treated controls but did not significantly differ among the 3 compartments (Figure 4B,H). Despite this, the total TREC number in the LNs was increased 2-fold in rmIL-7-treated mice, whereas total TREC numbers in the spleen and PBL compartments were not as dramatically altered (Figure 4D,J). In rmIL-7-treated thymectomized mice, the spleen TREC number was significantly increased but less dramatically than the LN TREC number. Consequently, after 7 days of rmIL-7 treatment in thymus-intact and thymectomized mice, the LNs constituted the largest reservoir of naive T cells and RTEs, whereas in the diluent-treated groups, naive cells and RTEs were found in the spleen and LNs in equal numbers. Further evidence of preferential RTE accumulation in the LNs with 7 days of rmIL-7 treatment is that despite equally elevated levels of Ki67 expression in naive cells in all 3 compartments, TREC frequency in LNs was only decreased to 80% of diluent control, whereas in the spleen and PBLs TREC frequency was more substantially decreased to 20% of that of diluent controls (Figure 4C,I).
Short-term IL-7 administration results in preferential accumulation of naive T cells and TRECs in the lymph nodes of thymus-intact and thymectomized mice. Thymus-intact (A-F) and thymectomized (G-L) mice were given rmIL-7 (▪) by continuous infusion at a dose of 5 μg per day, or diluent only (▦), for 7 days or 14 days. Following the 7-day treatment course, numbers of naive (CD44lo) CD4+ and CD8+ cells (A,G), Ki67 expression (B,H), organ TREC frequency (C,I), and total organ TREC number (D,J) were determined. TREC frequency (E,K) and total organ TREC number (F,L) following 14 days of rmIL-7 treatment are also shown. Shown are combined data from 2 to 3 experiments, with mean plus or minus SD of 7 to 12 mice per group. *P < .05 when comparing rmIL-7 treatment to diluent by the Mann-Whitney U test. In both thymus-intact and thymectomized mice, statistically significant differences in naive cell number and total TRECs between the lymph nodes and spleen of rmIL-7-treated mice (A,D; G,J) are indicated by brackets after 7 days of rmIL-7 treatment, and after 14 days of rmIL-7 treatment in thymus intact mice (F).
Short-term IL-7 administration results in preferential accumulation of naive T cells and TRECs in the lymph nodes of thymus-intact and thymectomized mice. Thymus-intact (A-F) and thymectomized (G-L) mice were given rmIL-7 (▪) by continuous infusion at a dose of 5 μg per day, or diluent only (▦), for 7 days or 14 days. Following the 7-day treatment course, numbers of naive (CD44lo) CD4+ and CD8+ cells (A,G), Ki67 expression (B,H), organ TREC frequency (C,I), and total organ TREC number (D,J) were determined. TREC frequency (E,K) and total organ TREC number (F,L) following 14 days of rmIL-7 treatment are also shown. Shown are combined data from 2 to 3 experiments, with mean plus or minus SD of 7 to 12 mice per group. *P < .05 when comparing rmIL-7 treatment to diluent by the Mann-Whitney U test. In both thymus-intact and thymectomized mice, statistically significant differences in naive cell number and total TRECs between the lymph nodes and spleen of rmIL-7-treated mice (A,D; G,J) are indicated by brackets after 7 days of rmIL-7 treatment, and after 14 days of rmIL-7 treatment in thymus intact mice (F).
Treatment with rmIL-7 for 14 days resulted in shifting RTE distribution patterns that differed between thymus-intact and thymectomized mice. In thymus-intact mice, the total TREC number in the LNs was increased 2-fold in rmIL-7-treated mice. Total TREC numbers in the spleen and PBL compartments were also significantly increased, but not to the same degree as that observed for LNs (Figure 4F). TREC frequency in LNs was only decreased to 60% of diluent control, whereas in the spleen and PBLs TREC frequency was more substantially decreased to 20% of that of diluent controls (Figure 4E). In contrast to thymus-intact mice, thymectomized mice given rmIL-7 for 14 days resulted in an accumulation of RTEs in both spleen and LNs, with both total spleen and LN TRECs significantly increased after IL-7 treatment. However, TREC frequency in the LNs was not significantly decreased relative to the diluent control, whereas in the spleen and PBLs the decrease in TREC frequency was to a level 40% and 8% of diluent control levels, respectively (Figure 4E-F), suggesting that the accumulation of RTEs relative to overall increases in naive cell number may occur to a greater degree in LNs than in spleen despite significant increases in the total TREC number in both organs. Also of note, the total PBL TREC number was significantly decreased in mice treated with 14 days of rmIL-7 compared with controls, in contrast to thymus-intact mice where PBL TREC numbers were increased (Figure 4L).
Exogenous IL-7 increases CD11a expression on naive T cells
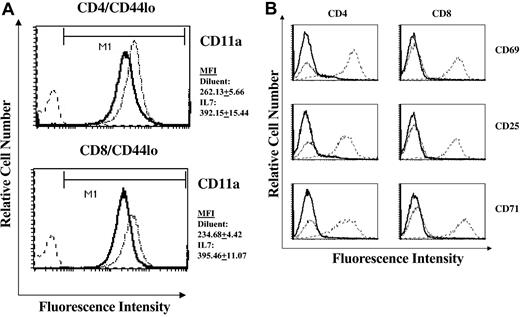
The ability of T cells to traffic specifically into peripheral immune organs is dependent on the coordinate expression of cell adhesion molecules.29 Particularly relevant to the observed accumulation of naive cells and RTEs in the LNs of rmIL-7-treated mice is the expression level of the adhesion molecule CD11a (LFA-1), a member of the β2 family of integrin receptors. CD11a expression on T cells has been shown to be critically important in the migration of T cells through the high endothelial venules of LNs.30,31 We therefore measured the expression of CD11a on naive T cells isolated from rmIL-7-treated mice. Expression of the CD11a in naive CD4+ and CD8+ cells of thymus-intact and thymectomized mice treated with rmIL-7 was significantly increased in naive cells derived from LNs (Figure 5A), spleen, and PBLs. Alterations in CD11a expression with rmIL-7 administration occurred in the absence of overt T-cell activation as measured by CD69, CD25, and CD71 expression (Figure 5B).
Short-term IL-7 administration alters the expression of CD11a (LFA-1) in naive cells in the absence of T-cell activation. Histogram plots of CD11a expression in lymph node-derived naive CD44lo CD4+ and CD8+ cells in diluent control and rmIL-7-treated (5 μg per day for 7 days) thymus-intact mice are shown (A). Mean fluorescent intensity (MFI) of CD11a was consistently higher in cells from rmIL-7-treated mice. Numerical values represent the MFI plus or minus SD of 7 mice per group. Similar increases in CD11a MFI were observed in spleen- and PBL-derived naive T cells (data not shown). In contrast, administration of rmIL-7 did not induce expression of the T-cell activation markers CD69, CD25, or CD71 (B). Solid lines represent histograms of diluent-treated control mice, dotted lines represent histograms of rmIL-7-treated mice, and dashed lines represent histograms of normal splenocytes that were stimulated in vitro with anti-CD3 (2C11) antibody serving as a positive control for CD69, CD25, and CD71 expression. Shown are representative data from 7 mice. Similar patterns of CD11a and T-cell activation marker expression were observed in rmIL-7-treated thymectomized mice and in thymus-intact and thymectomized mice treated with rmIL-7 for 14 days (data not shown).
Short-term IL-7 administration alters the expression of CD11a (LFA-1) in naive cells in the absence of T-cell activation. Histogram plots of CD11a expression in lymph node-derived naive CD44lo CD4+ and CD8+ cells in diluent control and rmIL-7-treated (5 μg per day for 7 days) thymus-intact mice are shown (A). Mean fluorescent intensity (MFI) of CD11a was consistently higher in cells from rmIL-7-treated mice. Numerical values represent the MFI plus or minus SD of 7 mice per group. Similar increases in CD11a MFI were observed in spleen- and PBL-derived naive T cells (data not shown). In contrast, administration of rmIL-7 did not induce expression of the T-cell activation markers CD69, CD25, or CD71 (B). Solid lines represent histograms of diluent-treated control mice, dotted lines represent histograms of rmIL-7-treated mice, and dashed lines represent histograms of normal splenocytes that were stimulated in vitro with anti-CD3 (2C11) antibody serving as a positive control for CD69, CD25, and CD71 expression. Shown are representative data from 7 mice. Similar patterns of CD11a and T-cell activation marker expression were observed in rmIL-7-treated thymectomized mice and in thymus-intact and thymectomized mice treated with rmIL-7 for 14 days (data not shown).
Discussion
Our results indicate that increases in spleen and LN RTE populations in mice given short courses of rmIL-7 result from extrathymic effects of IL-7, and not from enhanced thymic function. The finding of increased total naive T-cell and TREC numbers in spleen and LN cells in rmIL-7-treated thymectomized mice is compelling evidence in support of this hypothesis. Although residual thymic function following incomplete thymectomy is possible, all thymectomized mice were carefully inspected to verify the completeness of the procedure. In addition to establishing a mechanistic role of IL-7 in RTE homeostasis, the results confirm that TREC frequency is affected by changes in T-cell turnover and that only by determining total TREC numbers could changes in the size of the RTE population be accurately assessed.
rmIL-7 treatment of thymus-intact mice did not affect thymic function by multiple parameters. Clinical studies in HIV+ patients and in patients receiving chemotherapy suggest a direct relationship between thymic size and thymic output.32,33 Given the absence of changes to thymic size or phenotype, and the fact that thymocyte development kinetics and proliferation as measured by BrdU incorporation were unchanged, there is no evidence of enhanced thymic function with IL-7 administration in these experiments. Interestingly, the absence of altered thymic function with exogenous rmIL-7 is similar to that found in mice expressing the IL-7 transgene under the control of the major histocompatibility complex (MHC) class II Ea promoter. Despite a marked increase in IL-7 production by thymic epithelium, there were no significant alterations in thymic phenotype, while at the same time, profound increases in the size of peripheral T-cell populations due to systematic IL-7 production by peripheral epithelium were observed.34 This is in contrast to mice expressing the IL-7 transgene driven by the Ig promoter/enhancer35 or the pLCK promoter (N. El Kassar and R.E.G., unpublished observations, 2002), where marked perturbations in thymocyte development were observed. The differences among these thymic phenotypes likely relate to different quantitative and temporal-spatial patterns of IL-7 expression and regulation. That such profound effects of exogenous IL-7 could be seen with respect to the changes in peripheral T-cell population size in the absence of changes to thymic function underlies the role of IL-7 administered short-term as an important regulator of peripheral T-cell homeostasis, while minimizing its role as a dominant regulator of thymic output in otherwise normal mice, at least at the dose used in these experiments. These results support the role of IL-7 in T-cell reconstitution following HSCT in enhancing peripheral maintenance of RTE and other peripheral T-cell populations in addition to restoration of thymic function.9,14,15,36
Examining the distribution of naive T cells and TRECs among separate peripheral immune compartments supports the potential role of short-term IL-7 administration in increasing the spleen and LN RTE number through altered trafficking of RTEs into these organs. No clear evidence supporting other plausible mechanisms that could increase peripheral RTEs/TRECs with rmIL-7 treatment was found. Distribution patterns of RTEs differed in thymus-intact and thymectomized mice depending on the duration of rmIL-7 treatment. In both thymus-intact and thymectomized mice, 7 days of rmIL-7 treatment resulted in a predominant increase in LN RTEs. In contrast, with 14 days of rmIL-7 treatment RTE numbers in LNs in thymus-intact mice remained high along with significant, though more modest, increases in RTEs in the spleen. In thymectomized mice, the patterns of RTE localization with exogenous IL-7 were different in that the RTE number was prominently increased in both spleen and LNs, yet the absence of changes in TREC frequency in LNs and the significant decreases in spleen TREC frequency suggests that RTE accumulation accounted for a greater proportion of overall T-cell population increases in the LNs than in the spleen. The depletion of RTEs from PBLs in these mice further supports the role of IL-7 in redirecting circulating RTEs into secondary lymphoid organs, in contrast to IL-7-treated thymus-intact mice, where continuous production of RTEs by the thymus may compensate for the exit of RTEs from the circulation. A similar pattern of preferential RTE accumulation in the LNs has also been observed in HIV-infected patients.37 Although not measured, it is possible that these findings were due in part to elevated IL-7 levels in this setting of T-cell depletion.19
The mechanisms by which IL-7 controls RTE trafficking require further characterization. Evidence exists that IL-7 plays at least an indirect role in controlling lymphocyte migration to the extent that it has been shown to act as a mobilizing agent for pluripotent hematopoietic stem cells in a murine bone marrow transplant model.38 At the cellular level, IL-7 has been implicated in modulating expression of surface adhesion molecules and chemokine receptors in vitro including CD11a expression.39-41 Our data comparing relative expression levels of CD11a in naive T cells are evidence that short-term treatment with IL-7 may modulate CD11a expression in vivo. It remains a possibility that increased CD11a expression in naive cells and RTEs with rmIL-7 treatment may play an important role in the preferential trafficking of these cells to LNs. Completely consistent with our observations, adoptively transferred lymphocytes from CD11a-deficient mice exhibited markedly impaired homing into LNs, whereas their homing into the spleen was unaffected.31 Additional evidence for the role of CD11a in the homing of T cells to LNs is the observation that the aforementioned accumulations of naive cells and TRECs in the LNs of HIV+ patients were associated with increases in CD11a expression.37 Of note, in these experiments, alterations in CD11a expression occurred in the absence of overt T-cell activation as assessed by the expression of surface activation markers, although the possibility that this is the result of IL-7-induced homeostatic proliferation leading to a partially activated phenotype36 cannot be ruled out. Moreover, the relationship between IL-7 treatment and expression of other cell adhesion molecules involved with lymphocyte trafficking requires further experimentation.
While the redistribution of RTEs among LNs, spleen, and PBLs after IL-7 treatment is clear, the aggregate number of RTEs in these organs is greater in IL-7-treated thymectomized mice than in diluent-treated controls. This suggests that RTEs upon exit from the thymus not only migrate to the spleen and LNs, but must also traffic into other peripheral lymphoid or nonlymphoid organs. Upon treatment with IL-7, RTEs that initially migrated to these sites would then traffic to LNs and spleen. That RTEs migrate to organs other than LNs runs counter to the currently accepted notion that newly generated naive T cells exclusively traffic to LNs.42 Yet, adoptively transferred 51Cr-labeled naive T cells were found to migrate to nonlymphoid tissue in substantial numbers up to 24 hours after their injection.31,43 Together with the results reported here, extrathymic tissue may in fact represent a significant reservoir of RTEs, and entry and exit of RTEs from extrathymic tissues may be controlled at least in part by cytokines such as IL-7. As these cell populations were not analyzed in these experiments, it will be necessary to isolate T cells from lymphoid and nonlymphoid organs not included in this study and demonstrate a decrease in RTE number by enumeration of total TRECs in these organs concomitant with an increase in RTE number in the spleen and lymph node. Alternatively, trafficking patterns of naive T cells and/or RTEs with or without IL-7 administration could be monitored by adoptively transferring appropriately labeled cells that would permit real-time in vivo trafficking studies in whole animals.44,45
In conclusion, short-term exogenous IL-7 administration affects RTE populations in spleen and LNs not by enhancement of thymic function, but by altering peripheral RTE trafficking. These findings provide insights into the biology of RTEs and interpretation of TREC data.
Prepublished online as Blood First Edition Paper, May 6, 2004; DOI 10.1182/blood-2003-10-3635.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Crystal Mackall for critical reading of the manuscript and Larry Granger and Tony Adams for expert technical assistance.

![Figure 2. Short-term administration of IL-7 does not affect thymic cytoarchitecture, but greatly affects spleen and lymph node cytoarchitecture. Hematoxylin and eosin-stained sections were prepared of thymus (cortex [C] and medulla [M]), spleen (arrows indicating arterioles within periarteriolar T-cell zones), and mesenteric lymph node (B-cell follicles [B] and T-cell parafollicular zones [T]). Mice treated with rmIL-7 (5 μg per day for 14 days) were compared with diluent controls. Magnification: ×100 in all sections. Sections shown here are representative of 6 mice in each group.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/4/10.1182_blood-2003-10-3635/6/m_zh80160465010002.jpeg?Expires=1769126703&Signature=RmEfD-8jAdvtZX8MLwvM~s5bTCXp7G3nul2Dsu5VeGlPpgRd~sP~dSNe6BXxhULSNtEcNrt-o8WCvdZcUbDKU~iA2HnvX34FxilkCqAF1v7DRnAfMY6fmCLjnnQl4LS9qE5IwmzGcm1uhafaqbNNvmW-y6mFB-m7RW-2vZpi8yzFK8zQs9GQj8nEMW0jVFGTmSLSpGUAxEPj~k8bZ9Gwyap8FJE4ItZGTYtyk4ppIuFSHpykzelQaJuWOCDbqBRCuorb6FXa1IOIX4bRUa~xMrkfq2Ev6le4ZcMclH26bfcpfeDZ5TECT1Zw5Wa6zfYKFASJoAgXDhIS3JJCTqMkeA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal