Abstract
Antineutrophil cytoplasmic autoantibodies (ANCAs) recognizing human proteinase 3 of neutrophil granules are a diagnostic hallmark of Wegener granulomatosis, an autoimmune systemic vasculitis with predilection for the respiratory tract and kidneys. In vitro experiments have implicated several mechanisms by which ANCAs may lead to tissue injury. However, little is known about the pathogenic significance of proteinase 3–specific antibodies in vivo. In vivo models for ANCA-mediated proinflammatory effects have not been forthcoming, primarily because ANCA epitopes on human proteinase 3 are not shared by the murine homolog. In this study we generated ANCAs against recombinant murine proteinase 3 in proteinase 3/neutrophil elastase–deficient mice that recognized the murine antigen on the surface of neutrophils. Local inflammation induced by intradermal injection of tumor necrosis factor α triggered a stronger subcutaneous panniculitis in the presence of passively transferred systemic proteinase 3–ANCAs than in the presence of mock immune serum. When we transferred mouse proteinase 3-ANCA serum to systemically lipopolysaccharide-primed wild-type mice, mice treated with proteinase 3-ANCAs did not develop significantly stronger signs of inflammation of the lungs or kidneys than the respective mock immune serum-treated animals. In conclusion, our in vivo study provides the first evidence for a pathogenic effect of proteinase 3–specific ANCAs at local sites of inflammation.
Introduction
Circulating antineutrophil cytoplasmic autoantibodies (ANCAs), producing a granular cytoplasmic staining pattern on fixed neutrophils by binding to human proteinase 3 (PR3) of cytoplasmic granules, are well-accepted diagnostic markers for the idiopathic autoimmune vasculitis Wegener granulomatosis (WG).1-4 In most patients levels of PR3-ANCAs correlate with activity and severity of the disease and decrease as the disease improves.5-7 Persistently high titers or significant titer rises of PR3-ANCAs have repeatedly been reported to be positive predictors of clinical relapses.8-10 Although increases in PR3-ANCAs are not necessarily followed by a clinical relapse, relapses have been rarely observed in the absence of PR3-ANCAs.
Although PR3-ANCAs may occasionally occur in other inflammatory conditions including certain infections,11-14 they are highly specific for WG.15 Many in vitro experiments suggest a pathogenic role of PR3-specific autoantibodies; nevertheless, their in vivo relevance has been disputed since their discovery.2,16 The prevailing view of PR3-ANCA pathogenicity at present is experimentally supported by a number of in vitro studies demonstrating a proinflammatory effect of PR3-ANCAs on neutrophils, monocytes, and endothelial cells. The interaction of human ANCAs with tumor necrosis factor α (TNF-α)–primed neutrophils and monocytes results in their strong activation in vitro.17,18 ANCA-opsonized apoptotic neutrophils enhance the phagocytotic activity of macrophages and production of proinflammatory cytokines.19-21 Furthermore, ANCAs directly trigger the release of proinflammatory mediators from neutrophils and monocytes and impair the barrier function of endothelial cells.22-26 The in vitro experiments indicate that many of the proinflammatory effects of PR3-ANCAs require their binding to target antigen expressed on the surface of primed effector cells.16 It is, therefore, thought that if patients with ANCAs are exposed to an inflammatory stimulus such as an infection, ANCAs can interact with their target antigen expressed on the surface of primed neutrophils and monocytes.27 Several clinical findings support the hypothesis that microbial infections may provide a primary stimulus necessary for disease induction in WG.27-29
In contrast to the other small-vessel vasculitis-associated ANCA target antigen, human myeloperoxidase (MPO), which can be used to generate cross-reacting antibodies in rats, human PR3 is unable to trigger an antibody response against the endogenous murine proteinase 3 (mPR3).30-32 In addition, we observed that the endogenous mPR3 did not elicit a significant immune response in wild-type mice. To cover a wide spectrum of epitopes on mPR3, we developed a mPR3/neutrophil elastase (mNE) double-deficient mouse strain for active immunization with recombinant mPR3. Then, mPR3-specific polyclonal antibodies (mPR3-ANCAs) were successfully generated in these antigen-deficient mice. When transferred to wild-type mice, these autologous autoantibodies did not induce disease-specific symptoms but significantly aggravated the inflammatory response elicited by local TNF-α administration. Here we provide the first direct evidence in support of PR3-ANCA–mediated tissue damage in vivo.
Materials and methods
Recombinant expression, refolding, and purification of mPR3
By primer mutagenesis and the polymerase chain reaction, we introduced the helper sequence Met-Glu at the N-terminus of mature mPR3 (Met-Glu-mPR3, called pro-mPR3) and optimized the first 15 codons for usage by Escherichia coli. Recombinant pro-mPR3 was expressed from the plasmid pET24c(+) (Novagen, Madison, WI) in E coli and harvested as inclusion bodies essentially as desribed.33
For refolding, solubilized protein was diluted in at least 100 volumes 50 mM Tris (tris(hydroxymethyl)aminomethane)–HCl, 0.6 M l-arginine, 20 mM CaCl2, 1 mM EDTA (ethylenediaminetetraacetic acid), 100 mM NaCl, and 0.5 mM l-cysteine, pH 8.5. The solution was incubated overnight at 4° C and subsequently dialyzed against 50 mM sodium acetate and 100 mM NaCl, pH 5.5 (acetate buffer). Recombinant mPR3 was purified by hydrophobic interaction chromatography using phenyl-Sepharose (Amersham-Pharmacia, Freiburg, Germany) and running a gradient from 50 mM sodium acetate to 50 mM sodium acetate/30% isopropanol, pH 5.5. Protein concentrations were determined spectrophotometrically at a wavelength of 205 nm.
Processing by cathepsin C and activity assay
Refolded precursor protein was concentrated and converted to mature mPR3 by the dipeptidyl aminopeptidase cathepsin C (Sigma, Deisenhofen, Germany) in acetate buffer containing 1 mM dithioerythritol. Enzymatic activity was assessed by using 1 mM methoxysuccinyl-alanyl-alanyl-prolylvalyl-paranitroanilide in 100 mM Tris-HCl, pH 8.1, and 0.05% Triton X-100. After enzymatic activity reached a plateau, the reaction mixture was dialyzed against acetate buffer and subjected to hydrophobic interaction chromatography at 4° C. Inhibition assays were performed by incubating inhibitors with recombinant mPR3 for 30 minutes at 37° C prior to the substrate addition.
Casein-induced peritonitis
Mice received intraperitoneal injections of 1 mL of a 9% sterile casein solution and peritoneal cells were harvested 3 hours later by peritoneal lavage with phosphate-buffered saline (PBS)/0.02% EDTA. When cells from peritoneal exudates were used for Western blotting, an additional casein injection was given the day before.
Immunization
We generated mPR3/mNE double-deficient mice by homologous recombination of the mPR3/mNE locus with a targeting construct inactivating both tandemly organized genes (L. F. F. and D. E. J., unpublished data, January 1999) and used these mice for immunizations with mPR3 to overcome any immunologic tolerance toward mPR3 and potential epitopes shared by mPR3 and mNE.
Two- to 3-month-old mPR3/mNE-deficient 129/SvEv mice were immunized subcutaneously with 4 to 5 μg recombinant mPR3 (antiserum pool 1) or recombinant pro-mPR3 (antiserum pool 2) in complete Freund adjuvant (Sigma) and boosted 3 and 5 weeks later in incomplete Freund adjuvant. Blood was collected 10 to 13 days after each boost and finally another 5 to 7 days later. Antiserum pool 1 was obtained from 9 mice immunized with mature mPR3, antiserum pool 2 from a group of 6 mice immunized with murine pro-PR3. Mock immune serum was prepared under identical conditions without antigen. Mice were kept in a conventional animal facility. All animal experiments were performed with approval of the Institutional Animal Care and Use Committee.
SDS-PAGE and immunoblotting
Granulocytes were enriched from the peritoneal exudate of mice after a casein-induced peritonitis by discontinuous polysucrose gradient (Histopaque; Sigma) centrifugation. Total lysates from 4.8 × 104 cells and 200 ng recombinant mPR3 were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes, incubated with specific antiserum at a dilution of 1:100, and bound antibodies were visualized using peroxidase-conjugated sheep anti–mouse immunoglobulins (Roche, Mannheim, Germany) and chemiluminescence reagents (Amersham-Pharmacia).
Flow cytometry
Granulocytes were enriched from the peritoneal exudate of mice after a casein-induced peritonitis by Percoll centrifugation and incubated with diluted preimmune serum (1:20 in PBS, 1% bovine serum albumin [BSA], 0.1% azide) followed by unlabeled F(ab′)2 goat anti–mouse IgG. Cells were then probed with mPR3 antiserum or mock immune serum diluted 1:20 for 1 hour on ice, followed by fluorescein isothiocyanate (FITC)–conjugated F(ab′)2 goat anti–mouse IgG (Dianova, Hamburg, Germany). Fluorescence was analyzed on a FACSCalibur flow cytometer (Becton Dickinson, Heidelberg, Germany). Granulocytes were identified by their light scatter characteristics and dead cells were excluded by propidium iodide counterstaining.
Indirect immunofluorescence
A granulocyte-enriched leukocyte suspension obtained by dextran sedimentation was smeared on a glass slide, air dried, and fixed for 10 minutes in ice-cold 4% paraformaldehyde (PFA). After permeabilization (10 minutes 0.05% Triton X-100) and blocking (90 minutes 1.25% mouse albumin) diluted sera were added to the slides for 30 minutes. Bound antibodies were detected with cyanine 3 (Cy3)–labeled F(ab′)2 goat anti–mouse IgG.
ELISA for anti-mPR3 sera
Immulon4HBX plates (Dynex, Chantilly, VA) were coated with 2.5 μg/mL mPR3, and mPR3-specific antibodies were detected using standard enzyme-linked immunosorbent assay (ELISA) procedures. The antibody titers against mPR3 were determined from optical density (OD) measurements using p-nitrophenylphosphate as the alkaline phosphatase substrate and serial dilutions of the antiserum pools. The antibody titer was 1:300 for antiserum pool 1 and 1:7000 for antiserum pool 2 and represents the serum dilution at the turning point of the OD curve. Serum pool 2 was used to determine the half-life of circulating anti-PR3 immunoglobulins and to study the effects of mPR3-ANCAs over 70 days. In all other experiments the antiserum pool 1 against mature mPR3 was injected, because we regarded the antibody repertoire and the moderate high levels of polyclonal antibodies against mature mPR3 most appropriate for the human disease.
In contrast, antibody levels after passive transfer into wild-type recipients were determined by ELISA at the 2 serum dilutions of 1:200 and 1:2000. The antibodies were detectable at serum dilutions of 1:200 in all animals. The antibody levels that we achieved in the recipient mice were consistent with titers encountered in the vast majority of patients with active WG. Titers in excess of 1:256 (highest serum dilution at which OD readings are above background) are only found in a minority of patients with active disease.
For the experiments without TNF-α treatment, mice were injected intravenously with 250 μL antiserum pool 2 (titer 1:7000) followed by 3 repetitive doses of 100 μL antiserum 14, 35, and 63 days later. The half-life of transferred mPR3-specific IgG was estimated from antibody titers determined on days 2 and 14 before the second injection.
Serum transfer and TNF-α–mediated skin inflammation
Eight- to 12-week-old female mice (129/SvEv wild type) were given injections with 100 μL antiserum pool 1 or mock immune serum diluted 1:2 in PBS via the tail vein. Then, 50 ng murine recombinant TNF-α (Roche) in 40 μL PBS/0.1% BSA was injected daily into a ventrolateral area of shaved skin. Injection on the contralateral side with PBS or TNF-α solvent served as a control in all experiments. Twenty-four hours after the fourth injection the entire skin areas from both sides were used to prepare a complete set of serial sections (thickness of 5 μm, intervals of 45-70 μm) that were stained with hematoxylin and eosin. The maximum of the inflammatory reaction was identified by analyzing all consecutive sections using image analysis software (KS 300; Carl Zeiss, Jena, Germany) The degree of inflammation was quantified as the average size of the inflammatory infiltrate on 5 to 8 sections representing 300 μm across the peak zone of inflammation after subtracting the values of the sham-treated side (Figure S1; see the Supplemental Figure link at the top of the online article on the Blood website).
To identify neutrophil granulocytes in the inflammatory infiltrate, selected deparaffinized tissue sections were incubated with a rat anti–Ly-6G antibody (BD PharMingen, Heidelberg, Germany) followed by a biotinylated goat antirat antibody (BioGenex, San Ramon, CA). Bound antibodies were labeled with streptavidin-alkaline phosphatase and visualized with fast red (BioGenex). Sections were finally stained with Mayer hematoxylin solution (Mayer acid hemalaun; 1 g/L certified hematoxylin, 0.2 g/L sodium iodate, 50 g/L aluminum potassium sulfate dodecahydrate, 50 g/L chloral hydrate, 0.91 g/L citric acid; all from Merck Eurolab, Darmstadt, Germany). Histochemically stained sections were mounted with Histofluid (PSI Grünewald, Laudenbach, Germany), and immunostained sections were mounted with Kaiser glycerol gelatin (Merck Eurolab).
Systemic priming with LPS and evaluation of laboratory parameters of primed mice
Bacterial lipopolysaccharide (LPS; E coli serotype 055:B5; Sigma) was injected intraperitoneally into 8- to 12-week-old male mice (0.1 mg/kg body weight) at an interval of 48 hours. In a second set of experiments 4- to 6-month-old mice received a single LPS dose of 2.5 mg/kg body weight. Three hours after the first LPS injection, all animals received 50 μL antiserum pool 1 or mock immune serum diluted to 100 μL in PBS and a booster dose of 30 μL diluted with 70 μL PBS 2 days later. To study the dependence of ANCA pathogenicity on LPS stimulation, ANCA/LPS-treated mice were compared with control groups that received mock immune serum and the same LPS dose. Three days after the first injection mice were placed on a warm plate to collect urine. Urine was analyzed for hematuria and leukocyturia using dipsticks (Analyticon Biotechnologies, Lichtenfels, Germany). Total urinary protein content was determined by the Lowry method after trichloroacetic acid precipitation (Sigma). Urinary albumin was quantified using the albumin reagent bromcresol green (Sigma). Blood was collected from the orbital sinus before the mice were killed. Blood and urine creatinine levels were determined using a creatinine quantification kit (Sigma). The lung and kidney were fixed in 4% paraformaldehyde and processed for light microscopy. All specimens were stained with hematoxylin and eosin.
Statistical analysis
The Mann-Whitney U test was used to evaluate the differences in median lesion sizes among the different groups of mice. A P value less than .05 was considered significant.
Results
Recombinant expression and renaturation of mPR3
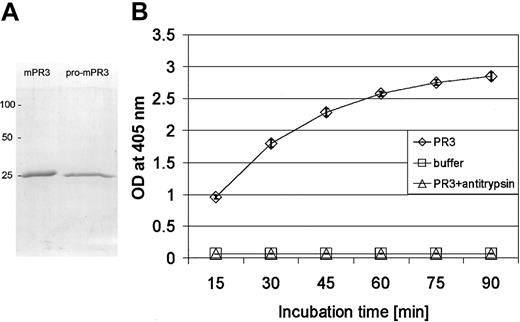
To explore the pathogenic potential of autologous autoantibodies to the endogenous PR3 in wild-type mice, we expressed the murine homolog of mature PR3 as a zymogen (pro-mPR3) with the propeptide Met-Glu in E coli and converted it to the catalytically active, natively folded enzyme by refolding and N-terminal trimming. The granzyme K–derived propeptide sequence Met-Glu was removed by cathepsin C treatment to obtain the correct N-terminus (starting with Ile-Val-Gly-Gly) of mature mPR3.33 Isoleucine was identified as the first residue by N-terminal amino acid sequencing. The material was then purified and concentrated by hydrophobic interaction chromatography. Recombinant pro-mPR3 and mature mPR3 migrated as a single band of approximately 25 kDa on reducing SDS-polyacrylamide gels (Figure 1A). Recombinant mPR3, but not its precursor pro-mPR3, hydrolyzed N-methoxysuccinyl-Ala-Ala-Pro-Val-pNA, a peptide substrate specific for NE and PR3. The hydrolytic activity was completely blocked by α1-antitrypsin, the most important physiologic inhibitor of PR3 in human plasma (Figure 1B).
Purification and characterization of recombinant catalytically active mPR3. (A) Purified recombinant mPR3 and pro-mPR3 after SDS-PAGE separation and staining with Coomassie brilliant blue; molecular mass markers are on the left. (B) Enzymatic activity of recombinant mPR3 (⋄), which was determined by measuring the increase of light absorption at 405-nm wavelength during hydrolysis of the paranitroanilide substrate MeOSuc-Ala-Ala-Pro-Val-pNA. Enzymatic activity is completely inhibited by an excess of bovine α1-antitrypsin (▵). Pro-mPR3 does not hydrolyze the substrate (not shown). Data points represent the mean of triplicate measurements after various incubation times. Error bars indicate the SD of the mean. □ indicates data points for the buffer solution.
Purification and characterization of recombinant catalytically active mPR3. (A) Purified recombinant mPR3 and pro-mPR3 after SDS-PAGE separation and staining with Coomassie brilliant blue; molecular mass markers are on the left. (B) Enzymatic activity of recombinant mPR3 (⋄), which was determined by measuring the increase of light absorption at 405-nm wavelength during hydrolysis of the paranitroanilide substrate MeOSuc-Ala-Ala-Pro-Val-pNA. Enzymatic activity is completely inhibited by an excess of bovine α1-antitrypsin (▵). Pro-mPR3 does not hydrolyze the substrate (not shown). Data points represent the mean of triplicate measurements after various incubation times. Error bars indicate the SD of the mean. □ indicates data points for the buffer solution.
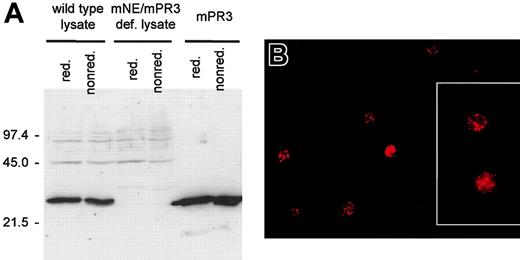
To circumvent mechanisms of tolerance to this highly abundant peripheral antigen, we produced ANCA-like antibodies in mPR3/mNE double-deficient mice that had been generated by homologous recombination (L. F. F. and D. E. J., unpublished data, January 1999). In using double-knockout animals, we avoided the potential suppression of certain murine anti-PR3 ANCA species as a consequence of the tolerogenic action of the closely related endogenous antigen mNE and aimed at inducing a spectrum of antibodies to mPR3 as broad as possible. Nevertheless, a monospecific antibody response to mPR3 was obtained as ascertained by testing the antibody reactivity against human leukocyte elastase. Both sera against mature mPR3 (antiserum pool 1) and pro-mPR3 (antiserum pool 2) reacted with pro-mPR3 and mPR3, respectively (not shown). Immunization of wild-type mice with mPR3 or pro-mPR3 was unsuccessful and yielded only very low antibody titers. We confirmed the specificity of antiserum pools 1 and 2 by Western blotting using purified antigen and neutrophil lysates of wild-type and knockout mice (Figure 2A) and by immunofluorescence microscopy using PFA-fixed murine neutrophils. Dilutions of 1:30 (antiserum pool 1) and 1:250 (antiserum pool 2) produced an intense granular fluorescence pattern in the cytoplasm (Figure 2B), similar to PR3-ANCAs on human neutrophils.
Antibodies against mPR3 generated in mPR3/mNE-deficient mice recognize mPR3 from mouse neutrophils. (A) Western blot analyses of murine neutrophil lysates derived from wild-type mice (lanes 1-2), knockout mice lacking mPR3 (lanes 3-4), and of recombinant mPR3 (lanes 5-6) resolved by SDS-PAGE under reducing (lanes 1, 3, and 5) and nonreducing conditions (lanes 2, 4, and 6) using antiserum pool 1; molecular mass markers are given on the left. Murine antibodies recognize mPR3 in wild-type neutrophils, but do not stain proteins in neutrophil lysates of mPR3/mNE-deficient mice (mPR3/mNE-def). The faint band of approximately 20 kDa in lanes 5 and 6 is a minor degradation product of mPR3 as determined by N-terminal amino acid sequencing. Equivalent results were obtained with antiserum pool 2 (not shown). (B) Indirect immunofluorescence microscopy using PFA-fixed murine neutrophils from wild-type and mPR3/mNE-deficient mice as a substrate and mPR3-specific antiserum pool 1 to demonstrate a PR3-ANCA–like pattern. The cytoplasm of wild-type granulocytes is stained with strong granular accentuation. Equivalent results were obtained with PR3 antiserum pool 2, whereas neither mock immune serum-treated wild-type neutrophils nor PR3 antiserum-treated neutrophils from mPR3/mNE-deficient mice stained positively (not shown). The fixed cells were embedded in Movid (Calbiochem, CA). Microscopy and photography were performed with an Axioplan 2 microscope (Zeiss, Göttingen, Germany) equipped with a 10 × /2.5 ocular, 20 × /0.5 neofluar, 40 × /0.75 neofluar, and 63 × /1.4 oil (inset) objectives, and a SpotRT color digital camera (Diagnostic Instruments, Sterling Heights, MI) Fluorescence images were electronically recorded with Metamorph software (Visitron Systems, Puchheim, Germany). Original magnification × 400; inset × 630.
Antibodies against mPR3 generated in mPR3/mNE-deficient mice recognize mPR3 from mouse neutrophils. (A) Western blot analyses of murine neutrophil lysates derived from wild-type mice (lanes 1-2), knockout mice lacking mPR3 (lanes 3-4), and of recombinant mPR3 (lanes 5-6) resolved by SDS-PAGE under reducing (lanes 1, 3, and 5) and nonreducing conditions (lanes 2, 4, and 6) using antiserum pool 1; molecular mass markers are given on the left. Murine antibodies recognize mPR3 in wild-type neutrophils, but do not stain proteins in neutrophil lysates of mPR3/mNE-deficient mice (mPR3/mNE-def). The faint band of approximately 20 kDa in lanes 5 and 6 is a minor degradation product of mPR3 as determined by N-terminal amino acid sequencing. Equivalent results were obtained with antiserum pool 2 (not shown). (B) Indirect immunofluorescence microscopy using PFA-fixed murine neutrophils from wild-type and mPR3/mNE-deficient mice as a substrate and mPR3-specific antiserum pool 1 to demonstrate a PR3-ANCA–like pattern. The cytoplasm of wild-type granulocytes is stained with strong granular accentuation. Equivalent results were obtained with PR3 antiserum pool 2, whereas neither mock immune serum-treated wild-type neutrophils nor PR3 antiserum-treated neutrophils from mPR3/mNE-deficient mice stained positively (not shown). The fixed cells were embedded in Movid (Calbiochem, CA). Microscopy and photography were performed with an Axioplan 2 microscope (Zeiss, Göttingen, Germany) equipped with a 10 × /2.5 ocular, 20 × /0.5 neofluar, 40 × /0.75 neofluar, and 63 × /1.4 oil (inset) objectives, and a SpotRT color digital camera (Diagnostic Instruments, Sterling Heights, MI) Fluorescence images were electronically recorded with Metamorph software (Visitron Systems, Puchheim, Germany). Original magnification × 400; inset × 630.
mPR3-ANCAs bind to the surface of mouse neutrophils
Many of the postulated pathogenic effects of PR3-ANCAs in humans hinge on their ability to interact with the target antigen expressed on the surface of neutrophils and monocytes.16 To obtain evidence that mPR3-ANCAs recognize epitopes on the surface of primed neutrophils, we isolated granulocytes directly from the peritoneal exudate after casein injection. An acute casein-induced peritonitis is associated with a rapid increase of TNF-α levels in the peritoneal cavity and TNF-α is directly involved in neutrophil recruitment.34 Accordingly, peritoneal neutrophils are an excellent substrate to assess ANCA binding on neutrophils being stimulated in vivo during an acute inflammation.
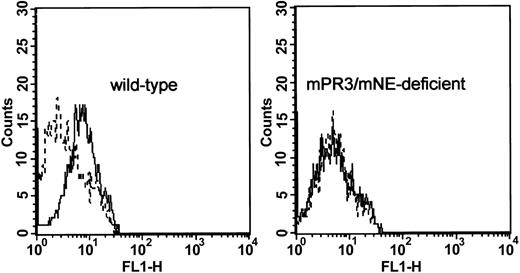
The surface of wild-type, but not of mPR3/mNE-deficient granulocytes, was stained with the antiserum pool 1 at a dilution of 1:20 (Figure 3) and with antiserum pool 2 at even higher dilutions (not shown). Thus, the binding properties of autologous mPR3-ANCA sera fulfilled an important prerequisite for transfer experiments and subsequent investigations of their pathogenic potential in animals.
Cytofluorometric detection of mPR3-ANCAs on the surface of primed murine neutrophils. Wild-type peritoneal granulocytes were incubated with mPR3-antiserum pool 1 (solid line) or preimmune serum (dashed line). Enhanced cell surface binding of monospecific antiserum pool 1 in comparison to the binding of a preimmune serum was visualized with a Cy3-conjugated F(ab′)2 goat antimouse (Fab) antibody (left). The right panel shows the absence of staining of peritoneal granulocytes that were prepared from an mPR3/mNE-deficient mouse.
Cytofluorometric detection of mPR3-ANCAs on the surface of primed murine neutrophils. Wild-type peritoneal granulocytes were incubated with mPR3-antiserum pool 1 (solid line) or preimmune serum (dashed line). Enhanced cell surface binding of monospecific antiserum pool 1 in comparison to the binding of a preimmune serum was visualized with a Cy3-conjugated F(ab′)2 goat antimouse (Fab) antibody (left). The right panel shows the absence of staining of peritoneal granulocytes that were prepared from an mPR3/mNE-deficient mouse.
Whereas variably small amounts of membrane-associated human PR3 have been reported for human neutrophils, we have not been able to detect mPR3 on freshly isolated murine neutrophils from murine blood with our current reagents. The biologic or technical reasons for this negative result are not clear at present. Neutrophils from the peritoneal lavage fluid, however, are fully activated, which may explain why mouse PR3 is detectable on their surfaces (Figure 3 left panel).
Passive transfer of mPR3-ANCA to naive congenic wild-type mice
To determine the half-life of mPR3-ANCA in the circulation and whether their presence alone had adverse effects, continuous mPR3-ANCA levels were generated in 6 naive wild-type mice by repeated intravenous injections of antiserum pool 2 over 10 weeks. During this time period, mPR3-ANCAs were detectable in all samples of plasma at dilutions of 1:2000 or higher (ELISA data not shown). The half-life of the antibodies was 11.1 days (± 2.2 days). This is in line with the half-life of passively transferred normal IgG (11.7 ± 0.535 days)35 and indicates that mPR3-ANCAs were not rapidly cleared by immune complex formation in recipient mice. One week after the fourth injection all mice were humanely killed and analyzed by a pathologist. No mPR3-ANCA–induced inflammatory changes were detected in the lung or kidneys (not shown). This finding is consistent with the clinical observation that PR3-ANCAs alone are not sufficient to trigger a relapse of disease activity in patients with WG.2,16
Significant augmentation of a TNF-α–induced skin inflammation by systemic mPR3-ANCAs
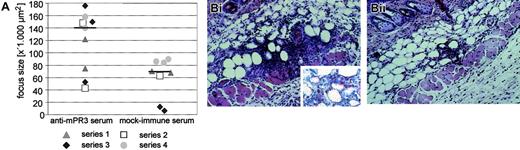
In consideration of locally produced TNF-α as a putative important condition for ANCA pathogenicity, we injected this proinflammatory cytokine intradermally into the same cutaneous area on 4 consecutive days. The TNF-α dose chosen had no obvious systemic side effects in any of the recipient animals, regardless of the type of antiserum coadministered intravenously. In those animals treated with serum from mock-immunized knockout mice, TNF-α injection caused a mild inflammation of the dermal and subcutaneous tissue (local panniculitis). In contrast, the average inflammatory focus size at the site of TNF-α injection was significantly increased in the presence of systemically delivered mPR3-ANCAs. Histomorphometric comparison of the TNF-α–treated versus the sham-treated skin area in the same mouse revealed a net increase of the inflammatory infiltrate (P = .043; Figure 4) by approximately 100% in the presence of autologous mPR3-ANCAs. Separate analysis of each of the 4 independent experimental series supports this robust difference even better. Eight of 9 animals treated with antiserum pool 1 had a stronger TNF-α–induced panniculitis as compared to the corresponding mock immune serum-treated animals of the same experimental series. Staining of skin biopsies with an antibody for the neutrophil marker Ly-6G showed that the inflammatory infiltrate at the site of local TNF-α injection contained a substantial number of neutrophils (Figure 4). These data clearly demonstrate a distinct proinflammatory effect of systemic mPR3-ANCA on local inflammatory responses.
Exacerbation of a TNF-α–induced panniculitis by mPR3-ANCAs. Mice treated intravenously with either autologous mPR3-ANCA serum (antiserum pool 1) or serum from mock-immunized mice received TNF-α locally in the same skin area on 4 consecutive days. The contralateral side was treated either with PBS or PBS/0.1% BSA (TNF-α solvent) as a control. (A) The degree of cellular infiltrations in the subcutaneous adipose tissue was measured with digital image analysis software. The plotted data show the net focus size increase between TNF-α–treated and control-treated skin in 17 mice belonging to 4 independent experimental series, which are distinguished by squares, circles, triangles, and diamonds. Nine mice were treated with autologous mPR3-ANCA serum (left column), and 8 mice received serum from mock-immunized mice (right column). Horizontal lines indicate the median of the 2 experimental groups. The difference between mPR3-ANCA serum and mock immune serum–treated animals is statistically significant (P = .043, Mann-Whitney U test). (B) Histologic sections of biopsies from an mPR3-ANCA serum-treated mouse (i) and a mock immune serum-treated mouse (ii) are shown (original magnification × 200; inset × 400). Note the dense cellular infiltrate in the subcutaneous adipose tissue that is accompanied by an apparent local increase of the septal volume. The cellular infiltrate consisted of neutrophils, eosinophils without leukocytoclasia, and mononuclear cells. Staining with an antibody against the neutrophil marker Ly-6G confirms the presence of neutrophils in the inflammatory infiltrate (red-stained cells in the inset). Images were acquired using the same equipment described in the Figure 2B legend.
Exacerbation of a TNF-α–induced panniculitis by mPR3-ANCAs. Mice treated intravenously with either autologous mPR3-ANCA serum (antiserum pool 1) or serum from mock-immunized mice received TNF-α locally in the same skin area on 4 consecutive days. The contralateral side was treated either with PBS or PBS/0.1% BSA (TNF-α solvent) as a control. (A) The degree of cellular infiltrations in the subcutaneous adipose tissue was measured with digital image analysis software. The plotted data show the net focus size increase between TNF-α–treated and control-treated skin in 17 mice belonging to 4 independent experimental series, which are distinguished by squares, circles, triangles, and diamonds. Nine mice were treated with autologous mPR3-ANCA serum (left column), and 8 mice received serum from mock-immunized mice (right column). Horizontal lines indicate the median of the 2 experimental groups. The difference between mPR3-ANCA serum and mock immune serum–treated animals is statistically significant (P = .043, Mann-Whitney U test). (B) Histologic sections of biopsies from an mPR3-ANCA serum-treated mouse (i) and a mock immune serum-treated mouse (ii) are shown (original magnification × 200; inset × 400). Note the dense cellular infiltrate in the subcutaneous adipose tissue that is accompanied by an apparent local increase of the septal volume. The cellular infiltrate consisted of neutrophils, eosinophils without leukocytoclasia, and mononuclear cells. Staining with an antibody against the neutrophil marker Ly-6G confirms the presence of neutrophils in the inflammatory infiltrate (red-stained cells in the inset). Images were acquired using the same equipment described in the Figure 2B legend.
Passive transfer of mPR3-ANCAs to LPS-primed wild-type mice
To evaluate the sequelae of systemic neutrophil priming in the presence of PR3-ANCAs, we intravenously injected mPR3-ANCA or mock immune serum into mice that had been primed with a low or high dose of bacterial LPS. Three days after the first LPS and serum injection, the lungs and kidneys were removed and stained tissue sections were analyzed by 2 independent pathologists blinded to the treatment.
In line with our observations in animals exposed to antiserum pool 2 for 10 weeks (see “Passive transfer of mPR3-ANCA to naive congenic wild-type mice”), ANCA-treatment alone did not induce inflammation in the lung or kidneys after 3 days (Table 1 rows 1-2). One of the 3 animals, however, showed some signs of capillaritis in the lung. Similarly, injection of bacterial LPS (0.1 mg/kg body weight; Table 1 rows 3-4) and mock immune serum induced some capillaritis in 1 of the 5 mice. The combination of ANCAs and LPS did not potentiate these effects. In addition to the capillaritis detected in one ANCA/LPS-treated mouse, alveolar hypercellularity and hematuria were observed in one other mouse each. Laboratory kidney parameters (urinary protein and urinary albumin; Table 1 columns 5-8) did not differ significantly between control and experimental groups.
Effects of murine PR3-ANCAs compared to mock immune serum in LPS-primed and nonprimed mice
Treatment . | No. of affected mice/total no. . | No. with lung capillaritis or alveolar hypercellularity . | No. with glomerulonephritis . | No. with hematuria or leukocyturia . | Urinary protein, mg/dL, mean (SD) . | Urinary albumin, mg/dL, mean (SD) . | Urinary albumin-protein ratio . | Urinary protein-creatinine ratio . |
|---|---|---|---|---|---|---|---|---|
| mPR3-ANCAs | 1/3 | 1 | 0 | 0 | 645 (368) | 94 (29) | 0.18 | 13.5 |
| Mock immune serum | 1/3 | 0 | 1 | 0 | 670 (289) | 103 (37) | 0.16 | 16.5 |
| mPR3-ANCAs + 0.1 mg/kg LPS | 3/5 | 2 | 0 | 1 | 936 (588) | 118 (24) | 0.16 | 12.0 |
| Mock immune serum + 0.1 mg/kg LPS | 1/5 | 1 | 0 | 0 | 847 (362) | 130 (45) | 0.14 | 14.2 |
| mPR3-ANCAs + + 2.5 mg/kg LPS | 3/4 | 2 | 3 | 0 | 744 (292) | 336 (359) | 0.40 | 8.7 |
Treatment . | No. of affected mice/total no. . | No. with lung capillaritis or alveolar hypercellularity . | No. with glomerulonephritis . | No. with hematuria or leukocyturia . | Urinary protein, mg/dL, mean (SD) . | Urinary albumin, mg/dL, mean (SD) . | Urinary albumin-protein ratio . | Urinary protein-creatinine ratio . |
|---|---|---|---|---|---|---|---|---|
| mPR3-ANCAs | 1/3 | 1 | 0 | 0 | 645 (368) | 94 (29) | 0.18 | 13.5 |
| Mock immune serum | 1/3 | 0 | 1 | 0 | 670 (289) | 103 (37) | 0.16 | 16.5 |
| mPR3-ANCAs + 0.1 mg/kg LPS | 3/5 | 2 | 0 | 1 | 936 (588) | 118 (24) | 0.16 | 12.0 |
| Mock immune serum + 0.1 mg/kg LPS | 1/5 | 1 | 0 | 0 | 847 (362) | 130 (45) | 0.14 | 14.2 |
| mPR3-ANCAs + + 2.5 mg/kg LPS | 3/4 | 2 | 3 | 0 | 744 (292) | 336 (359) | 0.40 | 8.7 |
In view of the negative outcome of these experiments we provided a stronger LPS stimulus by administration of a single dose of 2.5 mg LPS/kg body weight. This dose already induced a lung capillaritis in one animal and hypercellularity of the alveolar wall in another. The third mouse of this control group exposed to high-dose LPS alone presented weak leukocyturia, whereas the fourth mouse did not show any histologic and laboratory abnormality. The effects of the high LPS dose in combination with either murine PR3-ANCAs or mock serum were not different, and both treatments led to inflammatory infiltrates in the lung to about the same extent (Table 1 rows 4-5). In the kidneys, we observed signs that could be interpreted as incipient glomerulonephritis in 3 of 5 mice exposed to ANCA/LPS, but also in 1 of the 5 mice in the ANCA-negative control group. Clear-cut crescent formation or fibrinoid necrosis could not be identified in mice that received PR3-ANCAs and LPS. Furthermore, urine total protein and albumin levels did not differ between the experimental and control groups. Serum creatinine levels were in the normal range in all experimental groups receiving no or a high dose of LPS. Focal hemorrhages of the lung observed in 3 (2 ANCA-treated, 1 mock immune serum–treated, not shown) of the 24 animals are probably due to traumatic tissue damage that occurred when the animals were killed. PR3-ANCAs were detectable in all recipient animals by ELISA at serum dilutions of 1:200 (not shown).
Discussion
ANCAs have been studied extensively and characterized in relationship to WG since the identification of their target antigen in 1990.3 Despite the many lines of circumstantial evidence that seem to indicate a pathogenic role for ANCAs in WG, the mechanistic link between PR3-ANCAs and small-vessel vasculitis and glomerulonephritis, characteristic features of WG, remains an enigma and has been questioned from different viewpoints. Patients who develop antibodies reacting with PR3 in a different clinical context generally do not develop small-vessel vasculitis or glomerulonephritis like patients with WG. PR3-ANCAs have been reported to occur transiently in a variety of infections, but the development of systemic vasculitis in these patients remains an exception.11-13 Cocaine abusers may also develop ANCAs, some of which react with PR3.36 Although systemic vasculitis or glomerulonephritis was not observed in these patients, the clinical features prompting them to seek medical attention were their dramatic localized inflammatory tissue destruction. These clinical observations are in agreement with our experimental finding that murine PR3-ANCAs, either in systemically primed or unprimed mice, did not induce symptoms typically found in WG. Given the clinical heterogeneity of WG and the abundance of in vitro experiments demonstrating a general proinflammatory effect of PR3-ANCAs on neutrophils, monocytes, and endothelial cells, our primary goal was to design an experimental model to demonstrate a local proinflammatory activity of PR3-ANCAs in vivo.20,26 We have chosen the skin as the first target organ for practical reasons and because it is also relevant for WG manifestations. Skin involvement has been reported in 35% to 50% of patients and may be an initial sign of the disease.37 Our experimental observations in the dermis of PR3-ANCA–positive mice exposed to TNF-α clearly support that PR3-ANCAs play a pathogenic role in dysregulated local inflammation in WG and possibly other disorders in which PR3-ANCAs have been detected.
Our experimental PR3-ANCA model is the first to investigate the effect of species-specific PR3-ANCAs as a single variable. The only other proposed mouse model to study the pathogenicity of PR3-ANCAs is based on the hypothesis that activation of an idiotypic network underlies the induction of most systemic autoimmune diseases including WG.38 Anti-anti-idiotypic antibodies were generated in the mouse by active immunization with affinity-purified anti–PR3-IgG from patients with WG. However, the binding of anti-anti-idiotypic antibodies to mPR3 was not shown and is unlikely because polyclonal antibodies against human PR3 including human PR3-ANCAs are not known to cross-react with mPR3.32 In contrast, ANCAs with specificity for human MPO predominantly found in microscopic polyangiitis and Churg-Strauss syndrome do cross-react with the endogenous mouse and rat autoantigens. Consequently, human MPO can be used for immunization in animal systems to study the pathogenic contribution of MPO-ANCAs to these diseases.30,31
Our experimental findings are consistent with clinical experience in patients with WG, indicating that the mere presence or recurrence of PR3-ANCAs is not sufficient to cause active disease.2,16 However, if patients with PR3-ANCAs are exposed to an inflammatory stimulus such as an infection, their risk of disease relapse is high.27 Likewise, we observed a genuine pathogenic effect of PR3-ANCA in the animals after applying locally the well-known trigger of inflammation, TNF-α. TNF-α levels are elevated in patients with active WG, TNF-α is detectable in tissue lesions, and many in vitro effects of ANCAs depend on the initial priming with TNF-α.17,39,40 In vitro experiments have shown that degranulation and an oxidative burst are induced on binding of PR3-ANCAs to purified neutrophils primed with TNF-α.17 TNF-α has multiple functions in vivo, including the recruitment of granulocytes and monocytes to the site of its production, activation of mast cells and endothelial cells, and enhancement of the surface expression of PR3 on neutrophils in the circulation.41 TNF-α is produced in infections that presumably play an important role in the induction of many autoimmune diseases. TNF-α production can be experimentally induced in various organs including lung and kidney by systemic administration of bacterial LPS.42 Additionally, systemic LPS leads to neutrophil sequestration in the lung and has been shown to strongly exacerbate injury in antiglomerular basement membrane antibody-mediated nephritis in rats.43,44
Contrary to our expectations we did not observe significant amplification of the LPS effects by passively transferred mPR3-ANCAs in the lungs or the kidneys of mice systemically primed with a low dose (0.1 mg/kg body weight) or a higher dose of LPS (2.5 mg/kg body weight). We therefore conclude that mPR3-ANCAs have a proinflammatory activity only in conjunction with a primary inflammatory stimulus at local extravascular sites. Why mPR3-ANCAs fail to fully activate primed mouse neutrophils in whole blood can be explained in many ways and may be due to, for example, low membrane affinity of mPR3 or effective murine plasma inhibitors that quickly solubilize and neutralize the mPR3 from the circulation.
In vivo proof or disproof of the prevailing mechanistic concept that mPR3-specific ANCAs, on binding to mPR3 on the outer plasma membrane of granulocytes, initiate activation and degranulation of neutrophils faces significant technical challenges. Electron microscopy studies with differentially labeled antibodies against immunoglobulins and mPR3 would be required to identify (1) membrane association and (2) colocalization of the autoantigen and ANCAs and to distinguish pericellular, membrane-associated, and endocytosed immune complexes in the tissue. Binding sites for partially released mPR3 on the extracellular and pericellular matrix may well compete with binding sites on the granulocyte surface. In addition, clearance of functionally relevant mPR3-ANCA complexes could be quite rapid and thus positive as well as negative results could be easily misinterpreted. Because mPR3 cleaves several substrates and modulates the inflammatory response in multiple ways, it is conceivable that the interactions of ANCAs with a small mPR3 subfraction on the surface of granulocytes as observed by many investigators ex vivo is not a condition sine qua non for the observed proinflammatory response. In support of this view, clinical inspection and qualitative histomorphologic examinations of mPR3-ANCA–exposed, LPS-primed mice did not reveal pathologic effects beyond those triggered already by LPS and nonimmune mouse sera. Hence, we suggest that full activation of primed neutrophils by ANCAs did not play the critical role in our mouse model.
Our data concur with the observation that MPO-ANCAs cross-reacting with the endogenous antigen after active immunization of BN rats with human MPO are not sufficient to directly induce glomerulonephritis or pulmonary inflammation in the rat.45,46 These and our findings, however, appear to be at odds with a recent study demonstrating a direct nephritogenic effect of murine MPO (mMPO)–ANCAs in C57BL/6J wild-type mice.47 The authors provided convincing experimental evidence that mMPO-ANCAs alone were sufficient to induce focal crescentic glomerulonephritis and vasculitis in naive mice. MPO-ANCA–treated mice developed hematuria, proteinuria, and leukocyturia within 3 days and prominent lesions of the lung and kidney within 6 days without LPS or TNF-α administrations. The discrepancy between the effects of mPR3-ANCAs and mMPO-ANCAs on neutrophils in mice are hard to clarify at present. They may originate from differences in immunogenicity, tissue retention, and binding or functional behavior of the respective autoantigens in mice. We paid particular attention to the purity of the recombinant murine autoantigen and unique specificity of the ANCAs generated in antigen-deficient knockout animals and we used whole serum to avoid the formation of immunoglobulin oligomers and small aggregates during the purification process. Although we were able to demonstrate the binding of mPR3-ANCAs on live neutrophils after their in vivo activation and transmigration into the peritoneal cavity (Figure 3), mPR3-ANCAs do not appear to fully activate the autologous murine neutrophils in the circulation of LPS-exposed mice. In contrast to mMPO-ANCAs, mPR3-ANCAs did not directly cause capillaritis and glomerulonephritis in healthy mice. The pathogenic potential of mPR3-ANCAs, which we observed in vivo only after local stimulation of neutrophils, is, however, fully consistent with clinical observations and the concept of conditional pathogenicity of human PR3-ANCAs in WG.48,49
Technical differences between our study and the mMPO-ANCA model alone cannot explain the observed differential pathogenicity of mPR3- and mMPO-ANCA types in mice. We would also like to point out that the biophysical and structural properties of the 2 antigens from murine granulocytes are very different. mMPO is highly basic and has a theoretical isoelectric point (pI) of 10.2, whereas mPR3 is much less basic displaying a pI of 6.7. In contrast, the theoretical pI of mature human PR3 is 7.7 and thus human PR3 is more basic than mPR3. Differential ionic interactions with negatively charged proteoglycans and matrix components in the kidney and other tissues could account for the differences of experimentally induced lesions. Above all, mMPO shows strong similarity to eosinophil myeloperoxidase (eg, 67.5% residue identities), whereas mPR3 shares only 54.5% identities with mNE. Even the second closest relative of the murine genome, lactoperoxidase, shares another 51.0% residues with mMPO. All members of the peroxidase family are 3-fold larger than PR3 homologs, which again increases the chance of their epitope sharing and cross-reactivity with polyclonal mMPO antibodies. The differentiation between the 2 types of ANCAs and associated disease phenotypes is not controversial among vasculitis experts. In fact, our study strongly supports the clinical view that in vivo PR3-ANCAs are not functionally equivalent with MPO-ANCAs.
The limitations of both experimental systems, which in fact apply to many other autoimmune models, is the artificial generation of an autologous T- and B-cell repertoire in antigen-deficient knockout mice. Antibodies generated in mPR3-deficient knockout mice against the murine antigen do not share the PR3 3-dimensional structure-specific binding properties of PR3-ANCAs from patients with WG. As is known, the latter WG-associated autoantibodies do not cross-react with mPR3.32 The hypothesis that a restricted number of disease-promoting epitopes on human PR3 are recognized by WG autoantibodies mediating pathogenicity is attractive, but cannot be tested and proven at present in an animal system, because both the human PR3 antigen and the as yet poorly characterized human autoantibody repertoire of the B-cell compartment would be required in animals. However, potential interference of antibodies with the biologic and pathologic functions of PR3 can be investigated in an autologous animal system as we did. Some of the many mPR3-specific antibodies that we raised in knockout animals most likely target surface regions on mPR3 that are topologically and functionally equivalent with the human autoantigen. It is thus quite reasonable to assume that the proinflammatory actions of PR3 antibodies observed in our mouse model are relevant to the human disease.
Our in vivo findings, moreover, encourage new therapeutic strategies currently under investigation in patients with WG. One strategy is the specific therapeutic inhibition of TNF-α.50,51 This is thought to be beneficial by reducing the recruitment of inflammatory cells and by inhibiting the priming of neutrophils and monocytes and thus the expression of ANCA target antigens on their surface. The other therapeutic approach supported by our animal model is the removal of pathogenic ANCAs from the circulation. The partial benefits of plasma exchange, reduction of immunoglobulins or PR3-ANCAs by semiselective immunoadsorption, and anti-B-cell therapy reported so far also provide some confidence in this latter approach. Specific elimination of the proinflammatory ANCAs may help to overcome the limitations and adverse effects of conventional, immunosuppressive therapy in WG.52-56 To this end, the use of renatured recombinant antigen as the ANCA-adsorbing ligand, produced in E coli with the new method described here, may form the basis for improving the specificity of PR3-ANCA immunoadsorption.
Prepublished online as Blood First Edition Paper, May 18, 2004; DOI 10.1182/blood-2004-01-0267.
Supported by German Research Council grant SFB571 (D.E.J.) and National Institutes of Health grant AI-47572 (U.S.).
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank W. Lasinger, M. Skerhut, B. Maar, and B. Heuser for excellent technical assistance; M. Bradl and R. Hohlfeld for helpful discussions; and Prof H. Wekerle for his continuous interest and support of the work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal