Comment on Zhang and Lodish, page 1679
Expression of oncogenic H-ras in primary erythroid progenitors activates several signaling pathways of which the MEK/ERK pathway alone is sufficient and necessary in blocking terminal erythroid differentiation.
Ras signaling is required for normal erythropoiesis as gene knockouts in either the K- or N-ras genes in mice result in anemia and early embryonic lethality.1 Likewise, oncogenic mutations in the N- and K-ras genes are common in many myeloid malignancies2 and are associated with causing defects in erythroid differentiation.3,4 Understanding the molecular basis for how oncogenic Ras signaling deregulates erythropoiesis has been hampered by the technical difficulties in isolating enough primary erythroid progenitors for biochemical analyses.
Studies by Zhang and Lodish in this issue of Blood employ a previously described in vitro system of erythroid progenitors isolated from mouse fetal livers that supports their normal proliferation and terminal erythroid differentiation.5 Zhang and Lodish show that erythroid progenitors transduced by retroviral vectors expressing oncogenic H-ras (H-ras.V12) activate the mitogen-induced extracellular kinase (MEK)/extracellular signal-regulated kinase (ERK), phosphatidylinositol 3-kinase (PI3-kinase)/Akt, and RalGEF/RalA signaling pathways. Using constitutively active (ca) mutants, each of the 3 pathways was individually induced to determine which one is critical for blocking terminal erythroid differentiation and triggering erythropoietin (Epo)-independent proliferation. Interestingly, expression of constitutively active MEK1 (ca.MEK) was sufficient to mimic the effects of oncogenic H-ras in the erythroid progenitors. Thus, ERK signaling through oncogenic H-ras is critical for blocking terminal differentiation and triggering Epo-independent proliferation of primary erythroid precursors. While ca.Akt or ca.Rlf mutants alone showed little effect, both ca.Akt and ca.MEK together acted synergistically in extending Epo-independent proliferation of erythroid precursors well beyond that observed for H-ras.V12. In contrast, ca.Rlf antagonized the effects of the ca.MEK mutant on Epo-independent proliferation, indicating that a complex balance between all 3 pathways occurs in regulating proliferation of early-stage erythroid progenitors.FIG1
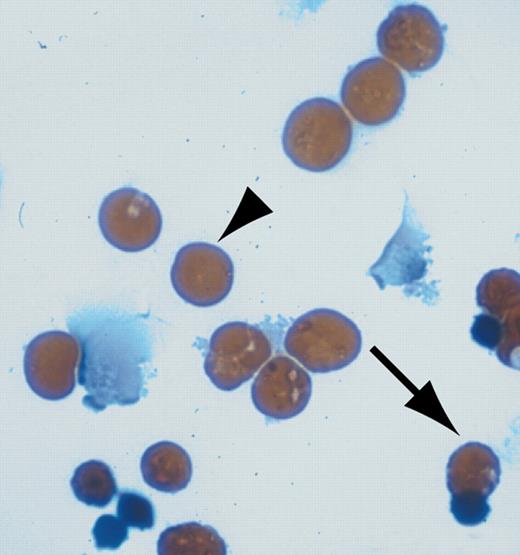
U0126 restores erythroid differentiation in H-ras.V12-transduced erythroid progenitors. See the complete figure in the article beginning on page 1679.
U0126 restores erythroid differentiation in H-ras.V12-transduced erythroid progenitors. See the complete figure in the article beginning on page 1679.
The most intriguing finding of this study came when primary erythroid progenitors expressing H-ras.V12 were treated with the specific MEK1/2 inhibitor UO126. Remarkably, inhibition of the ERK pathway restored normal terminal erythroid differentiation. This was shown by Benzidine-Giemsa staining that detects hemoglobin, a classic marker for terminal erythroid differentiation. Similar results were observed with overexpression of a dominant-negative MEK mutant. Interestingly, no significant increase of apoptosis was observed in erythroid cells expressing H-ras.V12 in the presence of the MEK inhibitor. Perhaps this is due to the survival effects of elevated levels of active Akt.
Although Ras mutations in patients with myeloid disorders occur predominantly in the N- and K-ras genes, the authors report that overexpression of oncogenic N-ras in isolated erythroid progenitors gave similar results to oncogenic H-ras expression, validating the physiologic relevance of their data. Overall, targeting the inhibition of the MEK/ERK pathway in patients with myeloid disorders provides an exciting possibility for restoring normal erythropoiesis.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal