Two studies provide a conceptual framework for understanding how statins overcome drug resistance in leukemia and myeloma, and furnish support for strategies incorporating such agents into the therapeutic armamentarium.
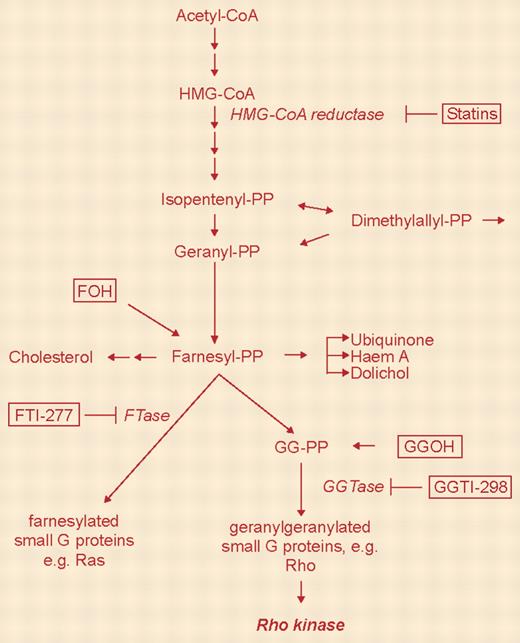
Two studies appearing in this month's issue of Blood suggest that perturbations in cholesterol biosynthesis may modulate cytotoxic drug resistance in malignant hematopoietic cells and provide support for incorporating statins into the therapeutic armamentarium. The rate-limiting step in the mevalonate pathway of cholesterol synthesis is catalyzed by βhydroxyβmethylglutaryl-coenzyme A (HMG-CoA) reductase, an enzyme that is inhibited by a variety of statins, including lovastatin, simvastatin, and cerivastatin, among others.1 However, HMG-CoA reductase is involved in the synthesis of various other molecules, including ubiquinone, dolichol, and, importantly, farnesyl and geranyl isoprenoids, which are involved in membrane binding and activation of several signaling proteins. Proteins requiring geranylgeranylation or farnesylation include guanosine 5′-triphosphate (GTP)-binding proteins such as Ras, as well as the Rho family members Rac1, RhoA, RhoB, and CDC42.2 Notably, oncogenic Ras mutations, which signal downstream to cyto-protective survival pathways such as Raf/mitogen-induced extracellular kinase (MEK)/extracellular signal-related kinase (ERK) and phosphatidylinositol 3-kinase (PI3-kinase)/Akt, occur frequently in hematologic malignancies.3 Such considerations have prompted the development of farnesyltransferase inhibitors for the treatment of these diseases. However, evidence is accumulating that statins, perhaps through analogous mechanisms, also kill malignant hematopoietic cells and/or increase their susceptibility to standard cytotoxic drugs.4-6 FIG1
The mammalian mevalonate pathway. See the complete figure in the article beginning on page 1825.
The mammalian mevalonate pathway. See the complete figure in the article beginning on page 1825.
The 2 studies described in this issue suggest that in addition to killing these cells, statins might also circumvent drug resistance. For example, Schmidmaier and colleagues report that the HMG-CoA reductase inhibitors simvastatin and lovastatin effectively overcome cell adhesion-mediated drug resistance (CAM-DR) in human multiple myeloma cells. Furthermore, this process involves geranylgeranylation, rather than farnesylation, of downstream targets such as Rho. The mechanism underlying CAM-DR is not known but may stem from activation of survival pathways related to integrin signaling cascades.7 Thus, by disrupting the mevalonate pathway, statins might act upstream of integrin pathways to permit proapoptotic signals to proceed un-opposed. One possible clinical implication of these findings is that cytotoxic drug resistance stemming from bone marrow stromal cell interactions might be overcome by coad-ministering statins.
In the second study, Banker and colleagues report that exposure of primary acute myeloid leukemia (AML) cells to cytotoxic drugs such as ARA-C (cytarabine) and daunorubicin resulted in increases in mRNA levels of HMGCoA reductase and the low-density lipoprotein (LDL) receptor; furthermore, coordinate changes in cholesterol levels and accumulation of low-density lipoproteins were noted in the case of daunorubicin-treated but not ARA-C-treated cells. In a subset of specimens, coadministration of the HMG-CoA reductase inhibitor mevastatin with cytotoxic drugs resulted in a net increase in LDL accumulation, consistent with its known actions. Collectively, these findings are consistent with a model in which leukemic cells exposed to certain antileukemic agents mount a cyto-protective response consisting of increases in LDL levels leading to enhanced cholesterol import. This model provides a theoretical foundation for combining statins with antileukemic agents based on the concept of disabling the critical cytoprotective lipid signaling pathway.
A key question is whether the end products of the mevalonate cascade (eg, cholesterol) directly protect cells from noxious stimuli or simply represent by-products stemming from activation of more proximal steps in related cytoprotective pathways. Definitive answers to this question may require specific inhibitors of more distal steps in cholesterol biosynthesis. However, it is unlikely to be coincidental that in addition to their effects on cholesterol levels, statins perturb a variety of signaling pathways that are intimately involved in cell survival decisions, particularly those related to ERK, Akt, and Rho pathways. Consequently, statins may mimic the actions of MEK or Akt inhibitors in potentiating the lethal effects of conventional agents. Whatever the mechanism of chemosensitization, it seems likely that interest in the use of statins in hematologic malignancies, either alone or in combination with established cytotoxic drugs, will only accelerate in the future.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal