Abstract
Primary cutaneous lymphomas have been successfully treated with interferons (IFNs), counterbalancing the T-helper 2 (Th2)-skewing state. We undertook a phase 1, open-label, dose-escalating trial of repeated intratumoral administration of TG1042 in patients with advanced primary cutaneous T-cell lymphomas (CTCLs) and multilesional cutaneous B-cell lymphomas (CBCLs). TG1042 is a third-generation, nonreplicating human adenovirus vector containing a human IFN-γ cDNA insert. Nine patients (7 CTCL, 2 CBCL) were enrolled at the following TG1042 doses: 3 × 109, 3 × 1010, and 3 × 1011 total particles. Local clinical response was observed in 5 of 9 treated patients (3 patients with complete response [CR] and 2 patients with partial response [PR]). Out of these, 3 patients showed systemic CR with the clearance of other noninjected skin lesions. Clinical response lasted for a median of 3 months (range, 1-6 months). Adverse events were mostly of grades 1 and 2. Seven of 9 treated patients had a detectable TG1042-derived IFN-γ message in injected lesions after the first treatment cycle. A TG1042-IFN-γ message was also detectable after several treatment cycles. We demonstrate the induction of humoral immune response to lymphoma tumor-antigen se70-2 after treatment. Our study shows that intralesional injections of TG1042 are both safe and well tolerated. (Blood. 2004;104:1631-1638)
Introduction
Primary cutaneous lymphomas (CLs) are a heterogeneous group of extranodal non-Hodgkin lymphomas with an incidence estimated to be around 1 in 100 000 per year.1 These lymphoproliferative disorders are characterized by an accumulation of clonal T or B lymphocytes preferentially homing to the skin.2 Extensive investigations have documented an imbalanced local cytokine milieu3 promoting the survival of the tumor cell populations by viability factors such as interleukin 7 (IL-7)4 and IL-15.5 Clonal T-cell populations of primary cutaneous T-cell lymphomas (CTCLs) transcribe and secrete T-helper 2 (Th2) cytokines such as IL-10.6-8 This could explain immunosuppressive phenomena such as reduced delayed-type hypersensitivity reactions, diminished natural killer (NK) cell activity,9 hypereosinophilia, and elevated immunoglobulin E (IgE) serum levels in patients with CL.2 An IL-10-dominated cytokine profile has been demonstrated in primary cutaneous B-cell lymphomas (CBCLs) as well.10 Therapeutic approaches supporting Th1-type immune response using interferon α (IFN-α),11 IFN-γ,12 or IL-1213 are shown to be clinically effective in this group of diseases.
However, therapy with recombinant cytokines is hampered by their short half-life and significant side effects. Based on the current concept of the CL immunobiology, we have tailored a new approach to target the tumor-bearing cutaneous compartment by intralesional injection of an optimized recombinant adenoviral vector permitting transfer of human IFN-γ cDNA. IFN-γ gene delivery resulted in the regression of injected and noninjected lymphoma lesions. We demonstrate intralesional transgene expression even after several injection cycles as well as systemic antitumor activity.
Patients, materials, and methods
Patients
Nine patients, 7 of whom presented with advanced CTCL and 2 with multilesional CBCL, were enrolled in a phase 1, open-label, dose-escalating trial of repeated intratumoral administration of TG1042 supported by Transgene S.A. (Strasbourg, France). The study was approved by the local institutional ethical committee, Swiss Agency for Therapeutic Products (Swissmedic), and Swiss Expert Committee for Biosafety (EFBS, former SKBS). Patient characteristics are presented in Table 1. In order to be eligible for the study, patients had to fulfill different criteria including histologically proven CTCL or CBCL; performance status of 0, 1, or 2 on the Eastern Cooperative Oncology Group (ECOG) scale; tumor-nodemetastasis (TNM) stage Ib or higher; failure of local tumor control by at least 2 first-line treatments; no systemic steroid, chemotherapy, or immunotherapy as well as no topical steroid or radiation therapy within 3 weeks preceding enrollment; and minimum life expectancy longer than 3 months. Prior to entering the study, all patients provided written informed consent.
Characteristics of the patients
. | . | . | . | . | . | . | . | . | Response . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | Diagnosis . | Stage . | Previous treatment . | Age, y . | Sex . | Cohort . | Dose, tp's . | No. of injections . | Injected lesion . | Noninjected lesion . | Overall . | Duration, mos.* . | |||
| 1 | Mycosis fungoides | Ib | Topical steroids, PUVA, IFN-α, acitretin, bexarotene | 84 | M | 1 | 3 × 109 | 3 | PR | SD | PR | 6 | |||
| 2 | Mycosis fungoides | IIa | Topical steroids, PUVA, imiquimod | 67 | F | 1 | 3 × 109 | 12 | CR | CR | CR | 1 | |||
| 3 | Granulomatous slack skin | IIb | Topical steroids, PUVA, IFN-α, acitretin, imiquimod | 26 | M | 1 | 3 × 109 | 12 | SD | SD | SD | NE | |||
| 4 | Mycosis fungoides | IIb | Topical steroids, PUVA, IFN-α, acitretin, radiotherapy | 54 | F | 2 | 3 × 1010 | 3 | SD | PD | PD | NE | |||
| 5 | Sézary syndrome | III | Topical steroids, calcipotriole, PUVA, IFN-α, acitretin, methotrexate | 57 | M | 2 | 3 × 1010 | 9 | SD | SD | SD | NE | |||
| 6 | Lymphomatoid papulosis | NA | Topical steroids, PUVA | 40 | F | 2 | 3 × 1010 | 5 | CR | CR | CR | 3 | |||
| 7 | Sézary syndrome | III | Topical steroids, PUVA | 68 | M | 3 | 3 × 1011 | 3 | SD | SD | SD | NE | |||
| 8 | Marginal zone B-cell lymphoma | NA | Topical steroids, antibiotics | 34 | M | 3 | 3 × 1011 | 9 | PR | NE† | NE† | 5 | |||
| 9 | Follicular center cell B-cell lymphoma | NA | Radiotherapy, rituximab | 34 | M | 3 | 3 × 1011 | 3 | CR | SD | PR | 1 | |||
. | . | . | . | . | . | . | . | . | Response . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | Diagnosis . | Stage . | Previous treatment . | Age, y . | Sex . | Cohort . | Dose, tp's . | No. of injections . | Injected lesion . | Noninjected lesion . | Overall . | Duration, mos.* . | |||
| 1 | Mycosis fungoides | Ib | Topical steroids, PUVA, IFN-α, acitretin, bexarotene | 84 | M | 1 | 3 × 109 | 3 | PR | SD | PR | 6 | |||
| 2 | Mycosis fungoides | IIa | Topical steroids, PUVA, imiquimod | 67 | F | 1 | 3 × 109 | 12 | CR | CR | CR | 1 | |||
| 3 | Granulomatous slack skin | IIb | Topical steroids, PUVA, IFN-α, acitretin, imiquimod | 26 | M | 1 | 3 × 109 | 12 | SD | SD | SD | NE | |||
| 4 | Mycosis fungoides | IIb | Topical steroids, PUVA, IFN-α, acitretin, radiotherapy | 54 | F | 2 | 3 × 1010 | 3 | SD | PD | PD | NE | |||
| 5 | Sézary syndrome | III | Topical steroids, calcipotriole, PUVA, IFN-α, acitretin, methotrexate | 57 | M | 2 | 3 × 1010 | 9 | SD | SD | SD | NE | |||
| 6 | Lymphomatoid papulosis | NA | Topical steroids, PUVA | 40 | F | 2 | 3 × 1010 | 5 | CR | CR | CR | 3 | |||
| 7 | Sézary syndrome | III | Topical steroids, PUVA | 68 | M | 3 | 3 × 1011 | 3 | SD | SD | SD | NE | |||
| 8 | Marginal zone B-cell lymphoma | NA | Topical steroids, antibiotics | 34 | M | 3 | 3 × 1011 | 9 | PR | NE† | NE† | 5 | |||
| 9 | Follicular center cell B-cell lymphoma | NA | Radiotherapy, rituximab | 34 | M | 3 | 3 × 1011 | 3 | CR | SD | PR | 1 | |||
PUVA indicates psoralen ultraviolet A treatment (psoralen and UVA light); PR, a > 50% decrease in the size of preexisting lesions, also assigned if > 50% of nodular or plaquelike lesions become macules without evidence of internal involvement; SD, any response that did not meet the criteria for CR, PR, or PD; CR, the absence of detectable residual disease; NE, not evaluated in patients with SD; PD, the appearance of new lesions, an increase of > 25% of preexisting lesions, a change from macular to plaquelike or nodular in > 25% of preexisting lesions, or any evidence of internal organ involvement; and NA, not applicable.
Response duration is defined as a recurrence-free period upon completion of TG1042 treatment.
Not evaluable due to the absence of other lesions.
Administration of the TG1042
TG1042 is a nonreplicating (E1 and E3 regions deleted) adenovirus type 5 (group C) vector containing a human IFN-γ cDNA insert. The cytomegalovirus (CMV) promoter-driven expression of IFN-γ gene should result in the prolonged local expression of IFN-γ. Patients received intratumoral TG1042 injections into the designated lesion once weekly for 3 weeks with no injection in the fourth week (defines 1 treatment cycle) and thereafter up to 4 cycles, if there was no evidence of progressive disease. TG1042 concentration was increased after every third patient from 3 × 109 to up to 3 × 1011 total particles (tp's) or to the maximum tolerated dose (MTD), whichever occurred first (Table 1). Patients were monitored clinically in the day care clinic ward until 6 hours after the injection and again 24 hours thereafter. The patients were seen for clinical tumor assessment 2 weeks after the third injection of each cycle. At that point, the lesions were measured accurately to decide whether the treatment should be continued. Clinical outcome was recorded for the injected lesion, noninjected lesions, and the overall status (for definition see Table 1).
Detection of TG1042 transgene expression in injected lesions
Expression of the TG1042-derived IFN-γ messenger RNA (mRNA) was detected in skin biopsies by quantitative polymerase chain reaction (PCR) using LightCycler technology. The lesion designated for TG1042 injection was biopsied before (baseline) and on day 16 of each treatment cycle. Total RNA was extracted from frozen biopsy specimens using TRIzol reagent (Invitrogen AG, Basel, Switzerland) according to manufacturer's recommendations. Approximately 1 μg of total RNA was reverse transcribed using the 1st Strand cDNA Synthesis Kit for RT-PCR (Roche Molecular Biochemicals, Mannheim, Germany). The primers to detect TG1042-derived IFN-γ were positioned within an intron, included in the expression cassette and specific to recombinant adenovirus, and IFN-γ cDNA. The specificity of the PCR product was confirmed by sequencing. Injected lesions were assessed for the production of total IFN-γ relative to CD4 and CD8 mRNA (CD4, CD8, and IFN-γ quantification kit; Search LC, Heidelberg, Germany).14 To compensate for the variations in quantity and quality of starting mRNA, the absolute IFN-γ, CD8, and CD4 mRNA copy numbers/μL were normalized with respect to the absolute glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA copy number/μL.15
Immunohistochemistry
Paraffin-embedded tissue sections were stained with the following primary antibodies: T-cell restricted intracellular antigen (TIA-1; clone 2G9), CD1a (clone O10; both from Immunotech, Marseille, France), CD3, CD4 (MT310), CD8 (DK25), CD20 (L26), CD21, CD56 (T199), CD79a (HM57; all from DakoCytomation, Glostrup, Denmark), anti-coxsackie adenovirus receptor (anti-CAR; used on cryostat tissue sections, kindly provided by Dr Sylvio Hemmi, Department of Molecular Biology, University of Zurich), and HLA class I (HCA-1; kind gift from S. Ferrone, Roswell Park Cancer Institute, Buffalo, NY). After antigen retrieval, immunohistochemistry was performed using alkaline phosphatase-antialkaline phosphatase (APAAP) technique, as previously described.15 Images were acquired using a Zeiss AxioSkop 2 plus, objective 20×/0.45 (Achroplan) or objective 40×/0.75 (Plan-Neo-Fluor); a Zeiss Axio Cam color camera; and Axio Vision 3.1.2.1 (Zeiss).
Immunology and virology evaluations
Patients' sera were assessed for IL-6, IFN-γ, and neopterin levels prior to (0 h), 6 hours, and 24 hours after each TG1042 injection. In addition, autoimmunity parameters (thyroid-stimulating hormone [TSH], anti-thyroperoxidase [anti-TPO], and anti-double-stranded DNA [anti-dsDNA] antibodies) were assessed prior to first TG1042 injection and on day 29 of each treatment cycle. The sera were tested in the certified laboratories of the Institute for Clinical Chemistry and Division of Clinical Immunology, Department of Internal Medicine, University Hospital Zurich. The titer of serum neutralizing antiadenovirus antibodies (NAAbs) and antibody titer against cutaneous lymphoma-associated antigen se70-2 were determined in plasma samples taken at baseline and on day 29 of each treatment cycle (after third injection). For the detection of NAAbs, dilutions of serum were assessed for their ability to block in vitro cellular infection by infectious particles of adenovirus, as previously described.16 Titer is expressed as the dilution of serum, which blocks infection by 50% of 300 infectious particles. The se70-2 represents a CL-associated antigen that has been recently defined using the Serological Analysis of Recombinant cDNA Expression Libraries (SEREX) approach.17 The se70-2 seroreactivity was assessed by a glutathione-S-transferase (GST)-capture enzyme-linked immunosorbent assay (ELISA) using a fusion protein composed of GST/se70-2/SV40-tag.
All patients were monitored for the presence of viral particles by PCR. Plasma was collected prior to each injection, 1 to 2 hours after injection for the first 3 injections, and at the end of the study; urine samples were collected prior to each injection, 2 to 4 hours after injection for the first 3 injections, and at the end of the study.
Results
Clinical outcome
Local clinical response was observed in 5 of 9 patients who received TG1042 intratumoral injections. Injected lesions showed complete response (CR) in 3 patients and partial response (PR) in 2 cases (Table 1). Systemic CR was seen in 2 patients who suffered from mycosis fungoides (MFs) and lymphomatoid papulosis, respectively, whereas systemic PR was seen in one patient with follicular center cell lymphoma and in one patient with MF. Two patients with Sézary syndrome (the leukemic variant of CTCL) and a patient with granulomatous slack skin remained stable. Only one patient with MF showed disease progression. In patients showing objective clinical response, response lasted for a median of 3 months (range, 1-6 months). It is of note that in patients who relapsed, relapse was less severe than prior to entering the TG1042 study (Table 1).
Safety
All 9 patients were assessable for toxicity evaluation (Table 2). No patient died as a result of TG1042 administration. No grade 3 or 4 dose-limiting toxicities were observed. One serious adverse event (AE) was considered as possibly related to the treatment. Patient 2, enrolled at dose level 1, experienced an unspecific colitis requiring 5 days hospitalization 10 days after the ninth injection. Patient recovered without sequelae. AEs were mostly grade 1 or grade 2 (the latter observed only in 4 patients). Most frequently reported AEs were injection site reaction, fatigue (malaise), fever, and headache (Table 2). Headache appears to be the only dose-dependent AE, with 1 patient affected at 3 × 109, 2 patients at 3 × 1010, and 3 patients at 3 × 1011 dose level (Table 2). All patients were evaluated for the presence of viral particles by PCR (Table 3). Urine samples were tested and returned negative for the presence of viral particles (data not shown).
Safety data
. | No. events by dose level*(no. of patients†) . | . | . | All patients, n = 9 . | ||
|---|---|---|---|---|---|---|
| Disorders (details) . | 3 × 109 tp's, n = 3 . | 3 × 1010 tp's, n = 3 . | 3 × 1011 tp's, n = 3 . | . | ||
| General disorders and the administration site conditions | 37 (3) | 23 (3) | 54 (3) | 114 (9) | ||
| Injection site reactions (burning, pain, edema, induration, erythema, vesicles, movement impairment, pruritus, others) | 19 (3‡ | 10 (2‡ | 30 (3‡‡ | 59 (8) | ||
| Asthenic conditions (fatigue, malaise) | 13 (3) | 4 (3) | 6 (3) | 23 (9) | ||
| Body temperature perception (chills, cold sensation) | 0 (0) | 4 (2) | 6 (3) | 10 (5) | ||
| Febrile disorders (fever) | 5 (2) | 5 (2) | 9 (3) | 19 (7) | ||
| General signs and symptoms (dyspnea) | 0 (0) | 0 (0) | 3 (1) | 3 (1) | ||
| Blood and lymphatic system (lymphadenopathy) | 0 (0) | 0 (0) | 1 (1) | 1 (1) | ||
| Cardiac (arrhythmia, tachycardia) | 0 (0) | 1 (1) | 1 (1) | 2 (2) | ||
| Gastrointestinal (diarrhea, nausea) | 3 (1) | 0 (0) | 2 (2) | 5 (3) | ||
| Musculoskeletal and connective tissue (limb pain) | 0 (0) | 2 (2) | 3 (2) | 5 (4) | ||
| Nervous system (dizziness, headache) | 1 (1) | 3 (2) | 9 (3) | 13 (6) | ||
| Psychiatric (sleep disorders) | 0 (0) | 0 (0) | 2 (2) | 2 (2) | ||
| Respiratory (nose bleeding, cough) | 3 (2) | 0 (0) | 1 (1) | 4 (3) | ||
| Skin (exfoliative dermatitis, sweating, erythema, rash, fissures, pruritus) | 1 (1) | 7 (2) | 2 (1) | 10 (4) | ||
| Vascular (flushing) | 0 (0) | 2 (1) | 2 (2) | 4 (3) | ||
. | No. events by dose level*(no. of patients†) . | . | . | All patients, n = 9 . | ||
|---|---|---|---|---|---|---|
| Disorders (details) . | 3 × 109 tp's, n = 3 . | 3 × 1010 tp's, n = 3 . | 3 × 1011 tp's, n = 3 . | . | ||
| General disorders and the administration site conditions | 37 (3) | 23 (3) | 54 (3) | 114 (9) | ||
| Injection site reactions (burning, pain, edema, induration, erythema, vesicles, movement impairment, pruritus, others) | 19 (3‡ | 10 (2‡ | 30 (3‡‡ | 59 (8) | ||
| Asthenic conditions (fatigue, malaise) | 13 (3) | 4 (3) | 6 (3) | 23 (9) | ||
| Body temperature perception (chills, cold sensation) | 0 (0) | 4 (2) | 6 (3) | 10 (5) | ||
| Febrile disorders (fever) | 5 (2) | 5 (2) | 9 (3) | 19 (7) | ||
| General signs and symptoms (dyspnea) | 0 (0) | 0 (0) | 3 (1) | 3 (1) | ||
| Blood and lymphatic system (lymphadenopathy) | 0 (0) | 0 (0) | 1 (1) | 1 (1) | ||
| Cardiac (arrhythmia, tachycardia) | 0 (0) | 1 (1) | 1 (1) | 2 (2) | ||
| Gastrointestinal (diarrhea, nausea) | 3 (1) | 0 (0) | 2 (2) | 5 (3) | ||
| Musculoskeletal and connective tissue (limb pain) | 0 (0) | 2 (2) | 3 (2) | 5 (4) | ||
| Nervous system (dizziness, headache) | 1 (1) | 3 (2) | 9 (3) | 13 (6) | ||
| Psychiatric (sleep disorders) | 0 (0) | 0 (0) | 2 (2) | 2 (2) | ||
| Respiratory (nose bleeding, cough) | 3 (2) | 0 (0) | 1 (1) | 4 (3) | ||
| Skin (exfoliative dermatitis, sweating, erythema, rash, fissures, pruritus) | 1 (1) | 7 (2) | 2 (1) | 10 (4) | ||
| Vascular (flushing) | 0 (0) | 2 (1) | 2 (2) | 4 (3) | ||
Adverse events shown according to the dose level and system organ class.
Adverse events occurring several times in a single patient are counted separately. Listed AEs were of grade 1.
Only injection site reaction presented as grade 2 in 4 patients.
Detection of the viral particles in plasma samples by PCR
. | No. copies per mL, by time point . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | Day 1, hour 0 . | Day 1, hour 1-2 . | Day 2 . | Day 8, hour 0 . | Day 8, hour 1-2 . | Day 15, hour 0 . | Day 15, hour 1-2 . | End of study . | |||||||
| 1 | 0 | — | 0 | 0 | NQ | 0 | NQ | 0 | |||||||
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| 3 | 0 | 1.92 × 102 | 0 | 0 | NQ | 0 | 0 | 0 | |||||||
| 4 | 0 | 1.82 × 102 | 0 | 0 | NQ | 0 | NQ | 0 | |||||||
| 5 | 0 | 0 | NQ | 0 | 0 | 0 | 0 | 0 | |||||||
| 6 | 4.08 × 102 | 0 | — | 0 | NQ | 0 | 0 | 0 | |||||||
| 7 | 0 | 1.17 × 103 | 1.80 × 104 ± 2.93 × 103 | 0 | 4.96 × 102 | NQ | — | 0 | |||||||
| 8 | 0 | 0 | 2.80 × 102 | 0 | NQ | — | — | — | |||||||
| 9 | 0 | 4.80 × 102 | 3.18 × 102 | 0 | 2.87 × 103 ± 2.67 | 0 | 0 | — | |||||||
. | No. copies per mL, by time point . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | Day 1, hour 0 . | Day 1, hour 1-2 . | Day 2 . | Day 8, hour 0 . | Day 8, hour 1-2 . | Day 15, hour 0 . | Day 15, hour 1-2 . | End of study . | |||||||
| 1 | 0 | — | 0 | 0 | NQ | 0 | NQ | 0 | |||||||
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||
| 3 | 0 | 1.92 × 102 | 0 | 0 | NQ | 0 | 0 | 0 | |||||||
| 4 | 0 | 1.82 × 102 | 0 | 0 | NQ | 0 | NQ | 0 | |||||||
| 5 | 0 | 0 | NQ | 0 | 0 | 0 | 0 | 0 | |||||||
| 6 | 4.08 × 102 | 0 | — | 0 | NQ | 0 | 0 | 0 | |||||||
| 7 | 0 | 1.17 × 103 | 1.80 × 104 ± 2.93 × 103 | 0 | 4.96 × 102 | NQ | — | 0 | |||||||
| 8 | 0 | 0 | 2.80 × 102 | 0 | NQ | — | — | — | |||||||
| 9 | 0 | 4.80 × 102 | 3.18 × 102 | 0 | 2.87 × 103 ± 2.67 | 0 | 0 | — | |||||||
—indicates no sample available; and NQ, positive but not quantifiable.
Expression of TG1042-derived IFN-γ mRNA in injected lesions
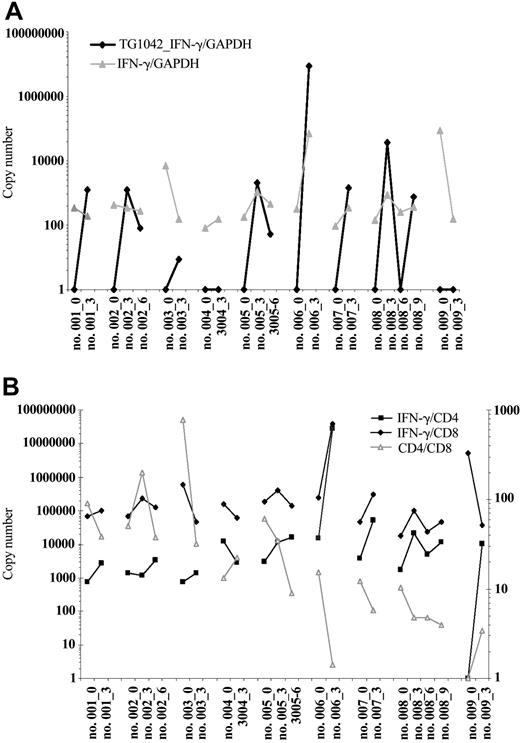
Seven of 9 treated patients had a detectable TG1042-derived IFN-γ message following the first treatment cycle as shown by quantitative PCR (Figure 1A). Patient 4 and patient 9 remained constantly negative for TG1042-IFN-γ mRNA expression. After second treatment cycle, 2 of 3 patients were positive for TG1042-IFN-γ mRNA expression (Figure 1A).
Detectable TG1042-derived IFN-γ messages following the first and second treatment cycles. Changes in expression of TG1042-derived IFN-γ and natural IFN-γ at baseline and after TG1042 injections (A). Results are presented as GAPDH-normalized ratios. IFN-γ production pertinent to CD8 and CD4 T cells assessed by the IFN-γ mRNA to CD8 and CD4 ratio, respectively (plotted on the left y-axis), at baseline and after TG1042 injections (B). The changes in CD4/CD8 ratio are plotted against the right y-axis. Number of injections follows the patient number (eg, no. 001_3).
Detectable TG1042-derived IFN-γ messages following the first and second treatment cycles. Changes in expression of TG1042-derived IFN-γ and natural IFN-γ at baseline and after TG1042 injections (A). Results are presented as GAPDH-normalized ratios. IFN-γ production pertinent to CD8 and CD4 T cells assessed by the IFN-γ mRNA to CD8 and CD4 ratio, respectively (plotted on the left y-axis), at baseline and after TG1042 injections (B). The changes in CD4/CD8 ratio are plotted against the right y-axis. Number of injections follows the patient number (eg, no. 001_3).
Immune response at the injection site
Intratumoral injection of TG1042 induced changes in the cellular infiltrate pattern as well as in expression of different cellular markers. Biopsies of injected lesions were characterized histologically by a change in infiltrate morphology differing from initial lymphoma. Signs of vasculitis and increase in eosinophil and neutrophil numbers were often seen (Figure 2; Table 4). Clear up-regulation of CD8, TIA-1, and CAR immunoreactivity was observed in all cases following the TG1042 treatment (Figure 2; Table 4). There were no significant changes in the expression of CD1a, CD3, CD4, CD20, CD21, CD56, and HLA class I molecules. Quantitative PCR showed a decrease in CD4/CD8 ratio in 6 of 8 patients (Figure 1B). IFN-γ/CD8 ratio increased in 6 of 9 patients (among which 5 showed objective clinical response), while IFN-γ/CD4 ratio increased either after first treatment cycle or later in 7 of 8 patients (Figure 1B).
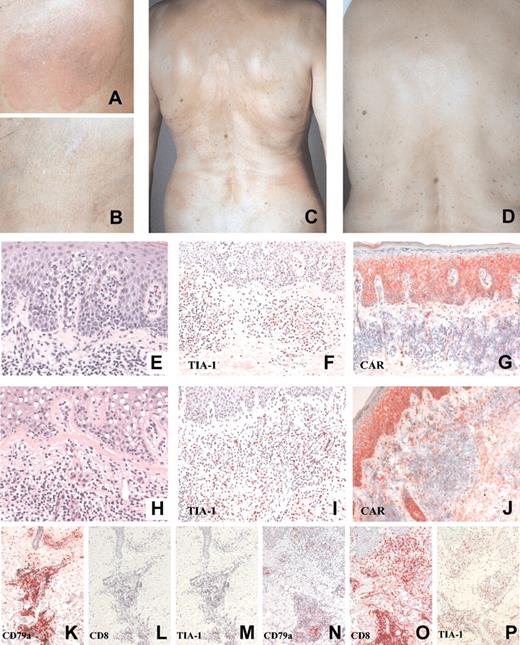
Treatment of patient 2. Patient 2 before (panel A, injected lesion; panel C, noninjected lesions) and after (panel B, injected lesion; panel D, noninjected lesions) TG1042 treatment. Changes in CTCL histology following TG1042 injections (hematoxylin-eosin staining: E, baseline; H, after treatment), TIA-1 (F, baseline; I, after treatment), and CAR (G, baseline; J, after treatment) expression in CTCL. Changes in CD79a, marker for neoplastic B cells (K, baseline; N, after treatment), CD8 (L, baseline; O, after treatment), and TIA-1 (M, baseline; P, after treatment) expression in CBCL. See Table 4. Original magnification, × 40 (E, H); × 20 (F, G, I, J, K-P).
Treatment of patient 2. Patient 2 before (panel A, injected lesion; panel C, noninjected lesions) and after (panel B, injected lesion; panel D, noninjected lesions) TG1042 treatment. Changes in CTCL histology following TG1042 injections (hematoxylin-eosin staining: E, baseline; H, after treatment), TIA-1 (F, baseline; I, after treatment), and CAR (G, baseline; J, after treatment) expression in CTCL. Changes in CD79a, marker for neoplastic B cells (K, baseline; N, after treatment), CD8 (L, baseline; O, after treatment), and TIA-1 (M, baseline; P, after treatment) expression in CBCL. See Table 4. Original magnification, × 40 (E, H); × 20 (F, G, I, J, K-P).
Immunohistochemistry data summary
Patient no. and sample . | CD4 . | CD8 . | CD79a . | TIA-1 . | CAR . |
|---|---|---|---|---|---|
| 1 | |||||
| Baseline | NC | SS | − | − | − |
| After 3 injections | NC | + | − | ++ | ++ |
| 2 | |||||
| Baseline | NC | + | − | + | − |
| After 3 injections | NC | ++ | − | ++ | ++ |
| After 6 injections | NC | ++ | − | +++ | +++ |
| 3 | |||||
| Baseline | NC | SS | − | SS | − |
| After 3 injections | NC | + | − | ++ | ++ |
| 4 | |||||
| Baseline | NC | + | − | + | − |
| After 3 injections | NC | ++ | − | ++ | + |
| 5 | |||||
| Baseline | NC | + | − | + | − |
| After 3 injections | NC | ++ | − | ++ | ++ |
| After 6 injections | NC | ++ | − | +++ | ++ |
| 6 | |||||
| Baseline | NC | − | − | SS | − |
| After 3 injections | NC | + | − | ++ | + |
| 7 | |||||
| Baseline | NC | + | − | SS | − |
| After 3 injections | NC | ++ | − | ++ | ++ |
| 8 | |||||
| Baseline | + | + | +++ | SS | − |
| After 3 injections | + | + | ++ | + | + |
| After 6 injections | ++ | ++ | + | ++ | + |
| After 9 injections | ++ | ++ | + | ++ | + |
| 9 | |||||
| Baseline | ++ | SS | ++ | SS | − |
| After 3 injections | +++ | +++ | + | +++ | + |
Patient no. and sample . | CD4 . | CD8 . | CD79a . | TIA-1 . | CAR . |
|---|---|---|---|---|---|
| 1 | |||||
| Baseline | NC | SS | − | − | − |
| After 3 injections | NC | + | − | ++ | ++ |
| 2 | |||||
| Baseline | NC | + | − | + | − |
| After 3 injections | NC | ++ | − | ++ | ++ |
| After 6 injections | NC | ++ | − | +++ | +++ |
| 3 | |||||
| Baseline | NC | SS | − | SS | − |
| After 3 injections | NC | + | − | ++ | ++ |
| 4 | |||||
| Baseline | NC | + | − | + | − |
| After 3 injections | NC | ++ | − | ++ | + |
| 5 | |||||
| Baseline | NC | + | − | + | − |
| After 3 injections | NC | ++ | − | ++ | ++ |
| After 6 injections | NC | ++ | − | +++ | ++ |
| 6 | |||||
| Baseline | NC | − | − | SS | − |
| After 3 injections | NC | + | − | ++ | + |
| 7 | |||||
| Baseline | NC | + | − | SS | − |
| After 3 injections | NC | ++ | − | ++ | ++ |
| 8 | |||||
| Baseline | + | + | +++ | SS | − |
| After 3 injections | + | + | ++ | + | + |
| After 6 injections | ++ | ++ | + | ++ | + |
| After 9 injections | ++ | ++ | + | ++ | + |
| 9 | |||||
| Baseline | ++ | SS | ++ | SS | − |
| After 3 injections | +++ | +++ | + | +++ | + |
NC indicates no change; SS, single cells positive; −, negative; +, 5%-35% cells positive; ++, 35%-70% cells positive; and +++, > 70% cells positive.
Systemic immune response
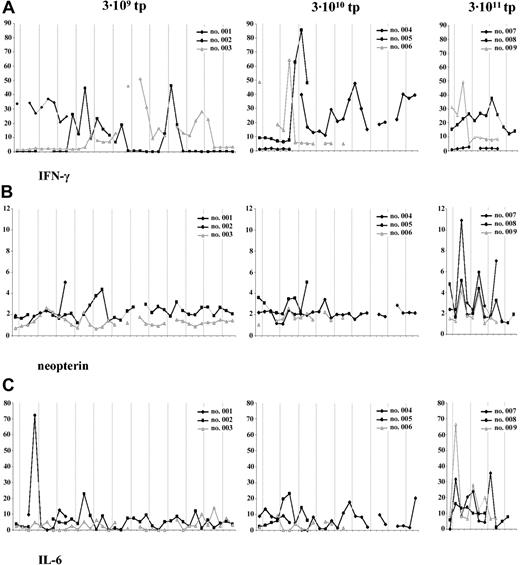
Serum IFN-γ levels showed a dynamic range of changes following TG1042 injections (Figure 3A). In the lowest-dosage cohort (3 × 109 tp's), serum IFN-γ levels in patient 2 started to peak at the beginning of the second treatment cycle, whereas in patient 3, IFN-γ levels peaked comparably at the beginning of the third treatment cycle. In patient 2, IFN-γ levels remained increased during the second treatment cycle as in patient 3, who demonstrated sustained IFN-γ levels during the third treatment cycle as well. In the next cohort (3 × 1010 tp's), changes in IFN-γ levels occurred earlier during the first injection cycle, reaching the maximum of 85.4 pg/mL in patient 4 (Figure 3A). Patient 5 exhibited relatively sustained IFN-γ levels throughout the second and third treatment cycle. In the highest-dosage cohort (3 × 1011 tp's), patient 9 peaked as early as after 24 hours following TG1042 injection (Figure 3A).
Serum levels. Serum levels of IFN-γ (A), neopterin (B), and IL-6 (C) assessed at 3 time points (preinjection, 6 h after injection, and 24 h after injection). Dashed line represents TG1042 injection. Patients are grouped according to the dose level.
Serum levels. Serum levels of IFN-γ (A), neopterin (B), and IL-6 (C) assessed at 3 time points (preinjection, 6 h after injection, and 24 h after injection). Dashed line represents TG1042 injection. Patients are grouped according to the dose level.
Neopterin, as a marker inducible by IFN-γ and thus indicative of IFN-γ presence in serum, increased 24 hours after each TG1042 injection only in the highest-dosage cohort (Figure 3B). In the case of dosage cohorts 1 and 2, neopterin levels did not show any specific changes with respect to TG1042 injections (Figure 3B).
Serum IL-6 levels changed continuously after each TG1042 injection, showing different kinetics with respect to dosage cohorts (Figure 3C). The patients from the highest-dosage cohort showed, in average, the most prominent changes, where IL-6 levels were peaking 6 hours after TG1042 injection.
Patient 2 exhibited an increase in anti-TPO titer following the second and third treatment cycle (data not shown). Apart from that, TSH, anti-dsDNA, and anti-TPO did not show any significant changes in other patients (data not shown).
Four (50%) of 8 patients had detectable preexisting NAAbs (titer > 1:10). Seven patients exhibited a significant increase in NAAbs after TG1042 treatment (Figure 4B).
Antibody titers. Dynamics of neutralizing antiadenovirus (A) and antise70-2 (B) antibody titers during TG1042 treatment. Samples were taken at baseline and after each treatment cycle (day 29).
Antibody titers. Dynamics of neutralizing antiadenovirus (A) and antise70-2 (B) antibody titers during TG1042 treatment. Samples were taken at baseline and after each treatment cycle (day 29).
By using ELISA to detect the humoral response against a CL-associated antigen se70-2, we observed an increase in antibody titers in 8 of 9 evaluated patients (Figure 4C). Two Sézary patients, patients 5 and 7, demonstrated significant increase in se70-2 seroreactivity. Patient 4, who presented with progressive disease, demonstrated a decrease in se70-2 seroreactivity.
Discussion
Cutaneous lymphomas are fragile neoplasms originating from the skin-associated lymphoid tissue. They depend on a specialized microenvironment characterized by the dominance of Th2-type cytokines. Immunotherapies using immune response modifiers (IFN-α, IFN-γ, IL-12) interfering with this cytokine network have shown antitumor activity.18,19 Extensive research in the past several years has demonstrated that IFN-γ plays a vital role in the generation of antitumor immune response. IFN-γ has been shown to inhibit Th2 cytokine production by Sézary cells in vitro.6 IFN-γ is also required for robust IL-12 secretion by antigen-presenting cells. IFN-γ enhances cell-mediated cytotoxicity by activating macrophages and NK cells. Through the up-regulation of major histocompatibility complex (MHC) molecules, transporter associated with antigen processing 1 (TAP-1), and the enhanced expression of tumor-associated antigens, IFN-γ increases the susceptibility of the tumor cells to MHC-restricted lysis by cytotoxic CD8+ T cells (reviewed in Ikeda et al20 ). IFN-γ enhances cytolytic activity of CD8+ T cells against autologous Sézary cells.21 Cutaneous lymphomas would therefore represent a good target for adenovirus-mediated IFN-γ gene delivery.
We conducted a phase 1, open-label, dose-escalating study of repeated intratumoral injections of TG1042 in patients with primary CTCL and multilesional CBCL. We observed clinical response of the injected lesion in 5 of 9 treated patients. Median duration of response was 3 months (range, 1-6 months). Four of these 5 patients demonstrated systemic response as well. Three patients experienced complete clearance of the injected lesion, whereas 2 of them presented with the complete clearance of all other noninjected lesions. The response rate achieved in this study is surprisingly superior to the rates reported in previous studies on the CTCL treatment with IFN-γ. In the study of Kaplan et al,12 which involved 16 patients with stage Ib to IVb CTCL that had received recombinant IFN-γ intramuscularly, 5 patients had partial responses, with median response duration of 10 months (range, 3 to more than 32 months). No complete responses were recorded. Jimbow et al22 treated 6 CTCL patients with intralesional administration of IFN-γ, out of which 3 had complete remission. The median duration of response observed in our study is shorter than that reported by Kaplan et al.12 It is of note, however, that we used an intralesional application of an adenovirus-IFN-γ construct for a minimum of 3 weeks, which is shorter in comparison to intramuscular application of maximally tolerated dose of IFN-γ for 8 weeks and longer used by Kaplan et al.12 It appears that patients who develop a clinical remission on IFN therapy have a high potential for relapse unless treatment is continued past clearing.18,19 Histologic clearance after IFN-γ treatment seems to lag behind clinical clearing and may explain this high relapse rate if IFN is stopped too soon after complete response.19 Literature suggests maintenance IFN treatment (taper) that could lead to prolonged remissions,19 which is a point that should be considered in the design of future trials with TG1042.
We have demonstrated that the TG1042 treatment of CL is both safe and well tolerated. No grade 3 or 4 toxicities were observed. Stool examination from patient 2, who suffered from unspecific colitis, did not demonstrate the presence of adenovirus, defining this serious adverse event (SAE) as possibly treatment related. In general, patients experienced mostly mild to moderate AEs within 24 hours after the injection. This may to be due to alternating levels of IFN-γ upon each injection, with lower systemic IFN-γ levels between injections, obviating many of the toxicities observed with systemic administration of IFN-γ.12,19
We could demonstrate successful IFN-γ gene transfer in 7 of 9 treated patients. A transgene-derived IFN-γ message was detectable even after several injection cycles, despite the presence of NAAbs in these patients. Irrespective of their NAAb titer prior to the treatment, all patients mounted a vigorous antibody response after the first injection. It appears that the presence of NAAbs does not significantly abrogate intralesional IFN-γ gene transfer in these patients. NAAbs may even prevent from vector spreading in the circulation. Three-dimensional structure of the injected lesion as well as the vector turnover in the lesion may be factors contributing to the biopsy material variability and thus to the different PCR results.
Intralesional injections of TG1042 resulted in changes of the initial lymphoma histology as well as in expression of various cellular markers. Adenoviral-IFN-γ gene transfer induced an influx of eosinophils and neutrophils, followed by CD8+ T cells. Marked increase in CD8 and TIA-1 immunoreactivity (expressed by granulocytic infiltrate as well) is indicative of immune activation following the TG1042 injection. CD8+ cytotoxic T lymphocytes (CTLs) are considered to be a critical component in the antitumor immune response in CTCL. The influx of CD8+ CTLs was seen in regressing MF tumors on intralesional administration of IL-12.13 Moreover, in vitro studies have consistently shown that malignant cells in MF display tumor-specific antigens that can be recognized by autologous CTLs.23-26 CD8+ CTL-mediated tumor-cell lysis is achieved, ie, through exocytosis of granules containing cytotoxic protein TIA-1. Following injection with adenovirus-engineered IL-2-expressing autologous plasma cells, Trudel et al27 reported the induction of local inflammatory response consisting predominantly of CD8+ and/or TIA-1+ T cells as well. Real-time PCR demonstrated decrease in CD4/CD8 ratio in 6 of 8 patients following TG1042 injections, confirming once again the increase in CD8+ cells. IFN-γ seems to be produced from both CD8+ as well as from CD4+ T cells according to the increase in IFN-γ/CD8 and IFN-γ/CD4 ratios. Although weak at baseline, CAR expression on infiltrating cells increased markedly upon TG1042 injections, remaining stable even after several injection cycles. Lymphocytes, which are known to be difficult targets for gene transfer because they normally express little or no CAR, develop the capacity to bind adenovirus following stimulation with phytohemagglutinin (PHA) and IL-2.28 In our case, the production of inflammatory cytokines29 seems to up-regulate CAR expression on infiltrate cells after TG1042 application, leading to the increased transfection efficiency of the next injection. This phenomenon might underlie the delay in IFN-γ serum peaks observed after first or second treatment cycle. Changes in serum IFN-γ levels did not consistently follow TG1042 injections, likely due to the sensitivity of this parameter to activation by other possible “intruders” including TG1042 vector itself. Neopterin, on the other hand, has shown to be quite a sensitive serum marker in the highest-dose cohort, reflecting either a response to the virus itself or systemic IFN-γ activity 24 hours after injection. Given the likelihood that the immediate immune response to adenovirus peaks around 6 hours after the injection, it is likely that these neopterin peaks reflect the activation of monocytes/macrophages30 by transgene-encoded IFN-γ in addition to adenoviral vector itself. IL-6 represents a reliable marker of the early innate response to adenovectors.31 At the 3 × 1011 dose level, our results show induction of systemic IL-6 response to TG1042 administration, with the maximum detected at 6 hours after injection. As with IFN-γ, this finding seems to be dose dependent, given that the most distinctive changes are seen in the highest-dose cohort.
Formation of thyroid autoantibodies after IL-2- and/or IFN-γ-based cancer immunotherapy has been associated with a favorable tumor response and prolonged survival.32,33 One of our patients showing systemic complete response to TG1042 therapy presented with increased anti-TPO titer after second and third treatment cycle. It is conceivable that the cytokines that enhance immune response to certain autoantigens are present on tumor cells as well. The humoral arm of antitumor immune response is responsible for the production of antibodies specific for different tumor antigens. Using SEREX methodology, we have previously described and defined several CTCL-associated tumor antigens, including se70-2.17 Following immunomodulatory treatment with TG1042, we observed an increase in anti-se70-2 titers in most patients (particularly in 2 Sézary patients), with the exception of one patient who progressed. This implicates systemic effects of local application of TG1042, even in patients with aggressive CTCL, such as Sézary syndrome. It is of note that the tendency to mount an antibody response against se70-2 increased with the number of cycles. It is conceivable that longer treatment may have resulted in better recognition or even unmasking of this antigen.
In summary, intralesional injections of adenovirus-IFN-γ appear to be safe and well tolerated in this study population of patients with primary CL. Treatment with TG1042 induced regressions in patients with T- and B-cell lymphoma at the injection site as well as systemically. Future clinical trials with adenovirus-IFN-γ will provide more information on the lymphoma subtype particularly responsive to this treatment as well as on the optimal schedules in terms of treatment frequency and duration.
Prepublished online as Blood First Edition Paper, May 25, 2004; DOI 10.1182/blood-2004-01-0360.
Supported in part by Swiss Cancer League and Gottfried and Julia Bangerter-Rhyner Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Micael De Meyer for his technical assistance during the study. We thank the Department of Dermatology histology laboratory team for lending us their expertise in immunohistochemistry.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal