Abstract
Cholesterol levels are abnormally increased in many acute myeloid leukemia (AML) samples exposed in vitro to chemotherapy. Blocking these acute cholesterol responses selectively sensitizes AML cells to therapeutics. Thus, defining the molecular mechanisms by which AML cells accomplish these protective cholesterol increments might elucidate novel therapeutic targets. We now report that the levels of mRNAs encoding the cholesterol synthesis-regulating enzyme, 3-hydroxy-3-methylglutaryl coenzyme A reductase, and the cholesterol-importing low-density lipoprotein (LDL) receptor were both increased by daunorubicin (DNR) or cytarabine (ARA-C) treatments in almost three fourths of cultured AML samples. However, less than one third of AML samples significantly increased LDL accumulation during drug treatments, suggesting that de novo synthesis is the primary mechanism by which most AML cells increase cholesterol levels during drug exposures. LDL increments were not correlated with cholesterol increments in ARA-C–treated AML samples. However, LDL and cholesterol increments did correlate in DNR-treated AML samples where they were measured, suggesting that a subset of AMLs may rely on increased LDL accumulation during treatment with particular drugs. Our data suggest that cholesterol synthesis inhibitors may improve the efficacy of standard antileukemia regimens, but that for maximum benefit, therapy may need to be tailored for individual patients with leukemia.
Introduction
In normal cells, cholesterol is synthesized via the mevalonate pathway and is also derived from circulating low-density lipoprotein (LDL) complexes via receptor-mediated endocytosis and lysosomal processing (for reviews, see Goldstein and Brown1 and Allayee et al2 ). Cellular cholesterol is essential to membrane structure and membrane protein function, and its homeostasis is achieved by complex feedback regulation of LDL receptors (LDLRs) and of key enzymes of the mevalonate pathway, including 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoAR) and squalene synthase (SS). Leukocytes from healthy individuals show increased cholesterol synthesis when deprived of lipoprotein and show decreased synthesis when exposed to complete serum or purified cholesterol-LDL.3,4 This homeostatic feedback is accomplished by coordinate transcriptional control of cholesterol-regulating genes. For example, both HMG-CoAR and LDLR mRNA levels are increased in normal leukocytes when HMG-CoAR activity is inhibited by lovastatin.5
On the other hand, cholesterol homeostasis is abnormal in acute myeloid leukemia (AML) cells. Both cholesterol synthesis and LDL processing are hyperactive in cultured AML cells,6-8 and the transcription of HMG-CoAR and LDLR genes is apparently not coordinated in AML cells.5 In addition, exogenous cholesterol-LDL does not reduce these processes as it does in normal leukocytes.3,4 Consistent with data showing that AML cells import abnormally high levels of LDL-cholesterol, hypocholesterolemia is common in patients with de novo AML but rarely seen when the same patients achieve remission.9
We have recently shown that cholesterol levels acutely increase in AML cells that are treated in vitro with sublethal doses of radiation or chemotherapeutics.10 We also showed that inhibiting cholesterol synthesis at the first committed step of the mevalonate pathway with an HMG-CoAR inhibitor (mevastatin) or at the final committed step of cholesterol synthesis with an SS inhibitor (zaragozic acid A), or blocking LDL import by serum deprivation, all radiosensitize and chemosensitize AML cells, and that exogenous LDL protects AML cells from the heightened cytotoxicity of combination treatments with a therapeutic drug plus mevastatin. We have shown that normal bone marrow cells do not make these same adaptive cholesterol responses and are relatively insensitive to statins, as others have also shown.11-13 Therefore, we hypothesize that AML cells require abnormally high levels of cholesterol for their survival, that acute cholesterol responses protect AML cells and contribute to therapy failures in patients with AML, and that cholesterol responses might be a rational target for the development of new antileukemia therapies.
Elucidating mechanisms by which AML cells acutely increase cholesterol to reduce drug sensitivity could help target new therapeutic strategies. For example, if AMLs primarily increase cholesterol by new synthesis, then it would be appropriate to pursue various cholesterol synthesis inhibitors as chemosensitizers in anti-AML regimens. Mouse xenograft analyses14 and preliminary clinical investigations15-18 have suggested that statins might have anticancer activities, and one trial has shown that statins can improve the efficacy of standard therapy in hepatocarcinoma.19 On the other hand, if AMLs primarily use imported LDL-cholesterol to survive chemotherapy, then regimens that block LDL accumulation or include LDL-formulated drugs would be supported, as others have already suggested.5 Finally, if different AMLs rely differentially on synthesis versus import processes, or if different drug treatments differentially affect these processes, then it might be necessary to develop robust laboratory tests to distinguish the mechanisms by which particular AMLs make cholesterol responses so that these laboratory findings could be used to tailor therapies for individual patients. Therefore, we have begun characterizing the mechanisms by which individual AML samples mount protective cholesterol increments. We now report the results of analyses in which we measured the levels of cholesterol-regulating mRNAs and of LDL accumulation in AML cells that were cultured with or without sublethal doses of the standard chemotherapeutic agents, daunorubicin (DNR) or cytarabine (ARA-C), because those drugs represent different classes of therapeutics that kill cells by different mechanisms and may therefore affect cholesterol metabolism by different mechanisms.
Materials and methods
Cell culture
Human NB4, KG1a, and HL-60 AML cells were obtained from the American Type Culture Collection (Rockville, MD). NB4 and KG1a cells were cultured in RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 5% heat-inactivated bovine calf serum (BCS; Hyclone, Logan, UT). HL-60 cells were cultured in Iscove modified Dulbecco medium supplemented with 10% cosmic calf serum (CCS; Hyclone). Human hepatocyte TAMH cells20 were received from Dr D. Hockenbery at the Fred Hutchinson Cancer Research Center (FHCRC) and were cultured in Dulbecco modified Eagle medium/Ham F-12 medium (Invitrogen) with 5 μg/mL insulin, 5 μg/mL transferrin, 5 ng/mL selenium, 100 nM dexamethasone, and 10 mM nicotinamide (all additives from Sigma, St Louis, MO). Drug treatments and assays were performed as described herein for primary cell analyses, except as noted in “Results” or figure legends.
Cryopreserved primary AML bone marrow samples were obtained from the Southwest Oncology Group (SWOG) cell repository (Dr C. Willman; Albuquerque, NM) and the Children's Oncology Group (COG) AML Reference Laboratory (Dr I. Bernstein; Seattle, WA) all with appropriate patient consent, as approved by the institutional review boards of the FHCRC, SWOG, and COG. Twenty AML primary cell samples thawed with 38% to 89% viability (median, 70%) and were resuspended into Iscove media supplemented with 20% CCS, 100 ng/mL human interleukin 3 (Biosource, Camarillo, CA), 100 ng/mL human stem cell factor (Biosource), 100 U/mL penicillin (Invitrogen), and 100 μg/mL streptomycin sulfate (Invitrogen). Primary cells were allowed to recover for 5 to 6 hours and in some cases dead cells were removed by centrifugation through Ficoll-Hypaque (density, 1.077) if thaw viabilities were less than 80%. Cells were then incubated for 18 to 24 hours in culture medium without drugs or with 0.05 μM DNR (ICN Biomedicals, Aurora, OH), 0.2 μM ARA-C (Sigma), 12.5 μM mevastatin (Sigma), or a combination of statin plus DNR or ARA-C at the same doses. As we previously demonstrated,11,21 the drug doses used were relatively nontoxic to primary AML cell samples during 18 to 24 hours of incubation whether viabilities were determined by trypan blue staining or flow cytometry (Tables 1 and 2). Neither cholesterol, LDLR RNA, HMG-CoAR RNA, SS RNA, nor LDL accumulation data correlated with viability indices (P > .15 for all).
Relative drug sensitivites and cholesterol and RNA assays performed with samples from AML patients
AML sample . | Relative Via + DNR . | Relative Via + ARA-C . | Chol . | Semi-Q RT-PCR . | Quant RT-PCR . |
|---|---|---|---|---|---|
| Adult 1 | 1.05 | 1.00 | x | ND | ND |
| Adult 2 | 0.89 | 0.91 | x | ND | ND |
| Adult 3 | 1.01 | 1.04 | x | x | x |
| Adult 4 | 0.92 | 0.95 | x | ND | ND |
| Adult 5 | 0.86 | 0.87 | x | x | x |
| Adult 6 | 0.95 | 0.84* | x | x | x |
| Adult 7 | 0.98 | 0.99 | x | x | x |
| Adult 8 | 0.88 | 1.01 | x | x | x |
| Adult 9 | 0.81* | 1.07 | x | x | x |
| Adult 10 | 0.97 | 0.94 | x | ND | ND |
| Adult 11 | 0.79* | 0.92 | x | x | x |
| Adult 12 | 0.90 | 1.00 | ND | x | x |
| Adult 13 | 0.97 | 0.97 | x | x | x |
| Pediatric 1 | 0.93 | 1.03 | x | x | x |
| Pediatric 2 | 0.99 | 1.01 | x | x | x |
| Pediatric 3 | 0.88 | 0.94 | x | x | ND |
| Pediatric 4 | 1.00 | 0.91 | x | x | x |
| Pediatric 5 | 1.00 | 0.91 | x | x | x |
| Pediatric 6 | 0.94 | 1.00 | x | x | x |
| Pediatric 7 | 0.83* | 0.88 | x | ND | ND |
| No. | 20 | 20 | 19 | 15 | 14 |
| Mean | 0.92 | 0.96 | — | — | — |
| SEM | 0.02 | 0.01 | — | — | — |
AML sample . | Relative Via + DNR . | Relative Via + ARA-C . | Chol . | Semi-Q RT-PCR . | Quant RT-PCR . |
|---|---|---|---|---|---|
| Adult 1 | 1.05 | 1.00 | x | ND | ND |
| Adult 2 | 0.89 | 0.91 | x | ND | ND |
| Adult 3 | 1.01 | 1.04 | x | x | x |
| Adult 4 | 0.92 | 0.95 | x | ND | ND |
| Adult 5 | 0.86 | 0.87 | x | x | x |
| Adult 6 | 0.95 | 0.84* | x | x | x |
| Adult 7 | 0.98 | 0.99 | x | x | x |
| Adult 8 | 0.88 | 1.01 | x | x | x |
| Adult 9 | 0.81* | 1.07 | x | x | x |
| Adult 10 | 0.97 | 0.94 | x | ND | ND |
| Adult 11 | 0.79* | 0.92 | x | x | x |
| Adult 12 | 0.90 | 1.00 | ND | x | x |
| Adult 13 | 0.97 | 0.97 | x | x | x |
| Pediatric 1 | 0.93 | 1.03 | x | x | x |
| Pediatric 2 | 0.99 | 1.01 | x | x | x |
| Pediatric 3 | 0.88 | 0.94 | x | x | ND |
| Pediatric 4 | 1.00 | 0.91 | x | x | x |
| Pediatric 5 | 1.00 | 0.91 | x | x | x |
| Pediatric 6 | 0.94 | 1.00 | x | x | x |
| Pediatric 7 | 0.83* | 0.88 | x | ND | ND |
| No. | 20 | 20 | 19 | 15 | 14 |
| Mean | 0.92 | 0.96 | — | — | — |
| SEM | 0.02 | 0.01 | — | — | — |
Twenty Ficoll-purified primary AML cell samples were analyzed for viability, cholesterol content, LDLR and HMG-CoAR RNA levels in semiquantitative, multiplex RT-PCR and quantitative, real-time RT-PCR after cells were cultured for 18 to 24 hours with and without drug treatments (DNR, 0.05 μM; ARA-C, 0.2 μM). Viability data represent trypan blue-negative cell fractions and are expressed relative to untreated controls for each sample. The number of samples analyzed and the mean relative viabilities are shown, as are SEMs; x represents assays that were performed with individual AML samples (see data in Figure 2).
Via indicates viability; chol, cholesterol, Semi-Q, semiquantitative, multiplex RT-PCR; Quant, quantitative, real-time RT-PCR; and ND, assays not performed.
Indicates viabilities that were reduced more than 15% by drug treatments.
Relative drug sensitivities and LDL accumulation assays performed with samples from AML patients
AML sample . | Relative Via + MEV . | Relative Via + DNR . | Relative Via + ARA-C . | LDL . |
|---|---|---|---|---|
| Adult 1 | 0.94 | 1.15 | 1.17 | x |
| Adult 2 | 0.79* | 0.89 | 0.91 | x |
| Adult 3 | 0.95 | 1.01 | 1.04 | x |
| Adult 4 | 1.00 | 0.92 | 0.95 | x |
| Adult 5 | 0.99 | 1.01 | 1.00 | x |
| Adult 6 | 0.78* | 0.92 | 0.95 | x |
| Adult 7 | 1.01 | 0.98 | 0.99 | x |
| Adult 8 | 0.94 | 0.88 | 1.01 | x |
| Adult 9 | 0.88 | 0.81* | 1.07 | x |
| Adult 10 | 0.90 | 0.97 | 0.94 | x |
| Adult 11 | 0.93 | 0.90 | 1.00 | x |
| Adult 12 | ND | ND | ND | ND |
| Adult 13 | 0.94 | 0.97 | 0.97 | x |
| Pediatric 1 | 1.00 | 0.93 | 1.03 | x |
| Pediatric 2 | 0.97 | 0.99 | 1.01 | x |
| Pediatric 3 | 0.89 | 0.88 | 0.94 | x |
| Pediatric 4 | 0.94 | 1.00 | 0.91 | x |
| Pediatric 5 | 0.94 | 1.00 | 0.91 | x |
| Pediatric 6 | 0.99 | 0.94 | 1.00 | x |
| Pediatric 7 | 1.03 | 0.83* | 0.88 | x |
| No. | 19 | 19 | 19 | 19 |
| Mean | 0.94 | 0.95 | 0.99 | — |
| SEM | 0.02 | 0.02 | 0.02 | — |
AML sample . | Relative Via + MEV . | Relative Via + DNR . | Relative Via + ARA-C . | LDL . |
|---|---|---|---|---|
| Adult 1 | 0.94 | 1.15 | 1.17 | x |
| Adult 2 | 0.79* | 0.89 | 0.91 | x |
| Adult 3 | 0.95 | 1.01 | 1.04 | x |
| Adult 4 | 1.00 | 0.92 | 0.95 | x |
| Adult 5 | 0.99 | 1.01 | 1.00 | x |
| Adult 6 | 0.78* | 0.92 | 0.95 | x |
| Adult 7 | 1.01 | 0.98 | 0.99 | x |
| Adult 8 | 0.94 | 0.88 | 1.01 | x |
| Adult 9 | 0.88 | 0.81* | 1.07 | x |
| Adult 10 | 0.90 | 0.97 | 0.94 | x |
| Adult 11 | 0.93 | 0.90 | 1.00 | x |
| Adult 12 | ND | ND | ND | ND |
| Adult 13 | 0.94 | 0.97 | 0.97 | x |
| Pediatric 1 | 1.00 | 0.93 | 1.03 | x |
| Pediatric 2 | 0.97 | 0.99 | 1.01 | x |
| Pediatric 3 | 0.89 | 0.88 | 0.94 | x |
| Pediatric 4 | 0.94 | 1.00 | 0.91 | x |
| Pediatric 5 | 0.94 | 1.00 | 0.91 | x |
| Pediatric 6 | 0.99 | 0.94 | 1.00 | x |
| Pediatric 7 | 1.03 | 0.83* | 0.88 | x |
| No. | 19 | 19 | 19 | 19 |
| Mean | 0.94 | 0.95 | 0.99 | — |
| SEM | 0.02 | 0.02 | 0.02 | — |
Ficoll-purified primary AML cells were analyzed for viability and LDL accumulation in flow cytometry assays performed after cells were cultured for 18 to 24 hours with and without drug treatments (MEV, 12.5 μM; DNR, 0.05 μM; ARA-C, 0.2 μM). Viability data represent flow cytometry assessments of live cell fractions in forward by side light-scatter plots (Figure 3A) and are expressed relative to untreated controls for each sample. The number of samples analyzed and the mean relative viabilities are shown, as are SEM values; x represents assays that were performed with individual AML samples (see data in Figure 5).
MEV indicates mevastatin; other abbreviations are explained in Table 1.
Indicates viabilities that were reduced more than 15% by drug treatments.
Cellular cholesterol assay
As in our published report,10 we measured cellular cholesterol levels with the Amplex Red assay (Molecular Probes, Eugene, OR), a fluorometric technique in which cholesterol is oxidized into a ketone and hydrogen peroxide, which then reacts stoichiometrically with the Amplex Red reagent (10-acetyl-3,7-dihydroxyphenoxazine) in the presence of horseradish peroxidase to form the fluorescent compound resorufin. To perform this assay, 5 × 105 cells were plated in wells of 24-well plates and exposed to drugs for 18 to 24 hours; viable cell fractions were determined by trypan blue staining. Both viable and nonviable cells were washed in phosphate-buffered saline (PBS), resuspended at 2000 cells/μL in reaction buffer, and dispensed into wells of a 96-well tissue culture plate (Falcon/Becton Dickinson, Franklin Lakes, NJ) with 50 μL Amplex Red working solution added to each well, per the manufacturer's instructions (Molecular Probes). After incubations for 90 minutes at 37° C, protected from light, fluorescence was measured on a CytoFluor II fluorescent plate reader (PerSeptive Biosystems, Framingham, MA) using an excitation wavelength of 530 nm and an emission wavelength of 590 nm. A cholesterol standard curve was determined for each plate using a cholesterol standard (Sigma) diluted at various concentrations in Amplex Red reaction buffer.
RT-PCR
The expression levels of LDLR, HMG-CoAR, and SS mRNAs were evaluated in AML cells by semiquantitative, multiplex reverse transcription-polymerase chain reaction (RT-PCR) with glyceraldehyde phosphate dehydrogenase (GAPDH) internal RNA controls and by quantitative, real-time RT-PCR with β2-microglobulin RNA signals used as standardization controls. For both types of RNA analyses, approximately 106 cells were untreated or treated with the drug, RNA extractions were performed using TRIZOL reagent, and oligo(dT)–primed cDNA was synthesized from total cellular mRNA by RT using Superscript II per the manufacturer's instructions (Invitrogen). A 1-μg mRNA input was added for AML cell sample assays or serially diluted mRNA inputs were added for standard curve production. In all cases, PCR products were sequenced to confirm identity.
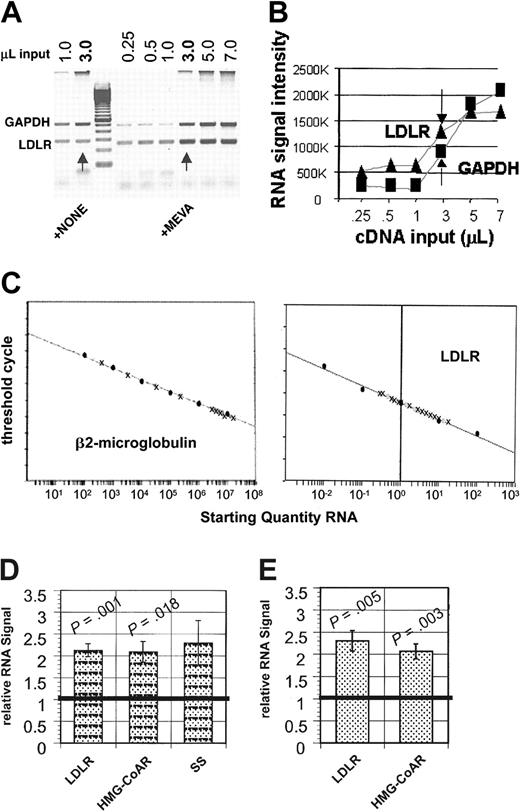
For multiplex, semiquantitative mRNA analyses, LDLR, HMG-CoAR, and SS primers were multiplexed one each with GAPDH primers so that GAPDH RNA levels could be used as a normalization factor for the measured levels of LDLR, HMG-CoAR, and SS RNAs. The intron-spanning primers used for PCR amplification and their size of PCR product were: LDLR, forward 5′-CAATGTCTCACCAAGCTCTG-3′ and reverse 5′-TCTGTCTCGAGGGGTAGCTG-3′; HMG-CoAR, forward 5′-TTACTCCTTGGTGATGGGAGCTTG-3′ and reverse 5′-TCCTGTCCACAGGCAATG-TAGATG-3′; SS, forward 5′-CCACTTTGGCTGCCTGTTAT-3′ and reverse 5′-CCT-AAACCGTGGCACTGAAT-3′; and GAPDH, forward 5′-GTCTTCACCACCATGGAGAAG-3′ and reverse 5′-GCTTCACCACCTTCTTGATGTCATC-3′. PCR amplification was performed using Platinum Taq DNA polymerase per the manufacturer's instructions, with cycles of 1 minute at 94° C, 1 minute at 55° C, and 1 minute at 72° C (Invitrogen). LDLR, HMG-CoAR, and SS primer pairs were optimized to ensure amplification in the linear range for both products (30, 29, and 28, respectively), and 2 μL GAPDH primers were added with 23 cycles remaining. PCR products were run on a 2% agarose gel and visualized by scanning the ethidium bromide–stained gel on a Typhoon 8600 Imager (Amersham Biosciences, Piscataway, NJ) using a 532-nm laser line and a 610-bandpass (BP) filter. Band densities were quantified using the software ImageQuant version 5.2 (Amersham Biosciences). The reproducibility of the multiplex RT-PCR assays was verified in repeated, independent assays of RNAs from untreated and mevastatin-treated NB4 cells (Figure 1).
The abundance of RNAs encoding cholesterol import (LDLR) and cholesterol synthesis-regulating (HMG-CoAR, SS) proteins are substantially increased by mevastatin treatments in NB4 AML cells. NB4 cells were incubated for 18 to 24 hours with 12.5 μM mevastatin, total cellular mRNAs were prepared from untreated and treated cells, and oligo(dT)–primed cDNAs were synthesized. For semiquantitative RT-PCR, LDLR intron-spanning primer pairs were multiplexed with GAPDH primers using conditions optimized to ensure amplification in the linear range for both products, as determined by image analysis of ethidium bromide-stained agarose gels (A-B). Arrows indicate 3-μL cDNA inputs. (C) For real-time RT-PCR, β2-microglobulin DNA and NB4 cell line RNAs were serially diluted into yeast RNA and processed to create standard curves. Quenched, fluorescent internal probes allowed the amount of PCR amplicon created to be quantitated per cycle as fluorescence on an ABI Prism 7700 detector. Standard curves were generated for β2-microglobulin using plasmid DNA and for β2-microglobulin, LDLR, and HMG-CoAR using the NB4 cell line. Standard curves are shown that plot the cycle number at which signals were detected above threshold (y-axis) against the starting quantities of β2-microglobulin plasmid (10-108 copies/μL) diluted into yeast RNA (x-axis) or against the starting quantity of NB4 cell RNA (0.01-100 ng/μL). (D) Methods similar to those used in panels A and B were used to optimize HMG-CoAR and SS RT-PCRs, as described more completely in “Materials and methods.” When standardized to GAPDH signals and normalized to untreated signals, mean LDLR and HMG-CoAR signals were significantly increased in mevastatin-treated samples (5 independent assays), and mean SS signals (n = 3) were also increased. (E) RNA quantities were normalized based on β2-microglobulin levels in real-time PCR, as shown in panel C, and LDLR and HMG-CoAR mRNA levels in mevastatin-treated and untreated NB4 cells are expressed relative to the untreated NB4 cell line controls. Means are plotted in panels C and E, and error bars represent SEM. Wilcoxon rank sum tests were used to compare means of treated and untreated AML samples along with 2-tailed tests of significance.
The abundance of RNAs encoding cholesterol import (LDLR) and cholesterol synthesis-regulating (HMG-CoAR, SS) proteins are substantially increased by mevastatin treatments in NB4 AML cells. NB4 cells were incubated for 18 to 24 hours with 12.5 μM mevastatin, total cellular mRNAs were prepared from untreated and treated cells, and oligo(dT)–primed cDNAs were synthesized. For semiquantitative RT-PCR, LDLR intron-spanning primer pairs were multiplexed with GAPDH primers using conditions optimized to ensure amplification in the linear range for both products, as determined by image analysis of ethidium bromide-stained agarose gels (A-B). Arrows indicate 3-μL cDNA inputs. (C) For real-time RT-PCR, β2-microglobulin DNA and NB4 cell line RNAs were serially diluted into yeast RNA and processed to create standard curves. Quenched, fluorescent internal probes allowed the amount of PCR amplicon created to be quantitated per cycle as fluorescence on an ABI Prism 7700 detector. Standard curves were generated for β2-microglobulin using plasmid DNA and for β2-microglobulin, LDLR, and HMG-CoAR using the NB4 cell line. Standard curves are shown that plot the cycle number at which signals were detected above threshold (y-axis) against the starting quantities of β2-microglobulin plasmid (10-108 copies/μL) diluted into yeast RNA (x-axis) or against the starting quantity of NB4 cell RNA (0.01-100 ng/μL). (D) Methods similar to those used in panels A and B were used to optimize HMG-CoAR and SS RT-PCRs, as described more completely in “Materials and methods.” When standardized to GAPDH signals and normalized to untreated signals, mean LDLR and HMG-CoAR signals were significantly increased in mevastatin-treated samples (5 independent assays), and mean SS signals (n = 3) were also increased. (E) RNA quantities were normalized based on β2-microglobulin levels in real-time PCR, as shown in panel C, and LDLR and HMG-CoAR mRNA levels in mevastatin-treated and untreated NB4 cells are expressed relative to the untreated NB4 cell line controls. Means are plotted in panels C and E, and error bars represent SEM. Wilcoxon rank sum tests were used to compare means of treated and untreated AML samples along with 2-tailed tests of significance.
Quantitative, real-time RNA analyses were also performed, using standard techniques.22 The RT reaction used 0.5 μg RNA and 400 U Superscript II, per the manufacturer's instructions (Invitrogen), with 50 minutes at 42° C and 15 minutes at 70° C. β2-Microglobulin and NB4 RNAs were serially diluted into yeast RNA for RT reactions. PCR was performed with 5 U Platinum Taq DNA polymerase (Invitrogen,), 2 μL cDNA, and the passive reference dye Rox. Taq polymerase was activated by heating for 5 minutes at 95° C, and PCR amplification began with cycles of 15 seconds at 95° C followed by 1 minute at 60° C times 40. The following primers and probes were used: β2-microglobulin, forward 5′-CATTCGGGC-CGAGATGTC-3′ and reverse 5′-CTCCAGGCCAGAAAGAGAGAGTAG-3′; probe, 5′-CCGTGGCCTAGC-TGTGCTCGC-3′); LDLR, forward 5′-ACAGAGGATGAGGTCCACATTTG-3′ and reverse 5′-AGATGT-TCACGCCACGTCATC-3′; probe, 5′-CTGACCATCTGTCTGCCGTCCTGGTT-3′), and HMG-CoAR, forward 5′-GGACGCAACCTTTATATCCGTTTC-3′ and reverse 5′-GTACAATAGTTACCACTAACG-GCTAGAATC-3′; probe, 5′-AAGTGCTTTCTCTGTACCCTTTGAAATCATGTTCA-3′. The β2-microglobulin, LDLR, and HMG-CoAR sequence-specific forward and reverse primers were synthesized by Qiagen (Alameda, CA). Sequence-specific probes were synthesized by Synthegen (Houston, TX) to include the FAM reporter dye at the 5′ end and the TAMRA quencher group at the 3′ end. The amount of PCR amplicon created per cycle was quantitated as unquenched fluorescence on an ABI Prism 7700 Sequence Detector (PE Applied Biosytems, Wellesley, MA).
Human β2-microglobulin DNA was cloned using the TOPO TA cloning kit (Invitrogen), and β2-microglobulin normalization curves were produced by serially diluting β2-microglobulin DNA into yeast RNA (at 108-102 copies/μL) for RT prior to PCR amplification. RNAs produced from mevastatin-treated NB4 cells were serially diluted in yeast RNA (∼0.01-100 ng/μL) to create standard curves for β2-microglobulin, LDLR, and HMG-CoAR RNA analyses. The starting quantity of sequence-specific RNA in patient samples was determined by extrapolating from these standard curves. DNA amplification and detection was prevented by using primers and probes that spanned introns.
LDL accumulation assays
Bodipy-LDL fluorescence is neither quenched at high concentration nor reduced by acidic pH as in the lysosomes to which LDL-bound LDLRs traffic, so it can be used to quantify intracellular LDL accumulation (Molecular Probes). To measure the capacity of cells to accumulate LDL, 5 × 105 cells were incubated overnight with or without drugs, and then exposed to warm 0.05% trypsin (Invitrogen) for 5 minutes before washing once with warm RPMI media to remove any exogenous LDL. Cell samples were then divided, and 1 aliquot was incubated with 10 μg/mL LDL-bodipy (Molecular Probes) in RPMI, whereas the second aliquot was incubated with 10 μg/mL LDL-bodipy plus 210 μg/mL (21-fold excess) unlabeled LDL (Calbiochem, San Diego, CA) in RPMI, to distinguish LDLR-specific binding. In some assays, 3% sodium azide was added to inhibit LDLR recycling. Cells were incubated in the dark at 4° C or 37° C for the time specified, and then washed once in cold RPMI media, and fixed in 2% formaldehyde before flow cytometry. Surface LDLRs were quantified after washing cells twice with PBS/2% BCS and then incubating samples with either 1 μg/mL mouse anti–human LDLR (C7; Maine Biotechnology Services, Portland, ME) or 1 μg/mL isotype control mouse IgG2b (Sigma) for 10 minutes at room temperature, washing twice with cold PBS/2% BCS, and then incubating with anti–mouse IgG-fluorescein isothiocyanate (FITC; 1:64 dilution; Sigma) for 10 minutes at room temperature in the dark. Cells were washed twice again and kept cold before flow cytometry. At least 10 000 cells were analyzed with a Becton Dickinson (Mountain View, CA) FACSCalibur bench-top flow cytometer, using light-scatter gating to distinguish AML blasts in patient samples and mononuclear cells in bone marrow samples from healthy individuals, and to exclude dead cells from the analyses. LDL-bodipy and FITC signals were recognized in the FL1 detector.
LDLR internalization and processing is energy dependent, and bodipy fluorescence was accumulated to a substantially higher level in NB4 cells at 37° C than at 4° C, and in the absence of mitochondrial respiration-inhibition as compared to the presence of the respiration inhibitor, sodium azide (data not shown). Bodipy-LDL accumulation was also dependent on dose and time, as expected. We found that 10 μg/mL bodipy-LDL was subsaturating in NB4 cells that showed increasing bodipy fluorescence across 15-minute to 1-hour incubation periods, whereas KG1a cells required nearly 3 hours to accumulate similar levels of bodipy-LDL as accumulated by NB4 cells in 1 hour (data not shown).
Statistical analyses
Instat3 software (GraphPad, San Diego, CA) was used to perform Wilcoxon matched pairs tests that compare means of treated and untreated aliquots of AML samples, and Pearson correlations were calculated, with 95% confidence intervals and 2-tailed tests of significance, to assess possible correlations of cholesterol increments with increments in specific RNA levels or with LDL accumulation increments.
Results
Many primary AML samples mount cellular cholesterol increments during treatments with standard therapeutic agents
We previously showed that AML cell viabilities are generally unaffected by in vitro treatments with DNR or ARA-C at doses that reproduce peak plasma concentrations reported for patients with AML undergoing induction chemotherapies, whereas normal bone marrow cells are typically more chemosensitive in the same in vitro assays.21 In considering mechanisms that promote this relative AML chemoresistance, we recently found that a majority of AML cells mount cholesterol increments during in vitro treatments with 0.05 μM DNR or 0.2 μM ARA-C, and that blocking these acute cholesterol responses increases AML chemosensitivity.10 To begin determining how AML cells increase cholesterol to survive drug treatments, and to address whether DNR and ARA-C have similar or different effects on cholesterol metabolism, we analyzed AML samples after in vitro treatments with 0.05 μM DNR or 0.2 μM ARA-C, rather than a DNR plus ARA-C drug combination that would mimic a standard anti-AML regimen. Consistent with our previous report, these DNR and ARA-C doses were minimally cytotoxic (Table 1), and cholesterol increments were not correlated with drug-reduced viabilities. As before,10 we used a fluorometric assay to measure cellular cholesterol and found that a majority of primary AML cell samples (n = 19) showed cholesterol increments after treatment with 0.05 μM DNR or 0.2 μM ARA-C. Eleven (58%) of 19 samples significantly increased cholesterol levels during ARA-C or DNR treatments; 9 (48%) showed cholesterol increments after DNR treatments (P = .0007) and 8 (42%) showed cholesterol increments after ARA-C treatments (P = .004). There was significant overlap in these groups such that 6 of 8 samples that showed cholesterol increments after ARA-C treatment also responded to DNR (r = 0.49, P = .03), suggesting that particular AMLs are more likely to mount cholesterol increments during treatments with different damaging agents.
Drug treatments can significantly increase the abundance of LDLR and HMG-CoAR mRNA in AML cells
To begin elucidating whether synthesis or import mechanisms contribute to cholesterol increments in drug-treated AMLs, we developed RT-PCR assays to measure the levels of mRNAs that regulate cholesterol synthesis (HMG-CoAR, SS) and LDL import (LDLR). We first tested our ability to detect biologically relevant mRNA increments using NB4 cells as an AML cell line model because we had already shown that NB4 cells are sensitive to mevastatin and substantially increase cholesterol during DNR and ARA-C treatments.10,11 We compared mRNA levels in NB4 cells before and after treatments with mevastatin because cholesterol-regulating gene transcription increased by cholesterol synthesis inhibition.5 We prepared polyadenylated mRNAs from NB4 cells and performed semiquantitative RT-PCR analyses in which LDLR, HMG-CoAR, or SS mRNA-specific signals were normalized to GAPDH mRNA-specific signals (Figure 1A-B), as more completely described in “Materials and methods.” The levels of LDLR mRNA and HMG-CoAR mRNA were reproducibly and significantly increased by statin treatments in 5 independent NB4 cell assays (Figure 1D). Levels of SS mRNA were also consistently increased in 3 assays, but SS mRNA increments were not statistically significant, due to the smaller sample size. We also found that SS mRNA levels were rarely or only minimally increased by drug treatments in 15 primary AML samples in which cellular cholesterol levels had already been measured and for which sufficient RNA was available. However, significant LDLR mRNA increments were measured in 13 (87%) AML samples after DNR or ARA-C treatments and HMG-CoAR mRNA increments were measured in 10 (75%) drug-treated AML samples (data not shown).
To more quantitatively assess LDLR and HMG-CoAR RNA increments, we developed real-time RT-PCR assays, using β2-microglubulin RNA levels as normalization controls. Experimental details are described in “Materials and methods.” RT-PCR results were linear across at least a 4-log range of input RNAs (Figure 1C and data not shown), and relative LDLR and HMG-CoAR RNA levels were reproducibly and significantly higher in 5 independent assays of mevastatin-treated NB4 cells as compared to untreated NB4 cells (Figure 1E). RNA increments determined in multiplex, semiquantitative and real-time, quantitative RT-PCR assays were very similar for both LDLR (2.12 ± 0.15, 2.29 ± 0.23) and HMG-CoAR (2.09 ± 0.24, 2.06 ± 0.17), documenting our ability to accurately detect specific RNA increments with both of these assays.
Additional mRNA materials were available for real-time RT-PCR analyses of 14 of the 15 AML samples in which cellular cholesterol levels had been measured and semiquantitative RT-PCR analyses had been performed. Substantial LDLR mRNA increments were measured in 5 drug-treated AMLs (36%; DNR increment range, 1.16-2.10; ARA-C increment range, 1.02-2.68) and HMG-CoAR mRNA increments were measured in 14 AMLs (100%) after DNR (increment range, 1.05-1.88) or ARA-C treatments (increment range, 1.03-2.49). Drug-treated LDLR and HMG-CoAR RNA levels were significantly greater than untreated levels in these responsive samples, except for LDLR RNA levels after ARA-C treatments, due to smaller sample number (Figure 2A). LDLR RNA increments were significantly correlated after DNR versus ARA-C treatments (r = 0.92; P = .03). In addition, LDLR and HMG-CoAR RNA increments were significantly correlated in ARA-C–treated AML samples that showed LDLR RNA increments (r = 0.92; P = .003). However, LDLR RNAs were not consistently increased in ARA-C–treated AML samples that showed HMG-CoAR RNA increments; 4 AML samples showed HMG-CoAR RNA increments but did not show LDLR RNA increments.
The abundance of LDLR and HMG-CoAR RNAs is frequently increased in primary DNR- and ARA-C–treated AML cell samples. (A) Specific RNA levels were measured by real-time RT-PCR in aliquots of 14 primary AML samples, and significant LDLR mRNA increments and HMG-CoAR mRNA increments were measured in a majority of AML samples after DNR and ARA-C treatments, except for measured LDLR RNA increments that were not significant, in association with a smaller sample number. Error bars represent SEM, and Wilcoxon rank sum tests were used to compare means of treated and untreated AML samples along with 2-tailed tests of significance. Bold horizontal line refers to mean. (B) Real-time RT-PCR data were used to show that cholesterol increments were positively correlated with both LDLR RNA increments (r = 0.99; 95% CI, 0.67-0.99) and HMG-CoAR RNA increments (r = 0.88; 95% CI, 0.43-0.98) in ARA-C–treated AMLs, whereas neither LDLR RNA increments nor HMG-CoAR RNA increments induced by DNR were significantly correlated with cholesterol increments (LDLR r = 0.809; 95% CI, –036-0.98; HMG-CoAR r = 0.45, 95% CI –0.92-0.36).
The abundance of LDLR and HMG-CoAR RNAs is frequently increased in primary DNR- and ARA-C–treated AML cell samples. (A) Specific RNA levels were measured by real-time RT-PCR in aliquots of 14 primary AML samples, and significant LDLR mRNA increments and HMG-CoAR mRNA increments were measured in a majority of AML samples after DNR and ARA-C treatments, except for measured LDLR RNA increments that were not significant, in association with a smaller sample number. Error bars represent SEM, and Wilcoxon rank sum tests were used to compare means of treated and untreated AML samples along with 2-tailed tests of significance. Bold horizontal line refers to mean. (B) Real-time RT-PCR data were used to show that cholesterol increments were positively correlated with both LDLR RNA increments (r = 0.99; 95% CI, 0.67-0.99) and HMG-CoAR RNA increments (r = 0.88; 95% CI, 0.43-0.98) in ARA-C–treated AMLs, whereas neither LDLR RNA increments nor HMG-CoAR RNA increments induced by DNR were significantly correlated with cholesterol increments (LDLR r = 0.809; 95% CI, –036-0.98; HMG-CoAR r = 0.45, 95% CI –0.92-0.36).
We used data from quantitative RT-PCR analyses to ask whether LDLR and HMG-CoAR RNA increments correlated with cholesterol increments in the same primary AML cell samples. We found that increments were significantly correlated in ARA-C–treated AML samples in which LDLR mRNA increments were measured (4 samples with both measurements, r = 0.99, P = .008), and positively correlated in DNR-treated AML samples (5 samples, r = 0.80), but not significantly (Figure 2B). Increments were also significantly correlated in ARA-C–treated AML samples in which HMG-CoAR mRNA increments were measured (7 samples, r = 0.88, P = .005), and correlated in DNR-treated AMLs (8 samples, r = 0.45), but not significantly.
LDL accumulation can be accurately measured in flow cytometry assays
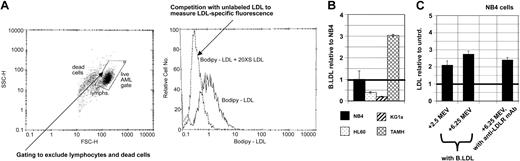
Unfortunately, no commercially available antibody specifically recognizes HMG-CoAR protein with enough sensitivity to accurately distinguish protein levels in different primary cell samples, and HMG-CoAR activity assays require larger cell numbers than are readily available from clinical AML samples (data not shown). Therefore, we have not yet determined whether HMG-CoAR RNA increments produce increased HMG-CoAR activity or protein expression. However, because the abundance of LDLR mRNA was increased in AML samples by both DNR and ARA-C treatments and LDLR RNA increments were correlated with cholesterol increments, and because hyperactive LDL import might have therapeutic utility,9,23-25 we asked whether LDLR activity was commonly increased in AML cells during in vitro drug treatments. To address this question, we developed a multiparameter flow cytometry assay using a fluorescent, bodipy-LDL derivative. AML cells were incubated for 1 hour at 37° C with 10 μg/mL bodipy-LDL with or without 20-fold excess unlabeled LDL (Figure 3A) to estimate the LDLR-specific binding fraction, as more fully described in “Materials and methods.”
Differential LDL accumulation can be accurately measured using flow cytometry assays that can also distinguish LDL accumulation increased by mevastatin or DNR in NB4 and KG1a AML cells. (A) Cells were incubated in the dark at 37° C to allow LDL binding and internalization and LDLR recycling, and LDL-bodipy signals were recognized by flow cytometry, after gating to exclude dead cells and nonblasts in analyses of primary AML cell samples, and using 20-fold molar excess unlabeled LDL to distinguish LDLR-specific binding, as more completely described in “Materials and methods.” (B) Untreated cells of AML cell lines showed reproducibly different levels of LDL accumulation, and these were lower than accumulation levels in TAMH liver cells. (C) LDL accumulation was increased in NB4 AML cells by mevastatin (MEV), in a dose-dependent manner (2.5 μM and 6.25μM MEV data shown here), and to a similar degree as anti-LDLR antibody binding was increased. Mean LDL accumulation increments are plotted for panels C and D, and error bars represent SEM. Bold horizontal lines indicate the mean.
Differential LDL accumulation can be accurately measured using flow cytometry assays that can also distinguish LDL accumulation increased by mevastatin or DNR in NB4 and KG1a AML cells. (A) Cells were incubated in the dark at 37° C to allow LDL binding and internalization and LDLR recycling, and LDL-bodipy signals were recognized by flow cytometry, after gating to exclude dead cells and nonblasts in analyses of primary AML cell samples, and using 20-fold molar excess unlabeled LDL to distinguish LDLR-specific binding, as more completely described in “Materials and methods.” (B) Untreated cells of AML cell lines showed reproducibly different levels of LDL accumulation, and these were lower than accumulation levels in TAMH liver cells. (C) LDL accumulation was increased in NB4 AML cells by mevastatin (MEV), in a dose-dependent manner (2.5 μM and 6.25μM MEV data shown here), and to a similar degree as anti-LDLR antibody binding was increased. Mean LDL accumulation increments are plotted for panels C and D, and error bars represent SEM. Bold horizontal lines indicate the mean.
Because we had previously shown that NB4, HL60, and KG1a AML cells are differentially sensitive to mevastatin and differentially mount cholesterol increments during DNR and ARA-C treatments,10,13 we used these AML cell lines to begin assessing the variability of baseline LDL accumulation in AML cells. Because liver cells are known to accumulate especially high levels of LDL,1,2 we also measured LDL accumulation in TAMH hepatocytes. As expected, LDL accumulation was consistently and substantially higher in TAMH hepatocytes as compared to AML cells (Figure 3B). Consistent with differential mevastatin sensitivity, HL60 and KG1a cells consistently accumulated lower levels of LDL than NB4 cells. Thus, our flow cytometry assay can reproducibly measure different levels of LDL accumulation in different cell types and in AML cell lines that are differentially sensitive to the cytotoxic effects of cholesterol synthesis inhibition.
AML cell lines show increased LDL accumulation after statin treatments and supra-additive increases after statin plus drug cotreatments
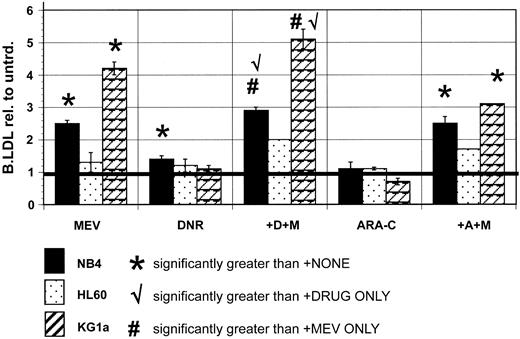
LDL processing is increased by statin treatments in normal leukocytes and AML cells.6-8 We found, as expected, that bodipy-LDL accumulation was increased in NB4 cells after treatments with sublethal doses of mevastatin. Similar statin-induced increments could be measured using the well-characterized anti–human LDLR monoclonal antibody, C7 (Figure 3C), suggesting that increased LDL accumulation in statin-treated cells corresponds to increased surface expression of LDLR. KG1a AML cells consistently showed even larger LDL accumulation increments after statin treatments, whereas HL60 AML cells reproducibly showed smaller statin-induced increments than NB4 cells (Figure 4 and Table 3).
LDL accumulation is differentially affected in AML cell lines by nontoxic treatments with mevastatin, DNR, and ARA-C, and DNR plus statin treatments can be used to uncover the ability of AML cells to mount LDL increments when new cholesterol synthesis is blocked. LDL accumulation was measured in untreated NB4, KG1a, and HL60 AML cells, and in the same cells after various drug treatments, as described more completely in “Materials and methods.” Wilcoxon rank sum tests were used to compare means of treated and untreated AML samples, with 2-tailed tests of significance. LDL accumulation was significantly increased by 6.25 μM mevastatin (MEV) treatments in NB4 (P = .0001) and KG1a (P = .02) cells. LDL accumulation was significantly increased by DNR (P = .001) but not ARA-C (P = .27) in NB4 cells. LDL accumulation was not increased by DNR or ARA-C in KG1a cells, but DNR plus mevastatin (+D+M) cotreatments produced LDL accumulation increments that were supra-additive (P = .05 with DNR only, P = .06 with MEV only) compared to increments measured in KG1a cells treated with statin or DNR alone. HL60 cells did not show increased LDL accumulation after mevastatin, DNR, or ARA-C treatments, and combination treatments did not produce supra-additive increments. Mean LDL accumulation increments are plotted, and error bars represent SEM. Bold horizontal lines indicate mean.
LDL accumulation is differentially affected in AML cell lines by nontoxic treatments with mevastatin, DNR, and ARA-C, and DNR plus statin treatments can be used to uncover the ability of AML cells to mount LDL increments when new cholesterol synthesis is blocked. LDL accumulation was measured in untreated NB4, KG1a, and HL60 AML cells, and in the same cells after various drug treatments, as described more completely in “Materials and methods.” Wilcoxon rank sum tests were used to compare means of treated and untreated AML samples, with 2-tailed tests of significance. LDL accumulation was significantly increased by 6.25 μM mevastatin (MEV) treatments in NB4 (P = .0001) and KG1a (P = .02) cells. LDL accumulation was significantly increased by DNR (P = .001) but not ARA-C (P = .27) in NB4 cells. LDL accumulation was not increased by DNR or ARA-C in KG1a cells, but DNR plus mevastatin (+D+M) cotreatments produced LDL accumulation increments that were supra-additive (P = .05 with DNR only, P = .06 with MEV only) compared to increments measured in KG1a cells treated with statin or DNR alone. HL60 cells did not show increased LDL accumulation after mevastatin, DNR, or ARA-C treatments, and combination treatments did not produce supra-additive increments. Mean LDL accumulation increments are plotted, and error bars represent SEM. Bold horizontal lines indicate mean.
Correlations for RNA increments
. | No. increments . | r2 . | P . |
|---|---|---|---|
| LDLR RNA + DNR | 5 | .64* | .57 |
| LDLR RNA + ARA-C | 4 | .98† | .008 |
| HMG.RNA + DNR | 7 | .29* | .21 |
| HMG.RNA + ARA-C | 8 | .76† | .005 |
. | No. increments . | r2 . | P . |
|---|---|---|---|
| LDLR RNA + DNR | 5 | .64* | .57 |
| LDLR RNA + ARA-C | 4 | .98† | .008 |
| HMG.RNA + DNR | 7 | .29* | .21 |
| HMG.RNA + ARA-C | 8 | .76† | .005 |
with chol + DNR.
with chol + DNR
Having documented that we could accurately measure constitutive differences and acutely increased levels of LDL accumulation, we next asked whether DNR and ARA-C treatments that induce cholesterol increments also induce LDL increments in AML cells. We found NB4 and KG1a cells significantly increased LDL accumulation after mevastatin treatments and that NB4 cells mounted small but significant LDL accumulation increments during DNR treatments, but that LDL accumulation was not increased by DNR in other cell lines (Figure 4). Cholesterol increment-inducing ARA-C treatments did not induce LDL accumulation increments in any of the AML cell lines analyzed. We hypothesized that statin cotreatments might force cholesterol-dependent cells to measurably increase LDL import. Consistent with this idea, NB4 and KG1a AML cells showed significantly supra-additive LDL accumulation after statin plus DNR cotreatments. However, these cells did not show supra-additive LDL accumulation after statin plus ARA-C treatments, and HL60 cells did not show LDL increments after either statin plus drug cotreatment.
LDL accumulation is consistently increased in primary AML cell samples by mevastatin treatments, but DNR and ARA-C responses are less common
Because NB4 cells accumulated higher levels of bodipy-LDL than other AML cell lines and showed significant LDL accumulation increments after mevastatin or DNR treatments, NB4 cells were used as interassay controls for analyses of primary AML cell samples. Eighteen of 19 AML samples that had been analyzed in cholesterol assays were available for LDL accumulation assays. LDL accumulation in untreated AML samples was variable, but consistently lower than in NB4 interassay controls (AML mean, 0.21 ± 0.05, relative to NB4 standards).
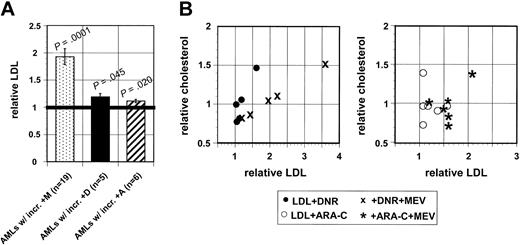
Nineteen AML samples (100%) showed significant LDL increments after mevastatin treatments (Figure 5), showing that primary AML cells can increase LDL accumulation during 18 to 24 hours of in vitro drug treatments. However, only 7 (39%) of 18 AML samples significantly increased LDL accumulation after DNR or ARA-C treatments. Four of these samples (22%) increased LDL accumulation during DNR and ARA-C treatments (r = 0.54, P = .025), suggesting that particular AMLs are more likely to increase LDL import during treatments with either cytotoxic drug. Seven additional AML samples (39%) showed supra-additive LDL increments when treated with mevastatin plus DNR or mevastatin plus ARA-C, and 3 of these (17%) showed supra-additive LDL increments after treatment with mevastatin plus DNR and mevastatin plus ARA-C (r = 0.75, P = .0004). However, LDL increments were significantly correlated with cholesterol increments only in DNR-treated and DNR/statin cotreated AML samples (Figure 5 and Table 4), suggesting that LDL increments may contribute to cholesterol increments in a subset of AMLs treated with particular drugs, including DNR.
LDL accumulation is consistently and significantly increased by mevastatin treatments in primary AML cell samples, but less frequently increased by DNR or ARA-C treatments, although LDL increments are significantly correlated with cholesterol increments in DNR-treated AMLs. (A) All 18 AML cell samples showed significant LDL increments after mevastatin treatments, but only 5 of these samples significantly increased LDL accumulation after DNR and only 6 samples significantly increased LDL accumulation after ARA-C treatment. Mean LDL accumulation increments are plotted, error bars represent SEM, and Wilcoxon rank sum tests were used to compare means of treated and untreated aliquots ofAML samples. Bold horizontal line indicates mean. (B) Cholesterol increments were positively correlated with LDL accumulation increments in DNR-treated (r = 0.93; 95% CI, 0.34-0.99) and DNR plus statin cotreated AML samples (r = 0.92; 95% CI, 0.21-0.99), but not in ARA-C–treated (r =–0.18; 95% CI, –0.87 to 0.74) or ARA-C plus statin cotreated AML samples (r =–0.21; 95% CI –0.87 to 0.72).
LDL accumulation is consistently and significantly increased by mevastatin treatments in primary AML cell samples, but less frequently increased by DNR or ARA-C treatments, although LDL increments are significantly correlated with cholesterol increments in DNR-treated AMLs. (A) All 18 AML cell samples showed significant LDL increments after mevastatin treatments, but only 5 of these samples significantly increased LDL accumulation after DNR and only 6 samples significantly increased LDL accumulation after ARA-C treatment. Mean LDL accumulation increments are plotted, error bars represent SEM, and Wilcoxon rank sum tests were used to compare means of treated and untreated aliquots ofAML samples. Bold horizontal line indicates mean. (B) Cholesterol increments were positively correlated with LDL accumulation increments in DNR-treated (r = 0.93; 95% CI, 0.34-0.99) and DNR plus statin cotreated AML samples (r = 0.92; 95% CI, 0.21-0.99), but not in ARA-C–treated (r =–0.18; 95% CI, –0.87 to 0.74) or ARA-C plus statin cotreated AML samples (r =–0.21; 95% CI –0.87 to 0.72).
Correlations for LDL accumulation
. | No. increments . | r2 . | P . |
|---|---|---|---|
| LDLR RNA + DNR | 5 | .88* | .01 |
| LDLR RNA + ARA-C | 5 | .85† | .03 |
| HMG.RNA + DNR | 6 | -.18* | .73 |
| HMG.RNA + ARA-C | 6 | -.21† | .68 |
. | No. increments . | r2 . | P . |
|---|---|---|---|
| LDLR RNA + DNR | 5 | .88* | .01 |
| LDLR RNA + ARA-C | 5 | .85† | .03 |
| HMG.RNA + DNR | 6 | -.18* | .73 |
| HMG.RNA + ARA-C | 6 | -.21† | .68 |
with chol + DNR.
with chol + ARA-C.
Discussion
We previously showed that cells in many AML samples acutely increase cellular cholesterol levels during in vitro radiation or chemotherapy exposures and are sensitized to therapeutics by agents that block cholesterol synthesis or block LDL-cholesterol import.10 Our current study confirms that many AMLs increase intracellular cholesterol levels during drug treatments, shows that the cholesterol increments produced during DNR and ARA-C treatments are often associated in individual AML samples, and suggests that a subset of AML is most likely to mount cholesterol increments after treatments with therapeutic agents. Based on these data, we hypothesize that acute cholesterol responses contribute to therapy failures in particular AML patients. To enhance the development of new cholesterol-focused, anti-AML strategies on the most relevant molecular targets, we investigated the mechanisms by which protective cholesterol increments might be achieved in AML cells. We used both real-time, quantitative RT-PCR and multiplex, semiquantitative RT-PCR assays to measure the levels of mRNAs that encode cholesterol synthesis-regulating enzymes (HMG-CoAR and SS) and the LDL-cholesterol receptor (LDLR), and measured LDL accumulation in primary AML cell samples that were untreated or treated with the standard chemotherapeutic agents, DNR and ARA-C.
Whether specific RNA levels were assessed relative to GAPDH or β2-micorglobulin RNA levels, our analyses showed that the levels of both LDLR and HMG-CoAR mRNAs were increased in many AML samples by ARA-C and DNR treatments, suggesting that both cholesterol synthesis and LDL-cholesterol import processes are acutely stimulated in AML cells by relevant drug treatments. In fact, LDLR and HMG-CoAR RNA increments were positively correlated in ARA-C–treated AML cells that showed LDLR RNA increments. However, LDLR and HMG-CoAR mRNA increments were not correlated in DNR-treated cells, and 4 samples showed HMG-CoAR RNA increments that did not show LDLR RNA increments after drug treatments. In addition, multiplex RT-PCR assays showed that SS mRNA levels were relatively unchanged in AMLs treated with either DNR or ARA-C. Our data are consistent with other data showing that cholesterol-regulating gene expression is frequently discordant in AML cells,5 and suggest that treatments with certain particular therapeutic agents increase the levels of particular cholesterol-regulating mRNAs, whereas the levels of other cholesterol-regulating mRNAs are rarely influenced by any drug treatment.
In our study, LDLR and HMG-CoAR mRNA increments were significantly correlated with cholesterol increments in AML samples treated with ARA-C but not DNR, suggesting that particular drugs are more likely to induce cholesterol responses in AML cells and that specific mRNA responses might have prognostic utility, if cholesterol increments decrease chemotherapeutic efficacy as our previously published in vitro data suggest.10 Others have shown that AML cells can increase LDL processing after statin treatments without measurably increasing LDLR mRNA levels and have concluded that AML cells regulate cholesterol levels by abnormally active posttranscriptional mechanisms.5 Our data are consistent with this idea but show instead that LDLR mRNA increments do not necessarily produce higher LDLR expression and activity. In either case, posttranscriptional regulation of cholesterol may contribute to the adaptive responses mounted by a subset of AMLs treated with particular antileukemia agents. This posttranscriptional mechanism will be the focus of future laboratory studies because such studies might elucidate new, leukemia-selective therapeutic targets.
Increased LDLR mRNA levels in AML cells were commonly measured in our multiplex and real-time RT-PCR assays of DNR- and ARA-C–treated AML samples, and hyperactive LDLR might provide therapeutic targets in AML.23-25 Therefore, we used a flow cytometry assay to ask how frequently LDL accumulation increases in primary AML cells during in vitro drug treatments. We found that LDL accumulation increased in every AML sample and in every cell line analyzed after mevastatin blockade of cholesterol synthesis. NB4 AML cells also significantly increased LDL accumulation during DNR treatments. Thus, AML cells can acutely increase LDL accumulation in response to in vitro drug treatments, and LDL accumulation increments can be measured in our flow cytometry assay. Cellular LDL levels were increased in 7 (39%) of the 18 AML cell samples that were exposed to DNR or ARA-C in our analyses, and supra-additive LDL increments were measured in an additional 7 AMLs when mevastatin was added to DNR or ARA-C treatments to prevent new cholesterol synthesis. Thus, more than three fourths of AML samples (78%) were able to acutely increase LDL accumulation during chemotherapy, which is very nearly the same fraction of AMLs (84%) that mounted cholesterol increments in our study, further supporting the idea that many AMLs require acute cholesterol increments to survive chemotherapy and that increased LDL import can contribute to cellular cholesterol increments in AMLs.
LDL accumulation increments were correlated with cholesterol increments in DNR-treated AML cells, but not in ARA-C–treated AML cells, suggesting that LDL import may be differentially used by AML cells to accomplish cholesterol increments, according to particular drug treatment. Larger studies are needed to test this idea, but if our preliminary findings are confirmed, serum LDL modulation or LDL-formulated therapeutics might be beneficial in particular AML patients. Others have already shown that LDL (and lipid emulsions resembling the lipid portion of LDL) is concentrated by leukemic blasts in patients and have suggested that LDL might be used as a carrier for cytotoxic drugs to improve therapeutic indices in AML and in other cancers.9,23-25
Approximately two thirds of the AML samples that we analyzed did not measurably increase LDL accumulation in the absence of statin cotreatments, and approximately one fourth did not increase LDL accumulation even during statin plus drug cotreatments. In addition, our real-time RT-PCR data suggest that HMG-CoAR RNA levels are more frequently increased in AML cells by drug treatments than are LDLR RNA levels. Although it is a formal possibility that we were unable to measure small LDL accumulation increments that were nonetheless sufficient to produce critical cholesterol increments in drug-treated AML cells, our data suggest that a majority of AML cells rely primarily on new cholesterol synthesis to mount protective cholesterol increments. We have not yet determined whether HMG-CoAR protein expression or activity increments significantly correlate with cholesterol increments in drug-treated AML cell samples, and future studies should test the utility of HMG-CoAR RNA measures as predictors of clinical drug response. However, our published data10,11 and the data herein combine to strongly support the idea that cholesterol synthesis-inhibiting drugs might improve the efficacy of antileukemia regimens for many patients with AML, as other have also suggested.12,14 Further, our data suggest that laboratory tests that accurately identify cholesterol-dependent AMLs, and identify the precise mechanism by which particular AMLs mount protective cholesterol increments, may have value in tailoring antileukemia therapies.
Larger laboratory studies are needed to confirm these ideas, but clinical trials testing the safety and chemosensitizing efficacy of statins in standard antileukemia regimens seem warranted. We have shown that lymphoma and myeloma cells also mount cholesterol increments during in vitro treatments with relevant chemotherapeutics and are statin sensitive (data not shown). Others have shown that, like AML cells, astrocytoma and glioblastoma cells, gastric adenocarcinoma cells, hepatocarcinoma cells, colon carcinoma cells, neuroblastoma cells, and hairy cell leukemia cells are sensitive to statins.16-18,26-28 A phase 1 trial showed that pravastatin plus 5-fluorouracil therapy was well tolerated and that pravastatin significantly improved the median survival of patients with hepatocarcinoma from 9 months to 18 months.19 Therefore, our findings may have broad significance for the development of new, effective anticancer regimens.
Prepublished online as Blood First Edition Paper, May 25, 2004; DOI 10.1182/blood-2004-01-0395.
Supported by National Institutes of Health grants R21-CA89491 (D.E.B.) and UO1-CA32102 (C.L.W.) and a Chuck Griffin Memorial Scholarship from the Leukemia and Lymphoma Society TR 6079-02 (D.E.B.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are grateful to Dr Derek Stirewalt for his help in developing the quantitative RT-PCR assays used in this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal