Abstract
Juvenile hemochromatosis is a rare autosomal recessive disorder characterized by the early onset of severe iron overload. We report the occurrence of compound heterozygous mutations in hemojuvelin (HJV), including a termination codon, in a patient with juvenile hemochromatosis but no family history of iron disorders. (Blood. 2004;104:2176-2177)
Introduction
Juvenile hemochromatosis is a rare autosomal recessive disorder characterized by severe iron overload that presents in the second and third decades of life. Recently, mutations causing juvenile hemochromatosis have been identified in a novel gene, hemojuvelin (HJV).1 Prominent clinical features include hypogonadotropic hypogonadism, cardiomyopathy, and arthropathy.2
Mutations in HFE, the gene responsible for hereditary hemochromatosis, cause a late-onset iron overload disorder that is found primarily in people of northern European origin. However, most patients with juvenile hemochromatosis do not have mutations in HFE; rather, they show linkage of the disease to chromosome 1q.3-5 Loss-of-function mutations in the HAMP gene, encoding hepcidin, were also found to cause juvenile hemochromatosis in several families.6-8
Papanikolaou et al1 recently reported the positional cloning of the 1q juvenile hemochromatosis gene. The normal function of the gene remains unknown, but it shares sequence homology with a molecule involved in axonal guidance in the central nervous system.
We report a novel termination mutation in HJV associated with autosomal recessive juvenile hemochromatosis in a Chinese family. We propose that compound heterozygosity for this mutation and a previously identified missense mutation resulted in severe iron overload in a patient with no family history of iron overload.
Study design
The patient, a 19-year-old student from China, initially presented to the University of Utah Hospital with a 1-week history of palpitations, chest pain, and dyspnea. Her medical history was significant for psoriasis and secondary amenorrhea, with the onset of menses occurring at age 11 and ceasing at age 14. Initial examination disclosed green-gray skin tone, hepatomegaly, and atrial fibrillation. Echocardiography revealed a dilated cardiomyopathy with an ejection fraction of 20%.
Blood counts were normal, but serum chemistry profiles revealed hyperglycemia (223 mg/dL) and elevated liver transaminases (aspartate aminotransferase, 131 U/L [normal, 14-50 U/L]; alanine aminotransferase, 64 U/L [normal, 6-38 U/L]). Her serum iron level was 239 μg/dL, with a transferrin saturation of 94%. The serum ferritin concentration was 7575 ng/mL.
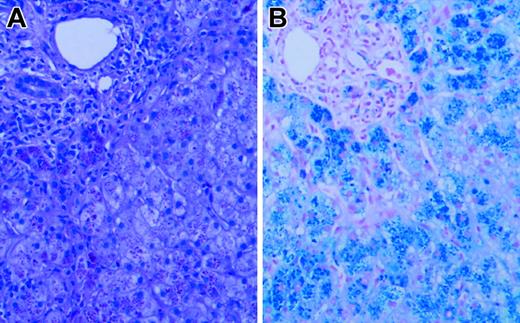
Liver biopsy revealed grade 4 hepatic parenchymal cell-stainable iron (Figure 1), portal and lobular inflammation (grade 3/4), and architectural distortion with portal-septal fibrosis (stage 3). Liver iron content by atomic absorption spectrophotometry was markedly elevated at 1500 μg iron/100 mg dry weight (normal, 1-17 μg iron/100 mg dry weight). Sequence analysis of HFE and HAMP revealed no mutations. Serum gonadotrophin analysis and intravenous glucose tolerance/insulin secretion test confirmed the presence of hypogonadotropic hypogonadism and diabetes, but endocrine function was otherwise normal.
Liver biopsy. Hematoxylin and eosin (A) and Prussian blue iron (B) stains of the patient's liver biopsy specimen at the time of diagnosis, demonstrating heavy (4+) hepatocellular iron deposition. Slides were viewed with a Nikon Eclipse E600 microscope equipped with a 40×/0.75 objective lens (Nikon, Melville, NY). An RT Slider SPOT 2.3.1. camera and SPOT advanced software (v.3.5.9; Diagnostic Instruments, Sterling Heights, MI) were used to capture images.
Liver biopsy. Hematoxylin and eosin (A) and Prussian blue iron (B) stains of the patient's liver biopsy specimen at the time of diagnosis, demonstrating heavy (4+) hepatocellular iron deposition. Slides were viewed with a Nikon Eclipse E600 microscope equipped with a 40×/0.75 objective lens (Nikon, Melville, NY). An RT Slider SPOT 2.3.1. camera and SPOT advanced software (v.3.5.9; Diagnostic Instruments, Sterling Heights, MI) were used to capture images.
The patient was an only child. Her parents, who are not related to each other, came from China for evaluation. Her mother, a 46-year-old premenopausal woman, was healthy and had normal findings on physical examination. Her serum iron level was 45 μg/dL, with a transferrin saturation rate of 10%, and a serum ferritin level of 19 ng/mL. The patient's 49-year-old father had psoriasis but was otherwise well. His serum iron level was normal (45 μg/dL), and he had a transferrin saturation rate of 34%, but his serum ferritin concentration was elevated at 554 ng/mL (normal, 25-320 ng/mL).
Therapy was initiated with digitalis, furosemide, captopril, insulin, and oral estrogen/progesterone replacement. Weekly phlebotomy of 500 mL blood was begun. After 53 weeks, the patient's serum ferritin concentration was 35 ng/mL; blood counts and liver function study results were normal. Administration of cardiac medications and insulin was discontinued. Phlebotomies were changed to 500 mL blood every 2 months. Normal cardiac function, euglycemia, and normal serum ferritin concentration have been maintained for the past 2 years.
Oligonucleotide primers were synthesized according to database genomic sequences for HJV (www.ensembl.org, NM_145277). Polymerase chain reaction (PCR) was performed on genomic DNA in a thermal cycler using 20 pM primers and 2 U Taq polymerase in a final volume of 25 μL. Primer sequences are available on request. Direct sequencing was performed on amplified PCR products using an ABI 3730 DNA Sequence Analyzer. Nucleotide changes in the HJV cDNA refer to GenBank accession number BK001576, encoding isoform A.1
Results and discussion
To identify the genetic defect responsible for the severe iron overload in this patient, we screened the proband and her parents for mutations in HJV. The mother was heterozygous for 2 novel mutations, a premature termination mutation (962G>A and 963C>A; C321X) and a mutation in the signal peptide (18G>C; Q6H). The father was heterozygous for a missense mutation (842T>C; I281T) in HJV that had been identified previously.1 The patient was a compound heterozygote for all 3 mutations (Figure 2). We propose that the coexistence of these mutations causes loss of function of hemojuvelin and impaired iron metabolism, resulting in severe iron loading. Because the abnormal maternal allele contains a missense change (Q6H) and a nonsense mutation (C321X) in cis, and because we did not analyze population-specific controls for the Q6H variant, we do not know whether the Q6H variant is functionally significant. However, we do note that this position is not conserved in the rat hemojuvelin protein.
Mutations in HJV. Positions of the nonsense and 2 missense mutations are shown diagrammatically. The Q6H and C321X mutations were present on the maternal allele; the I281T mutation (*) was found on the paternal allele. The signal peptide, RGD domain (RGD), von Willebrand factor D-domainlike (VWF type D), and putative transmembrane domain (TM) regions are indicated with colored boxes.
Mutations in HJV. Positions of the nonsense and 2 missense mutations are shown diagrammatically. The Q6H and C321X mutations were present on the maternal allele; the I281T mutation (*) was found on the paternal allele. The signal peptide, RGD domain (RGD), von Willebrand factor D-domainlike (VWF type D), and putative transmembrane domain (TM) regions are indicated with colored boxes.
The termination mutation we identified truncates hemojuvelin to encode a protein smaller than that encoded by the R326X mutant allele that has been previously identified.1 The missense mutation on the paternal allele has been reported previously,1 but not in Asian patients. Its occurrence in this family suggests that it has arisen on multiple occasions, possibly because of some aspect of the DNA sequence that makes this site hypermutable. Our findings support earlier genetic evidence that hemojuvelin plays a key role in iron homeostasis. Furthermore, they indicate a role for mutation detection in clinically sporadic cases of severe iron overload.
Juvenile hemochromatosis caused by mutations in HAMP or HJV are clinically and biochemically indistinguishable, suggesting that HJV and HAMP function in the same pathway. Reduced levels of hepcidin are found in patients with HJV mutations, suggesting that hemojuvelin may be involved in the induction of hepcidin production.1
Based on evidence of digenic inheritance of hereditary hemochromatosis, it has been proposed that the phenotype of patients with the HFE C282Y mutation may be modified by heterozygosity for mutations in HAMP and other iron metabolism genes that affect hepcidin function.9 If hemojuvelin is in the hepcidin pathway, it is possible that heterozygosity for mutations in HJV may modify iron disorders caused by mutations in HFE and other hemochromatosis disease genes.
Note added in proof. Since this paper was written, an additional series of mutations has been reported by Lanzara C, Roetto A, Daraio F, Rivard S, Ficarella R, Simard H, Cox TM, Cazzola M, Piperno A, Gimenez-Roqueplo AP, Grammatico P, Volinia S, Gasparini P, Camaschella C. Spectrum of hemojuvelin gene mutations in 1q-linked juvenile hemochromatosis. Blood. 2004;103:4317-4321.
Prepublished online as Blood First Edition Paper, May 11, 2004; DOI 10.1182/blood-2004-01-0400.
Supported in part by National Institutes of Health grants RR00064 (J.P.K.) and R01 DK066373 (M.D.F.). N.C.A. is an Associate Investigator of the Howard Hughes Medical Institute.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the staff at Xenon Genetics for providing information on hemojuvelin sequences before publication.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal