Abstract
Dendritic cells (DCs) are specialized antigen-presenting cells (APCs) that have an unequaled capacity to initiate primary immune responses, including tolerogenic responses. Because of the importance of DCs in the induction and control of immunity, an understanding of their biology is central to the development of potent immunotherapies for cancer, chronic infections, autoimmune disease, and induction of transplantation tolerance. This review discusses recent advances in DC research and the application of this knowledge toward new strategies for the clinical manipulation of DCs for cancer immunotherapy.
Introduction
The concept of tumor “immunosurveillance,” whereby the host immune system is thought to protect against the development of primary cancers, has been debated for decades and has been recently resurrected.1 Evidence in support of tumor immunosurveillance includes observations in mice that lymphocytes and molecules essential for immune function, such as interferon-γ (IFNγ) and perforin, collaborate to protect against the development of certain cancers. Additional corroboration has come from identification of numerous human tumor-associated or tumor-specific antigens recognized by T cells and from isolation of tumor antigen-specific T cells from metastatic lesions. Furthermore, infiltration of certain human cancers by T cells may correlate with dramatically improved survival.2 The accumulating evidence in favor of tumor immunosurveillance indicates that immunotherapies or “vaccines” may prove effective for the treatment of cancer. Indeed, numerous published reports have shown that vaccination of cancer patients with killed tumor cells, tumor cell lysates or tumor antigen proteins, peptides or DNA administered with cytokines or adjuvants can produce immunologic and clinical responses. However, the immune responses to these vaccines are often weak, and clinical responses are rarely complete and long lasting.3-5
Dendritic cells (DCs) are bone marrow–derived antigen-presenting cells (APCs) that play a critical role in the induction and regulation of immune responses. It has been proposed that the manipulation of DCs as a “natural” vaccine adjuvant may prove to be a particularly effective way to stimulate antitumor immunity.6,7 This hypothesis has been supported by experiments in mice. However, published reports of DC-based vaccine trials in humans have yet to demonstrate improved potency of DC vaccines over more traditional vaccine preparations.5,8,9 In this review we discuss the pitfalls of current DC vaccine approaches in the context of recent advances in DC biology and how improved understanding of DC biology can be applied to develop more effective immunotherapies for cancer.
DC biology
DC differentiation and subtypes
DCs are a heterogeneous population of cells produced in the bone marrow in response to growth and differentiation factors fms-like tyrosine kinase-3 ligand (Flt3L) and granulocyte-macrophage colony-stimulating factor (GM-CSF). There are 3 generally accepted stages of differentiation for all DC subtypes: DC precursors, immature DCs, and mature DCs.10 In human blood, immature DCs and DC precursors are lineage-negative (CD3–CD14–CD19–CD56–) HLA-DR+ mononuclear cells6 and are traditionally divided into 2 populations by staining with antibodies to CD11c and CD123 (interleukin 3 receptor α [IL-3Rα]). CD11c+CD123lo DCs have a monocytoid appearance and are called “myeloid DCs” (MDCs), whereas CD11c–CD123hi DCs have morphologic features similar to plasma cells and are thus called “plasmacytoid DCs” (PDCs). Although commonly used, this nomenclature is somewhat misleading. Experiments in mice indicate that both DC populations can be derived from Flt3-expressing myeloid and lymphoid progenitors.11,12 PDCs and MDCs differ in many ways, including their tissue distribution, cytokine production, and growth requirements. PDCs are important in innate antiviral immunity, are found primarily in blood and lymphoid organs, and are the principal interferon α (IFNα)–producing cells in the body. PDCs can activate antitumor and antiviral antigen responses,13,14 but their potential as immunotherapeutic adjuvants is largely unexplored because they are difficult to obtain in large quantities. MDCs, the focus of this review, are found in many tissues, where they may be classified into 2 principal subtypes: Langerhans cells (which express the C-type lectin Langerin, have unique intracellular organelles called Birbeck granules, and are found in the epidermis and oral, respiratory, and genital mucosa), and so-called interstitial, dermal, or submucosal DCs (variously named according to their anatomic location).15
Antigen uptake, processing, and presentation
DCs capture bacteria, viruses, dead or dying cells, proteins, and immune complexes through phagocytosis, endocytosis, and pinocytosis. They have an array of cell surface receptors for antigen uptake, many of which also function in signaling or cell-cell interactions (Table 1). DCs process captured proteins into peptides that are loaded onto major histocompatibility complex class I and II (MHC I and II) molecules, and these peptide-MHC complexes (pMHC I and II) are transported to the cell surface for recognition by antigen-specific T cells (pMHC I and pMHC II are recognized by CD8+ and CD4+ T cells, respectively).22 Antigens acquired endogenously (ie, synthesized within the DC cytosol) are typically processed and loaded onto MHC I, whereas antigens acquired exogenously (from the extracellular environment) are processed onto MHC II. Processing of endogenous proteins onto MHC I is through a cytosolic pathway that involves ubiquitination, degradation by proteasomes, and transport by TAP (transporters for antigen presentation) into the endoplasmic reticulum. In contrast, exogenously acquired proteins are typically degraded in endosomes/lysosomes, where the peptides are loaded onto MHC II following degradation of the MHC II–associated invariant chain (Ii, which blocks access to the peptide-binding pocket of MHC II).22
DC antigen uptake receptors
Receptor class . | Examples . | Ligands . | Notes . |
|---|---|---|---|
| C-type lectin-like receptors16 | DC-SIGN (CD209) | Viruses (HIV, Dengue, Ebola), mycobacteria | DC-SIGN also binds adhesion molecules (ICAM-2 and -3) that are important in DC trafficking and cell-cell interactions. |
| MMR (MRC1) | Mannosylated molecules | ||
| DEC-205 (LY75) | ? | ||
| BDCA-2 (CLECSF11) | ? | ||
| Langerin | ? | ||
| Dectin-1 | β-glucan | ||
| Fc receptors | FcγRI (CD32) | Immune complexes and opsonized cells | — |
| FcγRII (CD64) | |||
| Integrins | αVβ5 | Apoptotic cells | CR3 binds ICAM-1, found on activated lymphocytes and endothelial cells. |
| αMβ2 (CD11b/CD18, CR3) | Opsonized antigens (via iC3b), bacteria | ||
| αXβ2 (CD11c/CD18, CR4) | Opsonized antigens (via iC3b) | ||
| Scavenger receptors17 | CD36 | Apoptotic cells | Heat shock proteins (Hsps) are highly conserved molecules that are released by dying cells and that can mature DCs. Their normal function is to chaperone peptides between subcellular compartments.17,18 |
| LOX-1 (OLR1) | Hsp-peptide complexes | ||
| Other | CD9119 | Hsp-peptide complexes | — |
| Aquaporins | Fluids |
Receptor class . | Examples . | Ligands . | Notes . |
|---|---|---|---|
| C-type lectin-like receptors16 | DC-SIGN (CD209) | Viruses (HIV, Dengue, Ebola), mycobacteria | DC-SIGN also binds adhesion molecules (ICAM-2 and -3) that are important in DC trafficking and cell-cell interactions. |
| MMR (MRC1) | Mannosylated molecules | ||
| DEC-205 (LY75) | ? | ||
| BDCA-2 (CLECSF11) | ? | ||
| Langerin | ? | ||
| Dectin-1 | β-glucan | ||
| Fc receptors | FcγRI (CD32) | Immune complexes and opsonized cells | — |
| FcγRII (CD64) | |||
| Integrins | αVβ5 | Apoptotic cells | CR3 binds ICAM-1, found on activated lymphocytes and endothelial cells. |
| αMβ2 (CD11b/CD18, CR3) | Opsonized antigens (via iC3b), bacteria | ||
| αXβ2 (CD11c/CD18, CR4) | Opsonized antigens (via iC3b) | ||
| Scavenger receptors17 | CD36 | Apoptotic cells | Heat shock proteins (Hsps) are highly conserved molecules that are released by dying cells and that can mature DCs. Their normal function is to chaperone peptides between subcellular compartments.17,18 |
| LOX-1 (OLR1) | Hsp-peptide complexes | ||
| Other | CD9119 | Hsp-peptide complexes | — |
| Aquaporins | Fluids |
DC-SIGN indicates dendritic cell-specific ICAM-3-grabbing nonintegrin; ICAM-2, intracellular adhesion molecule 2; MMR, macrophage mannose receptor; BDCA, blood dendritic cell antigen; CR, complement receptor; LOX-1, low density lipoprotein, oxidized, receptor 1; and CLECSF, C-type lectin superfamily. Antigen uptake by way of DEC-205,20 Fcγ receptors,135 αVβ5 integrin,21 CD36,21 LOX-1,18 and CD9119 have all been associated with cross-presentation.
An alternative pathway also exists whereby DCs process exogenous antigens onto MHC I. This pathway, called “cross-presentation,” permits DCs to elicit CD8+ as well as CD4+ T-cell responses to exogenous antigens such as apoptotic or necrotic tumor cells, virus-infected cells, and immune complexes.23-25 Cross-presentation is linked to specific DC antigen uptake receptors (Table 1), which may be targeted in strategies to load exogenous antigens onto both MHC I and II.24
Lipid and glycolipid antigens expressed on pathogens or self tissues are presented by DCs to T cells on CD1 molecules (CD1a-d), which are structurally similar to MHC I but specialized to bind lipids instead of peptides.26,27 Processing of lipid antigens onto CD1 molecules is carried out in specialized intracellular compartments, much like antigen processing onto MHC II. CD1 molecules present lipid antigens to a variety of lymphocytes, including T cells with substantial T-cell receptor diversity as well as relatively invariant natural killer T (NKT) cells.
DC maturation
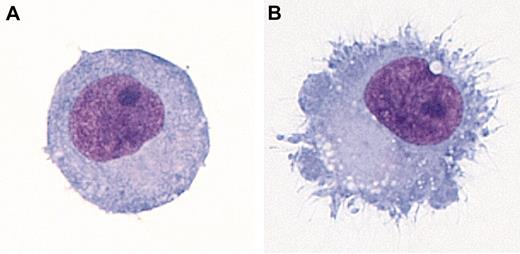
Maturation is a terminal differentiation process that transforms DCs from cells specialized for antigen capture into cells specialized for T-cell stimulation. DC maturation is induced by components of pathogens or by host molecules associated with inflammation or tissue injury. These stimuli are often collectively referred to as “danger signals.”28 Maturation is characterized by reduced phagocytic uptake, the development of cytoplasmic extensions or “veils” (Figure 1), migration to lymphoid tissues, and enhanced T-cell activation potential. Mature DCs express a number of characteristic markers, including CD83, a cell surface molecule involved in CD4+ T-cell development and cell-cell interactions,29,30 and DC-LAMP, a DC-specific lysosomal protein. Maturation signals act on DCs through receptors that trigger intracellular signaling, including receptors for host-derived inflammatory molecules such as CD40L, TNFα, IL-1, and IFNα (Table 2). Microbial products and molecules released by damaged host tissues transmit maturation signals through Toll-like receptors (TLRs), trans-membrane receptors expressed on DCs and other cell types related to Drosophila Toll protein.40 There are 11 known TLRs thus far, each with different expression patterns and each recognizing different sets of molecules. In humans, MDCs express TLRs 1 through 5 and, depending on the MDC subset, TLR 7 and/or 8. Human PDCs express TLRs 1, 7, and 9.41-43 Some TLRs act at the cell surface, whereas others such as TLRs 3, 7, 8, and 9 are found within endosomes and are presumably activated following capture and internalization of pathogens or their products.
DC morphology. (A) Immature monocyte-derived DC. (B) Monocyte-derived DC matured with IL-1β, IL-6, tumor necrosis factor α (TNFα), and prostaglandin E2 (PGE2). Dif-Quick–stained cytocentrifuge preparations are shown. Viewed at 1000× magnification using an Olympus BX51 microscope with an Olympus U Plan Fluorite 100×/1.30 NA oil immersion objective. Images were captured using an Optronics MicroFire digital camera and MicroFire image acquisition software and processed with Adobe Photoshop.
DC morphology. (A) Immature monocyte-derived DC. (B) Monocyte-derived DC matured with IL-1β, IL-6, tumor necrosis factor α (TNFα), and prostaglandin E2 (PGE2). Dif-Quick–stained cytocentrifuge preparations are shown. Viewed at 1000× magnification using an Olympus BX51 microscope with an Olympus U Plan Fluorite 100×/1.30 NA oil immersion objective. Images were captured using an Optronics MicroFire digital camera and MicroFire image acquisition software and processed with Adobe Photoshop.
DC maturation stimuli
Classes . | Examples . | Receptor on DC . | Notes . |
|---|---|---|---|
| TNF family molecules | TNFα (TNF) | TNFR (TNFRSF1A) | TNF family molecules are found on a variety of activated immune cells. |
| CD40L (CD154, TNFSF5) | CD40 (TNFSFR5) | ||
| FasL (TNFSF6) | Fas (TNFRSF6) | ||
| TRANCE (TNFSF11) | RANK (TNFRSF11A) | ||
| LIGHT (TNFSF14) | HVEM (TNFRSF14) | ||
| TLR agonists | Bacterial lipopeptides | TLR1 | Oligosaccharides of hyaluronan are breakdown products of host extracellular matrix. β-defensins are host-derived antimicrobial peptides. Imiquimod and R848 are synthetic antiviral compounds (imidazoquinolines). |
| Pathogen-associated peptidoglycans, lipoproteins, glycolipids, Hsp70 | TLR2 | ||
| dsRNA, polyl:C | TLR3 | ||
| LPS, Hsp60, Hsp70, oligosaccharides of hyaluronan, β-defensins | TLR4 | ||
| Flagellin | TLR5 | ||
| Imiquimod | TLR7 | ||
| R-848, ssRNA31,32 | TLR7, TLR8 | ||
| CpG DNA, HSV DNA33 | TLR9 | ||
| Cytokines | IL-1β | IL-1R | — |
| IL-6 | IL-6R | ||
| Growth factors | TSLP | IL-7Rα/TSLPR heterodimer | Expressed by inflamed epithelial cells and stimulates Th2 responses.34 |
| Interferons | IFNα35 | IFNAR1 | — |
| Adhesion molecules | Agonistic antibody | CEACAM-1 (CD66a) | — |
| Costimulatory molecules | Agonistic antibody | B7-DC36 | — |
| Immune complexes | Opsonized antigens | Fc receptors | Triggers signaling via Syk kinase37 |
| Microbes | Viruses (Influenza, HIV) | — | — |
| Bacteria | |||
| Activated lymphocytes | CD4+ and CD8+ T cells | — | — |
| NK cells | |||
| NKT cells | |||
| Vδ1+ γ:δ T cells | |||
| Other | Uric acid38 | ? | Uric acid is released from dying cells. Stimulation of TREM2 on DCs triggers signaling via DAP12.39 |
| Necrotic cells | |||
| Agonistic antibody | TREM2 |
Classes . | Examples . | Receptor on DC . | Notes . |
|---|---|---|---|
| TNF family molecules | TNFα (TNF) | TNFR (TNFRSF1A) | TNF family molecules are found on a variety of activated immune cells. |
| CD40L (CD154, TNFSF5) | CD40 (TNFSFR5) | ||
| FasL (TNFSF6) | Fas (TNFRSF6) | ||
| TRANCE (TNFSF11) | RANK (TNFRSF11A) | ||
| LIGHT (TNFSF14) | HVEM (TNFRSF14) | ||
| TLR agonists | Bacterial lipopeptides | TLR1 | Oligosaccharides of hyaluronan are breakdown products of host extracellular matrix. β-defensins are host-derived antimicrobial peptides. Imiquimod and R848 are synthetic antiviral compounds (imidazoquinolines). |
| Pathogen-associated peptidoglycans, lipoproteins, glycolipids, Hsp70 | TLR2 | ||
| dsRNA, polyl:C | TLR3 | ||
| LPS, Hsp60, Hsp70, oligosaccharides of hyaluronan, β-defensins | TLR4 | ||
| Flagellin | TLR5 | ||
| Imiquimod | TLR7 | ||
| R-848, ssRNA31,32 | TLR7, TLR8 | ||
| CpG DNA, HSV DNA33 | TLR9 | ||
| Cytokines | IL-1β | IL-1R | — |
| IL-6 | IL-6R | ||
| Growth factors | TSLP | IL-7Rα/TSLPR heterodimer | Expressed by inflamed epithelial cells and stimulates Th2 responses.34 |
| Interferons | IFNα35 | IFNAR1 | — |
| Adhesion molecules | Agonistic antibody | CEACAM-1 (CD66a) | — |
| Costimulatory molecules | Agonistic antibody | B7-DC36 | — |
| Immune complexes | Opsonized antigens | Fc receptors | Triggers signaling via Syk kinase37 |
| Microbes | Viruses (Influenza, HIV) | — | — |
| Bacteria | |||
| Activated lymphocytes | CD4+ and CD8+ T cells | — | — |
| NK cells | |||
| NKT cells | |||
| Vδ1+ γ:δ T cells | |||
| Other | Uric acid38 | ? | Uric acid is released from dying cells. Stimulation of TREM2 on DCs triggers signaling via DAP12.39 |
| Necrotic cells | |||
| Agonistic antibody | TREM2 |
TNF indicates tumor necrosis factor; TNFRSF, TNF receptor superfamily; CD40L, CD40 ligand; TNFSF, TNF ligand superfamily; TRANCE, TNF-related activation-induced cytokine; RANK, receptor activator of NF-κB (nuclear factor-κB); LIGHT, homologous to lymphotoxins, exhibits inducible expression, and competes with HSV glycoprotein D for HVEM, a receptor expressed by T lymphocytes; HVEM, Herpes virus entry mediator; TLR, Toll-like receptor; Hsp, heat shock protein; dsRNA, double-stranded viral RNA; LPS, bacterial lipopolysaccharide; ssRNA, single-stranded RNA; CpG DNA, bacterial unmethylated CpG motif DNA; HSV, Herpes simplex virus; TSLP, thymic stromal lymphopoietin; Th2, T helper cell type 2; IFN, interferon; CEACAM-1, CEA (carcinoembryonic antigen)—related cell adhesion molecule 1; TREM, triggering receptor expressed on myeloid cells; DAP12, DNAX activation protein 12.
TLRs signal through the adapter molecule MyD88, which recruits other signaling molecules in a pathway that activates NF-κB and mitogen-activated protein (MAP) kinases, inducing the transcription of genes encoding inflammatory mediators such as TNFα, IL-1, and IL-6.44 Stimulation of some TLRs can trigger additional, MyD88-independent, signaling pathways.44 In DCs, the distinct signaling pathways triggered can influence the direction of the resulting T-cell response.45 TLR agonists, therefore, can be used to target DC subsets to induce desired T-cell responses.
On maturation, DCs develop an enhanced ability to form pMHC II46 and pMHC I,22 and some maturation stimuli can also induce cross-presentation.47-49 Maturation also results in increased expression of adhesion and costimulatory molecules involved in the formation of the immunologic synapse (Figure 2) and induces DCs to secrete cytokines that are critical in determining the nature of the ensuing immune response (Figure 3). Another important effect of maturation is the induced secretion of chemokines that recruit monocytes, DCs, and specific subsets of T cells into the local environment (Table 3). Finally, maturation imparts on peripheral DCs the ability to migrate from the tissues to T-cell zones of lymph nodes. This is mediated, at least in part, through differential regulation of DC chemokine receptors such as CCR1, CCR5, and CCR7 (Table 3).
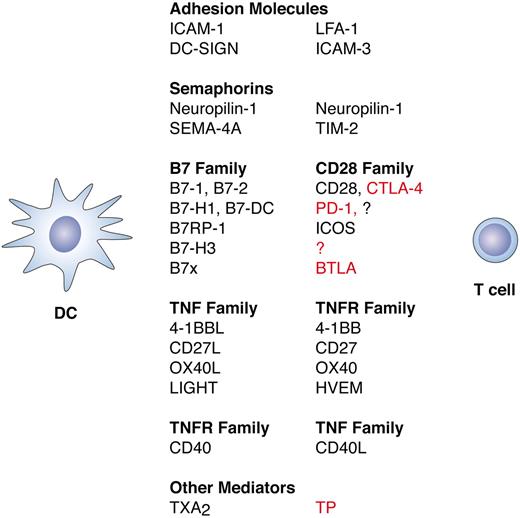
Molecules involved in the immunologic synapse between DCs and T cells. Molecules expressed on DCs are listed on the left, with their corresponding T-cell ligands listed on the right. In the right-hand column, stimulatory interactions are indicated in black text and inhibitory interactions are indicated in red. Signaling from T cells to DCs also occurs but is not shown here. Initial DC–T-cell interactions are mediated by adhesion molecules and semaphorins such as neuropilin-1.50 Following engagement of the T-cell receptor by pMHC complexes (signal 1) and engagement of CD28 by B7-1 and B7-2 (signal 2), additional molecules are up-regulated on both cell types that determine the nature of the ensuing T-cell response. Up-regulated molecules include semaphorins such as SEM4-A and members of the B7, CD28, TNF, and TNFR families of costimulatory molecules. Bidirectional signaling between these molecules results in either further T-cell activation or in attenuation of the T-cell response, depending on the molecules involved. Both B7-H1 and B7-DC interact with PD-1 to inhibit activated T cells, but B7-DC can also work synergistically with B7-1 and B7-2 to enhance T-cell activation through an unknown receptor.51,52 B7x transmits an inhibitory signal by way of BTLA (B and T lymphocyte attenuator),53 and B7-H3 can also transmit an inhibitory signal but through an unknown receptor.54 Thromboxane A2 (TXA2) secreted by the DCs also attenuates the DC–T-cell interaction by way of the thromboxane receptor (TP) on the T cell.55 Inhibitory molecules are thought to prevent excessive inflammation and autoimmunity. Alternative names for B7 family members are CD80 (B7-1), CD86 (B7-2), PD-L1 (B7-H1), PD-L2 (B7-DC), B7-H2 or ICOSL (B7RP-1), and B7-H4 (B7x).
Molecules involved in the immunologic synapse between DCs and T cells. Molecules expressed on DCs are listed on the left, with their corresponding T-cell ligands listed on the right. In the right-hand column, stimulatory interactions are indicated in black text and inhibitory interactions are indicated in red. Signaling from T cells to DCs also occurs but is not shown here. Initial DC–T-cell interactions are mediated by adhesion molecules and semaphorins such as neuropilin-1.50 Following engagement of the T-cell receptor by pMHC complexes (signal 1) and engagement of CD28 by B7-1 and B7-2 (signal 2), additional molecules are up-regulated on both cell types that determine the nature of the ensuing T-cell response. Up-regulated molecules include semaphorins such as SEM4-A and members of the B7, CD28, TNF, and TNFR families of costimulatory molecules. Bidirectional signaling between these molecules results in either further T-cell activation or in attenuation of the T-cell response, depending on the molecules involved. Both B7-H1 and B7-DC interact with PD-1 to inhibit activated T cells, but B7-DC can also work synergistically with B7-1 and B7-2 to enhance T-cell activation through an unknown receptor.51,52 B7x transmits an inhibitory signal by way of BTLA (B and T lymphocyte attenuator),53 and B7-H3 can also transmit an inhibitory signal but through an unknown receptor.54 Thromboxane A2 (TXA2) secreted by the DCs also attenuates the DC–T-cell interaction by way of the thromboxane receptor (TP) on the T cell.55 Inhibitory molecules are thought to prevent excessive inflammation and autoimmunity. Alternative names for B7 family members are CD80 (B7-1), CD86 (B7-2), PD-L1 (B7-H1), PD-L2 (B7-DC), B7-H2 or ICOSL (B7RP-1), and B7-H4 (B7x).
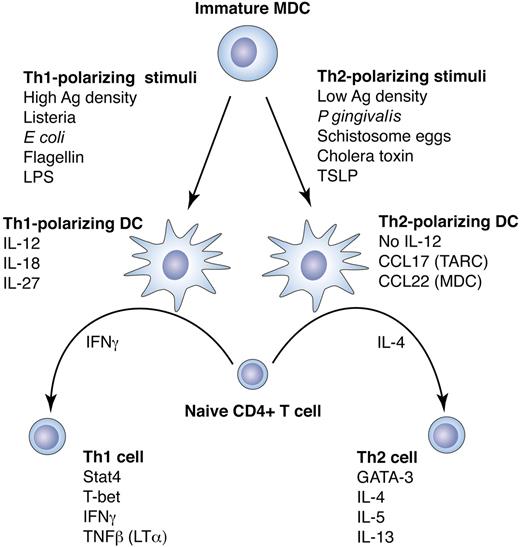
DC plasticity in response to different maturation stimuli directs Th polarization. DCs can direct the fate of naive CD4+ T cells, depending on the type of DC maturation stimulus. Following priming, CD4+ T cells may differentiate toward T-helper 1 (Th1) cells, which produce IFNγ and support CD8+ cytotoxic T lymphocyte (CTL) responses, or toward T-helper 2 (Th2) cells, which produce IL-4, IL-5, and IL-13, support humoral immunity, and down-regulate Th1 responses. The direction of Th polarization is determined by the secreted cytokine profile of the stimulating DCs, which in turn depends on the DC subtype, the anatomic location of the DCs, and the type of maturation stimulus.45,56 These factors control other characteristics of the T-cell response as well, such as tolerance induction57 or T-cell homing.58,59 Th1-polarizing stimuli such as LPS or flagellin direct a DC differentiation program that causes the DCs to secrete IL-12p70, which together with IFNγ potently induce CD4+ T cells to differentiate into IFNγ-secreting Th1 effector cells. This T-cell program is mediated largely by the transcription factors signal transducer and activator of transcription 4 (Stat4) and T-bet.60,61 Th1 polarization can also be induced in the absence of IL-12p70 by mechanisms that are not entirely known but may be due in part to IL-12–related cytokines such as IL-27. Other DC maturation stimuli such as cholera toxin or schistosome eggs can differentiate DCs that do not produce IL-12p70 and that, in the presence of IL-4, induce naive CD4+ T cells to differentiate into IL-4–secreting Th2 effector cells. It is not clear whether Th2 polarization is induced by specific DC cytokines or is rather a default program carried out in the absence of a Th1 polarization signal from the DCs. However, DC secretion of chemokines such as thymus and activation-regulated chemokine (TARC) and MDC can act to potentiate a Th2 response by preferentially attracting Th2 cells. The Th2 program in CD4+ T cells is dependent on transcription factors GATA-3 and c-Maf.60,61
DC plasticity in response to different maturation stimuli directs Th polarization. DCs can direct the fate of naive CD4+ T cells, depending on the type of DC maturation stimulus. Following priming, CD4+ T cells may differentiate toward T-helper 1 (Th1) cells, which produce IFNγ and support CD8+ cytotoxic T lymphocyte (CTL) responses, or toward T-helper 2 (Th2) cells, which produce IL-4, IL-5, and IL-13, support humoral immunity, and down-regulate Th1 responses. The direction of Th polarization is determined by the secreted cytokine profile of the stimulating DCs, which in turn depends on the DC subtype, the anatomic location of the DCs, and the type of maturation stimulus.45,56 These factors control other characteristics of the T-cell response as well, such as tolerance induction57 or T-cell homing.58,59 Th1-polarizing stimuli such as LPS or flagellin direct a DC differentiation program that causes the DCs to secrete IL-12p70, which together with IFNγ potently induce CD4+ T cells to differentiate into IFNγ-secreting Th1 effector cells. This T-cell program is mediated largely by the transcription factors signal transducer and activator of transcription 4 (Stat4) and T-bet.60,61 Th1 polarization can also be induced in the absence of IL-12p70 by mechanisms that are not entirely known but may be due in part to IL-12–related cytokines such as IL-27. Other DC maturation stimuli such as cholera toxin or schistosome eggs can differentiate DCs that do not produce IL-12p70 and that, in the presence of IL-4, induce naive CD4+ T cells to differentiate into IL-4–secreting Th2 effector cells. It is not clear whether Th2 polarization is induced by specific DC cytokines or is rather a default program carried out in the absence of a Th1 polarization signal from the DCs. However, DC secretion of chemokines such as thymus and activation-regulated chemokine (TARC) and MDC can act to potentiate a Th2 response by preferentially attracting Th2 cells. The Th2 program in CD4+ T cells is dependent on transcription factors GATA-3 and c-Maf.60,61
DC chemokine receptors and chemokines
Receptors . | Receptor expression on DCs . | Ligands . | Ligand expression by DCs . | Notes . |
|---|---|---|---|---|
| CCR1 and CCR5 | Immature DCs | CCL3 (MIP-1α) | Mature DCs (some stimuli only) | CCL3, 4, and 5 are also secreted by activated inflammatory cells and activated endothelium |
| CCL4 (MIP-1β) | ||||
| CCL5 (RANTES) | ||||
| CCR2 | Immature DCs | CCL2 (MCP-1) | Not expressed | CCL2 is secreted by activated monocytes, macrophages and endothelium |
| CCR4 | Immature DCs | CCL17 (TARC) | Mature DCs (some stimuli only) | CCR4 is preferentially expressed on Th2 cells, and TARC and MDC can promote Th2 responses. |
| CCL22 (MDC) | ||||
| CCR6 | Immature DCs (some subsets only) | CCL20 (MIP-3α) | Not expressed | CCL20 is expressed by activated monocytes and lymphocytes |
| CCR7 | Mature DCs | CCL19 (MIP-3β) | Not expressed | CCL19 and 21 are expressed in T-cell zones of lymph nodes and spleen, lymphatic endothelium, and lymph node high endothelial venules |
| CCL21 (SLC) | ||||
| CXCR1 and CXCR2 | Immature DCs | CXCL8 (IL-8) | Mature DCs | IL-8 is a mediator of neutrophil recruitment |
| CXCR3 | Not expressed | CXCL10 (IP-10) | Mature DCs (some stimuli only) | IP-10 attracts IFNγ-producing T cells, which express CXCR3, and can promote Th1 responses. |
| CXCR4 | Mature > Immature | CXCL12 (SDF-1) | Not expressed | SDF-1 is a chemoattractant for lymphocytes and monocytes. CXCR4 is also a coreceptor for T-cell-trophic strains of HIV |
Receptors . | Receptor expression on DCs . | Ligands . | Ligand expression by DCs . | Notes . |
|---|---|---|---|---|
| CCR1 and CCR5 | Immature DCs | CCL3 (MIP-1α) | Mature DCs (some stimuli only) | CCL3, 4, and 5 are also secreted by activated inflammatory cells and activated endothelium |
| CCL4 (MIP-1β) | ||||
| CCL5 (RANTES) | ||||
| CCR2 | Immature DCs | CCL2 (MCP-1) | Not expressed | CCL2 is secreted by activated monocytes, macrophages and endothelium |
| CCR4 | Immature DCs | CCL17 (TARC) | Mature DCs (some stimuli only) | CCR4 is preferentially expressed on Th2 cells, and TARC and MDC can promote Th2 responses. |
| CCL22 (MDC) | ||||
| CCR6 | Immature DCs (some subsets only) | CCL20 (MIP-3α) | Not expressed | CCL20 is expressed by activated monocytes and lymphocytes |
| CCR7 | Mature DCs | CCL19 (MIP-3β) | Not expressed | CCL19 and 21 are expressed in T-cell zones of lymph nodes and spleen, lymphatic endothelium, and lymph node high endothelial venules |
| CCL21 (SLC) | ||||
| CXCR1 and CXCR2 | Immature DCs | CXCL8 (IL-8) | Mature DCs | IL-8 is a mediator of neutrophil recruitment |
| CXCR3 | Not expressed | CXCL10 (IP-10) | Mature DCs (some stimuli only) | IP-10 attracts IFNγ-producing T cells, which express CXCR3, and can promote Th1 responses. |
| CXCR4 | Mature > Immature | CXCL12 (SDF-1) | Not expressed | SDF-1 is a chemoattractant for lymphocytes and monocytes. CXCR4 is also a coreceptor for T-cell-trophic strains of HIV |
CCR indicates CC motif chemokine receptor; CCL, CC motif chemokine ligand; MIP, macrophage inflammatory protein; RANTES, regulated on activation, normally T-expressed, and presumably secreted; MCP, monocyte chemoattractant protein; TARC, thymus and activation-regulated chemokine; MDC, macrophage-derived chemokine; SLC, secondary lymphoid chemokine; CXCR, CXC motif chemokine receptor; CXCL, CXC motif chemokine ligand; IP-10, IFNγ-inducible 10-kDa protein; SDF-1, stromal cell-derived factor 1; HIV, human immunodeficiency virus.
DC interactions with lymphocytes
DCs initiate or “prime” T-cell responses in secondary lymphoid organs such as lymph nodes, spleen, or mucosal lymphoid tissues.62-64 Effective priming of naive T cells is manifested by their clonal expansion and differentiation into memory cells and cytokine-secreting effector cells. The strength of the T-cell response is dependent on many factors, including the concentration of antigen on the DC, the affinity of the T-cell receptor for the corresponding pMHC, the state of DC maturation, and the type of maturation stimulus.65 For example, T-cell stimulation by immature DCs leads to initial T-cell proliferation but only short-term survival (“abortive proliferation”), whereas stimulation by mature DCs results in long-term T-cell survival and differentiation into memory and effector T cells.65 Enhanced survival following priming, referred to as T-cell “fitness,” is characterized by resistance to cell death in the absence of cytokines and by responsiveness to the “homeostatic” cytokines IL-7 and IL-15, which promote T-cell survival in the absence of antigen.65,66
Importantly, recent observations indicate that CD4+ T-cell help at the time of priming is required to generate CD8+ T-cell memory.67-69 This effect is thought to be mediated by CD40-CD40L interactions between CD4+ T cells and DCs.70 Other T-cell surface molecules are also involved in the generation of long-lived T-cell responses and T-cell memory and have corresponding ligands that are up-regulated on activated APCs such as DCs.71 Examples include members of the TNF receptor superfamily, including OX40 and 4-1BB, which may be critical for both initiating and sustaining long-lived T-cell immunity (Figure 2).71-75
DCs also interact directly with B cells and lymphocytes of the innate immune system. Activated MDCs can directly induce B-cell proliferation, immunoglobulin isotype switching, and plasma cell differentiation through the production of the B-cell activation and survival molecules BAFF (B-cell–activating factor belonging to the TNF family) and APRIL (a proliferation-inducing ligand),76-78 and activated PDCs can induce the differentiation of CD40-activated B cells into plasma cells through the secretion of IFNα/β and IL-6.79 DCs can also activate and induce the expansion of resting NK cells by mechanisms that are just beginning to be understood. Requirements for direct cell contact or soluble factors have both been described.80 Activated NK cells can kill immature, but not mature, DCs and can stimulate DCs to induce protective CD8+ T-cell responses.80,81 Finally, DCs presenting the synthetic glycolipid α-galactosylceramide (α-GalCer) on CD1d can activate NKT cells to produce IFNγ and promote resistance to tumors.82 Activated NKT cells can in turn rapidly induce the full maturation of DCs and can directly interact with DCs to enhance both CD4+ and CD8+ T-cell responses.82,83
DC induction of immune tolerance
Antigen presentation by immature DCs is considered to be an important pathway by which tolerance to self-antigens is maintained. Antigens targeted to immature DCs in vivo can induce tolerance through abortive proliferation and anergy of antigen-specific T cells, whereas simultaneous delivery of a DC maturation stimulus induces a full effector T-cell response (Figure 4).20,84,96 Immature DCs can also induce tolerance through the induction of CD4+ and CD8+ regulatory T (Tr) cells that suppress immune responses by way of secretion of cytokines such as IL-10 and TGFβ (Figure 4).97-99 This is in contrast to “naturally occurring” CD4+ Tr cells produced in the thymus, which constitutively express CD25 (IL-2Rα), CTLA-4, and Foxp3, and exert their immunosuppressive effect in a cell contact-dependent manner.84-86,97,100,101 Mature DCs can inhibit naturally occurring Tr cells through the production of IL-6.102 DC expression of CD40 may be an important factor in determining whether T-cell priming will result in immunity or Tr cell–mediated immune suppression. Antigen-exposed mouse DCs which lack CD40 prevent T-cell priming, suppress previously primed immune responses, and induce IL-10–secreting CD4+ Tr cells.90
Tolerogenic DCs. There are a number of pathways by which immature MDCs can be rendered tolerogenic. Some of these mechanisms may overlap. The 5 mechanisms summarized here (from left to right) include antigen presentation by resting (steady-state) DCs; exposure of DCs to “modulating” cytokines such as IL-10 and transforming growth factor-β (TGFβ) or to other modulating substances such as corticosteroids and vitamin D3; targeted inhibition of the RelB transcription factor (which controls CD40 expression) or direct inhibition of CD40; DC exposure to CD8+CD28– regulatory T (Tr) cells (which have been associated with graft tolerance in patients who received transplants); and the induction of indoleamine 2,3-dioxygenase (IDO)–expressing DCs (IDO-DCs) by ligating B7-1 and B7-2 molecules on the DC with a cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4)–immunoglobulin fusion protein or by CD4+CD25+ Tr cells. Immature DCs (left) can induce tolerance through the induction of abortive proliferation and anergy, as well as through the induction of IL-10–producing Tr cells.84,85,86 DCs that have been modulated by factors such as IL-10 or TGFβ (second from left) may also lead to the inhibition of effector T-cell expansion and the induction of IL-10–secreting Tr cells.87,88 Stat3 signaling within the DC appears to be critical for this effect.89 IL-10–producing Tr cells are also induced by DCs that are deficient in RelB or CD4090 (center). CD8+CD28– Tr cells induce tolerogenic DCs by up-regulating inhibitory receptors immunoglobulin-like transcript 3 (ILT3) and ILT4 on the DC surface, which ultimately leads to decreased DC expression of B7-1 and B7-2 and T-cell anergy91 (second from right). Finally, IDO-DCs (right) inhibit T-cell expansion and induce T-cell apoptosis by way of IDO-mediated tryptophan catabolism within the DCs.92-94 Both MDCs and PDCs can also be rendered tolerogenic by factors secreted by malignant tumors (not shown).95
Tolerogenic DCs. There are a number of pathways by which immature MDCs can be rendered tolerogenic. Some of these mechanisms may overlap. The 5 mechanisms summarized here (from left to right) include antigen presentation by resting (steady-state) DCs; exposure of DCs to “modulating” cytokines such as IL-10 and transforming growth factor-β (TGFβ) or to other modulating substances such as corticosteroids and vitamin D3; targeted inhibition of the RelB transcription factor (which controls CD40 expression) or direct inhibition of CD40; DC exposure to CD8+CD28– regulatory T (Tr) cells (which have been associated with graft tolerance in patients who received transplants); and the induction of indoleamine 2,3-dioxygenase (IDO)–expressing DCs (IDO-DCs) by ligating B7-1 and B7-2 molecules on the DC with a cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4)–immunoglobulin fusion protein or by CD4+CD25+ Tr cells. Immature DCs (left) can induce tolerance through the induction of abortive proliferation and anergy, as well as through the induction of IL-10–producing Tr cells.84,85,86 DCs that have been modulated by factors such as IL-10 or TGFβ (second from left) may also lead to the inhibition of effector T-cell expansion and the induction of IL-10–secreting Tr cells.87,88 Stat3 signaling within the DC appears to be critical for this effect.89 IL-10–producing Tr cells are also induced by DCs that are deficient in RelB or CD4090 (center). CD8+CD28– Tr cells induce tolerogenic DCs by up-regulating inhibitory receptors immunoglobulin-like transcript 3 (ILT3) and ILT4 on the DC surface, which ultimately leads to decreased DC expression of B7-1 and B7-2 and T-cell anergy91 (second from right). Finally, IDO-DCs (right) inhibit T-cell expansion and induce T-cell apoptosis by way of IDO-mediated tryptophan catabolism within the DCs.92-94 Both MDCs and PDCs can also be rendered tolerogenic by factors secreted by malignant tumors (not shown).95
DCs may actively be rendered tolerogenic by a number of mechanisms. In humans, a subset of monocyte-derived DCs has been described that expresses indoleamine 2,3-dioxygenase (IDO), inhibits T-cell proliferation, and induces T-cell death.92 IDO can be induced in DCs by ligation of their B7 molecules with CTLA-493,94 (Figure 4). Large numbers of “IDO DCs” can be found in tumor-draining lymph nodes, suggesting that they may be involved in the immunologic unresponsiveness seen in cancer patients.92 DCs may also be rendered tolerogenic by naturally occurring CD8+CD28– Tr cells, which up-regulate inhibitory receptors on DCs and disrupt CD40-induced B7-1 and B7-2 expression91 (Figure 4). Finally, DCs can be rendered tolerogenic in culture by the presence of IL-10, TGFβ, vitamin D3, or corticosteroids (Figure 4).87 DC Stat3 activity may be critical to the induction of antigen-specific T-cell tolerance. Stat3 is activated by tyrosine phosphorylation following DC exposure to IL-10 and other factors produced by tumor cells, and forced expression of activated Stat3 in DCs can result in impaired antigen-specific T-cell responses.89
Manipulation of DCs for cancer immunotherapy
Current approaches to DC vaccine design
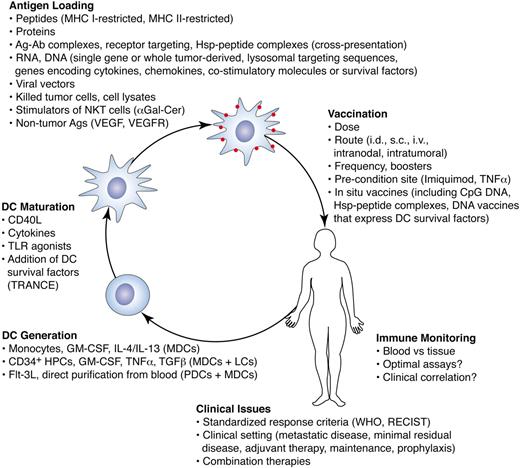
The most common approach to using DCs for vaccines is to prepare large numbers of autologous mature MDCs ex vivo, load them with antigens, and inject them back into the subject (Figure 5).103,104 Three general methods have been described, involving, respectively (1) differentiating DCs from leukapheresis-derived monocytes with GM-CSF and IL-4105,106 (the most popular approach; IL-13 has been used by some groups in place of IL-4), (2) GM-CSF and TNFα–mediated differentiation of CD34+ hematopoietic progenitor cells into mixtures of interstitial DCs and Langerhans cells107 (Flt3L or stem cell factor may be added to expand DC progenitors, and differentiation may be skewed toward Langerhans cells by adding TGFβ to the culture108 ), or (3) directly isolating DCs from leukapheresis products by density gradient centrifugation109 or with commercially available closed systems that use immunomagnetic beads. The yields of both plasmacytoid and classic myeloid-type DCs purified from blood can be significantly enhanced by stimulating patients with Flt3L prior to leukapheresis,110 although pharmaceutical-grade Flt3L is not currently available. All 3 types of DC preparations can stimulate antigen-specific T-cell responses in human subjects and have been associated with clinical responses in cancer patients. No direct comparisons have been performed in clinical trials, although one such trial is currently in progress.
Clinical DC vaccines. There are many alternative approaches for the preparation and use of DC vaccines to treat cancer. DCs may be prepared ex vivo following 3 general methods (lower left), each of which results in a different mixture of cells. DCs may be matured and loaded with antigens using a variety of techniques. Some of these techniques include the addition of DC survival factors, the use of substances that induce cross-presentation, or the use of stimulators of other innate immune cells such as NKT cells. DCs loaded with RNA or DNA can take advantage of sequences encoding cytokines, chemokines, or DC survival factors. Numerous variables such as antigen dose, cell dose, and route of administration also need to be optimized. In addition, less costly and labor-intensive approaches that stimulate and load DCs with antigen in situ are being explored. There are many unresolved issues regarding the monitoring of immune responses and in correlating these responses with clinical outcome. DC vaccines may prove most effective in the adjuvant setting or in combination with other treatments.
Clinical DC vaccines. There are many alternative approaches for the preparation and use of DC vaccines to treat cancer. DCs may be prepared ex vivo following 3 general methods (lower left), each of which results in a different mixture of cells. DCs may be matured and loaded with antigens using a variety of techniques. Some of these techniques include the addition of DC survival factors, the use of substances that induce cross-presentation, or the use of stimulators of other innate immune cells such as NKT cells. DCs loaded with RNA or DNA can take advantage of sequences encoding cytokines, chemokines, or DC survival factors. Numerous variables such as antigen dose, cell dose, and route of administration also need to be optimized. In addition, less costly and labor-intensive approaches that stimulate and load DCs with antigen in situ are being explored. There are many unresolved issues regarding the monitoring of immune responses and in correlating these responses with clinical outcome. DC vaccines may prove most effective in the adjuvant setting or in combination with other treatments.
DCs are frequently matured in culture prior to injection. Currently, many laboratories using monocyte-derived DCs induce maturation by the addition of a “cocktail” of IL-1β, IL-6, TNFα, and PGE2.111 Several groups have observed that DCs matured in this manner do not secrete detectable bioactive IL-12p70, but still express CCR7 and induce Th1 and CD8+ T-cell responses.112,113 How the DCs induce these T-cell responses is currently under investigation.
The choice of tumor antigen is important to consider (Table 4). Because vaccines may select for tumor cells that escape immune detection by loss of target antigen expression, antigens critical to tumor growth are preferred. MHC-restricted peptide antigens are frequently used, including altered or enhanced peptides that boost immunity to less immunogenic self-antigens or that improve antigen presentation or T-cell receptor affinity.3,104,107,110,116,117 A disadvantage to using peptides is that they must be compatible with the HLA type of the patient, often restricting peptide vaccination studies to individuals with common HLA types. In addition, the half-life of pMHC complexes may be short, and competition may prevent priming to lower-affinity epitopes when mixtures of peptides are used.
Classes of tumor antigens
Category . | Examples . | Notes . |
|---|---|---|
| Cancer-testis (CT) antigens | MAGE-1 (MAGEA1) | Expressed in germ cells, germinal tissues, tumor cells. |
| BAGE | ||
| GAGE-1 (GAGE1) | ||
| NY-ESO-1 (CTAG1) | ||
| Lineage-specific antigens | Melanocyte antigens: | Expressed in specific tissues or cells. |
| -Tyrosinase (TYR) | ||
| -Melan-A/MART-1 (MLANA) | ||
| -gp100/Pmel17 (SILV) | ||
| Tumor-specific altered gene products (amplified, aberrantly expressed, overexpressed or mutated genes, splice variants, gene fusion products, etc) | HER-2/neu (ERBB2) | Associated with a wide variety of tumors. KRAS2 is mutated in 30% to 40% of colorectal cancers, and p53 is mutated in up to 70% of all human cancers. Altered MUC1 glycosylation is seen in a variety of adenocarcinomas, and these altered glycopeptides can be presented by DCs to T cells.114 Myeloid leukemia cells can potentially be differentiated into DCs to vaccinate against endogenously expressed leukemia-specific antigens.115 |
| p53 (TP53) | ||
| Ras genes (KRAS2, HRAS, NRAS) | ||
| Mucin 1 (MUC1) | ||
| Beta-catenin (CTNNB1) | ||
| MUM1 (IRF4) | ||
| CDK4 | ||
| BCR-ABL fusion products | ||
| N-acteylglucosaminyltranferase V (MGAT5) | ||
| Survivin (BIRC5) | ||
| TERT | ||
| CEA | ||
| AFP | ||
| Immunoglobulin idiotypes | Multiple myeloma | Unique, tumor-specific idiotypes because of clonal rearrangements of immunoglobulin genes. Associated with B-cell malignancies. |
| B-cell lymphoma | ||
| Viral antigens | HPV E6 and E7 proteins | May be used for tumors such as cervical cancer that are induced by oncogenic viruses. |
| EBV (HHV4) LMP1 and LMP2 proteins |
Category . | Examples . | Notes . |
|---|---|---|
| Cancer-testis (CT) antigens | MAGE-1 (MAGEA1) | Expressed in germ cells, germinal tissues, tumor cells. |
| BAGE | ||
| GAGE-1 (GAGE1) | ||
| NY-ESO-1 (CTAG1) | ||
| Lineage-specific antigens | Melanocyte antigens: | Expressed in specific tissues or cells. |
| -Tyrosinase (TYR) | ||
| -Melan-A/MART-1 (MLANA) | ||
| -gp100/Pmel17 (SILV) | ||
| Tumor-specific altered gene products (amplified, aberrantly expressed, overexpressed or mutated genes, splice variants, gene fusion products, etc) | HER-2/neu (ERBB2) | Associated with a wide variety of tumors. KRAS2 is mutated in 30% to 40% of colorectal cancers, and p53 is mutated in up to 70% of all human cancers. Altered MUC1 glycosylation is seen in a variety of adenocarcinomas, and these altered glycopeptides can be presented by DCs to T cells.114 Myeloid leukemia cells can potentially be differentiated into DCs to vaccinate against endogenously expressed leukemia-specific antigens.115 |
| p53 (TP53) | ||
| Ras genes (KRAS2, HRAS, NRAS) | ||
| Mucin 1 (MUC1) | ||
| Beta-catenin (CTNNB1) | ||
| MUM1 (IRF4) | ||
| CDK4 | ||
| BCR-ABL fusion products | ||
| N-acteylglucosaminyltranferase V (MGAT5) | ||
| Survivin (BIRC5) | ||
| TERT | ||
| CEA | ||
| AFP | ||
| Immunoglobulin idiotypes | Multiple myeloma | Unique, tumor-specific idiotypes because of clonal rearrangements of immunoglobulin genes. Associated with B-cell malignancies. |
| B-cell lymphoma | ||
| Viral antigens | HPV E6 and E7 proteins | May be used for tumors such as cervical cancer that are induced by oncogenic viruses. |
| EBV (HHV4) LMP1 and LMP2 proteins |
A useful web site with links to current cancer antigen databases may be found at: http://www.cancerimmunity.org/statics/databases.htm. TERT indicates telomerase reverse transcriptase; AFP, alpha-fetoprotein; HPV, human papillomavirus; EBV, Epstein-Barr virus; LMP1, latent membrane protein 1.
DCs may be loaded with purified or recombinant proteins, transduced with nonreplicating recombinant viral vectors, or transfected with RNA or, less commonly, plasmid vectors encoding tumor-associated antigens.118-122 All of these approaches allow the host's MHC molecules to select epitopes from an antigen's entire amino acid sequence (Figure 5). Immunogenicity may be enhanced by using antigens coupled to or expressing other more immunogenic molecules such as foreign proteins (eg, keyhole limpet hemocyanin [KLH]), cytokines (IL-12, IL-15),118,123 costimulatory molecules (B7-2, CD40L), or chemokines (CCL21). DCs may also be loaded with whole tumor cells or tumor cell lysates or be transfected with whole tumor RNA, which permit vaccination with the complete antigenic content of the tumor.103,124-126
Studies to compare routes and frequency of injection, DC dose, and DC subset will be essential to optimize DC immunotherapy. DC vaccines may be stored frozen prior to vaccination103,104 and are typically injected intradermally, subcutaneously, or intravenously in numbers ranging from 2 to 100 million cells. Route of administration may directly affect the nature of T-cell priming. Skin injections may be required to induce immunity to cutaneous tumors, whereas intravenous injections may be less effective at Th1 induction but more effective at induction of humoral immunity.127,128 Injection into lymph nodes or lymphatics has also been attempted, because only 5% or fewer DCs may migrate to draining nodes following subcutaneous injection. Direct injection into tumors is also being investigated.
Lessons learned from early DC vaccine trials
DC vaccines have minimal side effects and have induced antigen-specific cytotoxic T lymphocyte (CTL) and Th1 responses in healthy volunteers and in patients with a variety of advanced cancers.5,9 Most of the trials in cancer patients have focused on the safety and immunogenicity of DC vaccines and were not designed to evaluate clinical responses. Larger controlled trials are now under way to objectively assess clinical efficacy by documenting responses following standardized criteria such as World Health Organization (WHO) or Response Evaluation Criteria in Solid Tumors Group (RECIST) guidelines.129
Some fundamental lessons have been learned from the smaller, published DC vaccine trials, although they have not led to a consensus on optimal antigen source or dose, DC dose, DC subset or frequency, or route of administration. Most investigators now avoid intravenous administration, as studies have suggested that subcutaneous or intradermal vaccination leads to improved DC migration to lymph nodes127 and enhanced Th1 polarization.128 Importantly, several studies indicate that DCs need to be matured to effectively generate antigen-specific immune responses in humans. Injection of healthy volunteers with antigen-loaded immature DCs has been associated with tolerogenic responses,99 and a randomized trial in patients with metastatic melanoma comparing peptide-pulsed immature DCs with peptides administered with adjuvant and GM-CSF demonstrated significantly lower immunogenicity in patients receiving the DC vaccine.130 In addition, a direct comparison of peptide-loaded immature and mature DCs in patients with metastatic melanoma showed that only mature DCs induced antigen-specific CTL responses.98
The availability of sensitive and specific techniques to monitor the induction of antigen-specific T-cell responses has provided insight into the capacity of DCs to induce primary responses to tumor antigens. For instance, it is clear that DC immunization can elicit Th1 and CD8+ T-cell responses specific to the immunizing antigens (as measured by enzyme-linked immunospot [ELISPOT], lymphocyte proliferation, cytolytic assays, and peptide-MHC tetramer staining), with a suggestion of epitope or antigen spreading in some cases.131,132 However, immune responses in most studies have been weak or undetectable, and durability has not been clearly established. Correlation with tumor regression or disease stabilization has been variable and needs to be established in larger trials. Furthermore, a general lack of standardization makes results difficult to assess or compare, especially when we have little concept of what magnitude of response correlates with protective immunity.
The results of DC-based clinical trials have been extensively reviewed5,9 ; therefore, our comments are limited to more recent studies. Although it is difficult to compare the results of the DC vaccine trials published to date, in our opinion the most impressive objective clinical responses have been associated with the use of whole proteins, killed tumor cells, or tumor lysates. This may be because these are exogenous antigen sources that target MHC II to generate CD4+ T-cell help and also target MHC I by way of cross-presentation to generate CD8+ CTLs. Using tumor-specific idiotype immunoglobulin-pulsed DCs in patients with follicular lymphoma, Timmerman et al118 reported 2 long-lasting complete responses (CRs) and 1 partial response (PR) among 10 patients with measurable disease in the pilot phase of the study. An additional 25 patients were vaccinated after their best clinical response was achieved by chemotherapy, and objective tumor regression was seen in 4 of 18 patients with residual disease. Holtl et al125 reported a trial of 35 patients with metastatic renal cell carcinoma who received monthly injections of autologous, mature monocyte-derived DCs loaded with tumor lysates. Of 27 evaluable patients, 2 had objective CR (as per WHO), 1 had a PR, and 7 had stable disease. Objective responses and disease stabilization were long lasting, ranging from 6 months to 3 years. Durable CRs were also reported by O'Rourke et al126 in a trial of 17 patients with metastatic melanoma who received mature monocyte-derived DCs loaded with autologous irradiated tumor cells. By WHO criteria there were 3 CRs (with durable remissions of over 3 years) and 3 PRs among 12 patients who completed the vaccinations. One patient with progressive disease was vaccinated every 6 weeks for more than 3 years, indicating that maintenance vaccinations may be useful even for patients with slowly progressive disease. Finally, another promising trial using autologous tumor lysate pulsed DCs showed objective responses in patients with refractory cutaneous T-cell lymphoma after intranodal vaccination.133 Larger studies will be important to confirm the results of the these trials.
New vaccine strategies that exploit DC biology
Tumors can evade immunity by a number of mechanisms, including mutations in genes encoding target antigens, loss of antigen expression, or immunosuppressive maneuvers such as secretion of TGFβ.1 This may be particularly true of large or metastatic tumors. Thus, DC vaccines may be most effective in the adjuvant setting for patients in remission but with a high risk of recurrence. However, DC biology may be exploited in many ways to generate more effective immunotherapies, and multimodality approaches may be used to enhance the effectiveness of these vaccines. Below we discuss some novel applications studied in murine models or in preclinical studies using human cells.
Provision of CD4+ T-cell help for CD8+ T cells
Vaccination studies in mice using MHC II–deficient DCs,134 as well as experiments that demonstrate the importance of CD4+ T cell help to generate CD8+ T-cell memory,67-69 call into question vaccine strategies that target only CD8+ T-cell responses. Peptideoaded DC vaccines should incorporate antigens targeting both CD4+ and CD8+ T cells, and a polyvalent approach should be considered. Peptide-loaded dendritic cells can clearly prime CD4+ T-cell responses,131 but a more practical approach that circumvents the problems of HLA-restricted peptides may be to target cross-presentation. For example, targeting antigens to Fc receptors on DCs using antibody-antigen complexes has been shown to activate both CD4 and CD8 effector responses and tumor immunity in mice.135 Coating myeloma cells with anti–syndecan-1 antibody similarly promotes cross-presentation.136 Pharmaceutical-grade antibodies already in use to treat human cancer (eg, anti-CD20, anti-HER-2/neu) may act in part through this mechanism and could be used in the preparation of DC vaccines. Cross-presentation can also be enhanced by targeting DC surface receptors such as DEC-205,20 loading DCs with killed cells or cell lysates or by stimulating DCs with TLR agonists that induce cross presentation49 (Figure 5). Transfected RNA, which primarily targets MHC I, may also be targeted to MHC II by incubating the transfected DCs with antisense oligonucleotides to the MHC II–associated Ii protein137 or by using fusion constructs carrying an endosomal/lysosomal sorting signal.138
Strategies to recruit, mature, and load DCs in situ
Existing DC vaccine methods require expensive facilities and labor-intensive cell processing. To avoid this, alternative approaches that simultaneously recruit, mature, and pulse DCs with antigens in vivo are being explored (Figure 5). To recruit DCs, locally implanted chemokines such as MIP-3β may be used to condition the injection site prior to vaccination.139 To mature DCs, simple vaccines that take advantage of CpG motif DNA (a TLR9 agonist) coinjected with or conjugated to a protein antigen have been used.140,141 Vaccination with heat shock protein-peptide complexes can similarly mature DCs in situ and may induce immunologic and clinical responses in melanoma patients.142
Another in situ approach uses CpG motif-containing DNA vaccines that encode tumor antigens. These vaccines can be engineered to carry xenogeneic antigens143 or to include DC-specific promoters to specifically target antigen expression to DCs. DNA vaccines may also be designed to drive the expression of survival factors such as Bcl-xL144 or to encode DC maturation signals145 or immunostimulatory cytokines.146
Certain microbes directly induce MDC or PDC maturation, even in nonreplicating form, and are being tested as recombinant vaccine vectors.3 One advantage of some viral vectors is that IFNα generated from virus-stimulated DCs may promote cross-priming of CD8+ T-cell responses.35 Microbial vectors may also be engineered to express adhesion molecules, costimulatory molecules, or cytokines that direct Th polarization, promote T-cell activation and longevity, and promote DC survival (Figure 5).
Ex vivo–derived DCs can also be matured in situ by preconditioning the injection site with TLR agonists,147 and DC migration can be enhanced by preconditioning the injection site with cytokines or with DCs themselves.7,148,149 This approach may be preferable to ex vivo maturation, because DC cytokines such as IL-12 are often expressed only briefly after exposure to many maturation stimuli, and local production of cytokines and chemokines induced by local application of TLR agonists may also promote DC viability and migration to draining lymph nodes.
Strategies to activate NKT cells
Vaccination with melanoma cells in adjuvant can activate CD1d-restricted NKT cells that recognize tumor-associated gangliosides,150 and intravenous delivery of a soluble antigen together with the synthetic CD1d-binding glycolipid α-GalCer can lead to in vivo activation of NKT cells and induction of antitumor T-cell immunity.83 Trials to test the activating potential of α-GalCer–pulsed DCs are under way in cancer patients.
Inhibition of immune tolerance
One way to enhance cancer vaccines is to simultaneously block inhibitory costimulatory molecules or Tr cells. For example, administering an inhibitory antibody to CTLA-4 in previously vaccinated cancer patients can result in effective antitumor immunity.151 Possible synergy of CTLA-4 blockade and concomitant tumor antigen vaccination has been observed in patients with metastatic melanoma.152 In this study, tumor regressions were accompanied by significant toxicity, including severe or life-threatening autoimmunity. Nevertheless, this approach is worth addressing in conjunction with DC vaccines, using different dosages or schedules to alleviate toxicity. In mice, blockade of the inhibitory costimulatory molecule B7-H1 has also been shown to improve DC-mediated antitumor T-cell responses.51
The activity of cancer vaccines may be enhanced through depletion or inhibition of Tr cells through the use of cytotoxic anti-CD25 antibodies or IL-2 coupled to cytotoxic molecules. In mice with poorly immunogenic tumors, depletion of Tr cells alone can slow tumor growth but does not efficiently reject the tumor.153 However, immune responses induced by antigen-pulsed mature DCs are significantly enhanced in CD25-depleted mice.154 The use of both of CTLA-4 blockade and CD25+ cell depletion may further potentiate the effectiveness of vaccines.155
Combination therapies
Multimodality approaches incorporating tumor vaccination have also shown promise in animal models, although it may prove difficult to translate some of these approaches into clinical use. For example, in one study the combination of vaccination with adoptively transferred T cells and administration of IL-2 resulted in tumor regression and long-term cures.156 Using another approach, Cui et al157 showed that transducing hematopoietic progenitor cells with a model tumor antigen and transplanting these cells into irradiated recipient mice resulted in expression of the antigen in donor-derived DCs in the host's lymphoid organs. When combined with systemic agents that generate and activate DCs and adoptive transfer of donor T cells, this treatment resulted in expansion of antigen-specific T cells and successful treatment of the antigen-bearing tumor. Antitumor vaccination in combination with therapies that target the tumor's vascular supply have also shown promise in mouse models,158 as has vaccination during lymphoid recovery following bone marrow transplantation.159
Use of “regulatory DCs” for the induction of transplantation tolerance
DC-based immunotherapy may also prove to be a highly selective way to induce graft tolerance in organ or hematopoietic stem cell transplantation or to induce tolerance in patients with autoimmune disease. Studies in mice and humans have shown that tolerogenic or “regulatory DCs” (rDCs) may be induced ex vivo by culturing immature DCs in modulating cytokines or growth factors such as IL-10 and TGFβ. In a mouse model for the treatment of leukemia, rDCs have been used to treat acute graft-versus-host disease and leukemia relapse in conjunction with allogeneic bone marrow transplantation.88
Conclusion
DC-based immunotherapy is still in its infancy. Two-arm trials are needed to assess the efficacy of DC vaccines compared with other immunotherapies and to optimize the use of DCs for vaccines. Until then, it is not truly meaningful to compare DC immunotherapy with standard cancer therapies in large randomized trials. The greatest clinical benefit of DC immunotherapy for cancer may be found in the adjuvant setting, although it is hoped that patients with advanced cancer will also benefit, at the very least, through disease stabilization. Eventually, it is possible that the most effective DC therapies may not necessarily involve the ex vivo manipulation of DCs. Multimodality approaches that include novel biologic agents may also help achieve effective, durable antitumor immune responses. With greater understanding of DC biology and of mechanisms to enhance DC immunogenicity, the answers will begin to come.
Prepublished online as Blood First Edition Paper, July 1, 2004; DOI 10.1182/blood-2003-12-4392.
Supported by grants from the National Institutes of Health (CA-84512, AI-44628), the Cancer Research Institute, the Burroughs Wellcome Fund, and the Doris Duke Charitable Foundation. N.B. is an Elizabeth Glaser Scientist of the Elizabeth Glaser Pediatric AIDS Foundation. D.W.O. is supported in part by an NYU Cancer Institute Translational Research Program Award. S.A. is supported in part by an American Society of Clinical Oncology Young Investigator Award.
We thank Teresita O'Neill for assistance with artwork; Stephen Schachterle for preparation of dendritic cells; and Giorgio Inghirami, Marie Larsson, Anne-Sophie Beignon, and Mojca Skoberne for helpful advice.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal