Abstract
The tetraspanin family member CD151 forms complexes with integrins and regulates cell adhesion and migration. While CD151 is highly expressed in megakaryocytes and to a lesser extent in platelets, its physiologic role in platelets is unclear. In this study, we investigate the physical and functional importance of CD151 in murine platelets. Immunoprecipitation/Western blot studies reveal a constitutive physical association of CD151 with integrin αIIbβ3 complex under strong detergent conditions. Using CD151-deficient mice, we show that the platelets have impaired “outside-in” integrin αIIbβ3 signaling with defective platelet aggregation responses to protease-activated receptor 4 (PAR-4) agonist peptide, collagen, and adenosine diphosphate (ADP); impaired platelet spreading on fibrinogen; and delayed kinetics of clot retraction in vitro. This functional integrin αIIbβ3 defect could not be attributed to altered expression of integrin αIIbβ3. CD151–/– platelets displayed normal platelet alpha granule secretion, dense granule secretion, and static platelet adhesion. In addition, CD151–/– platelets displayed normal “inside-out” integrin αIIbβ3 signaling properties as demonstrated by normal agonist-induced binding of soluble fluorescein isothiocyanate (FITC)–fibrinogen, JON/A antibody binding, and increases in cytosolic-free calcium and inositol 1,4,5 triphosphate (IP3) levels. This study provides the first direct evidence that CD151 is essential for normal platelet function and that disruption of CD151 induced a moderate outside-in integrin αIIbβ3 signaling defect.
Introduction
Integrin αIIbβ3 maintains an inactive conformation on the surface of resting platelets until it is converted to its high-affinity state by agonist induced “inside-out” signaling via G protein–coupled or tyrosine kinase–linked pathways. Once activated, integrin αIIbβ3 can bind its soluble ligands, fibrinogen, and von Willebrand factor (VWF). Subsequently, “outside-in” integrin αIIbβ3 signaling events induce integrin clustering that leads to cytoskeletal reorganization and postoccupancy events including clot retraction, platelet spreading, and platelet aggregation.1 The importance of integrin αIIbβ3 has been documented in Glanzmann thrombasthenia (GT) patients, who have bleeding complications despite a normal number of platelets. GT patients have dysfunctional platelets, which display quantitative or qualitative defects in integrin αIIb or β3. This gives rise to an integrin αIIbβ3-mediated platelet defect, where the platelets fail to aggregate, bind fibrinogen, or retract fibrin clots.2,3 This phenotype has been recapitulated in a complete β3 knock-out (KO) mouse model, which shows the cardinal features of Glanzmann thrombasthenia.4
The concept that transmembrane receptors including integrins can form multimolecular complexes with tetraspanin-4 superfamily members (TM4SF) was first demonstrated by the ability of integrin αIIbβ3 to associate with a tetraspanin superfamily member, CD9, in stimulated platelets.5 Subsequently, biochemical associations between tetraspanin superfamily members and other transmembrane receptors have been reported and clarified based upon associations in the presence of detergents with differing stringencies.6
The tetraspanin superfamily member CD151 (also known as platelet endothelial tetraspan antigen-3/PETA-3) is expressed on the surface of platelets, megakaryocytes, endothelial cells, activated T lymphocytes, Schwann cells, muscle cells, and epithelial cells.7 CD151 is found to be constitutively associated with a wide range of integrins including β1 from coimmunoprecipitation studies using hematopoietic cell lines.8 It appears to have strong physical associations with integrins α3, α6, and α7 chains, and α6β4 complexes.9 Biochemical studies have revealed that the CD151 associations with α3 and α6 integrins involve an integrin contact site, QRDHASNIYKVE194-205, located in the EC2 loop of CD151. Mutation of this integrin contact site resulted in loss of integrin association and disrupted integrin-dependent cell spreading.10 CD151 monoclonal antibodies (mAbs) have proved to inhibit endothelial cell migration and modify angiogenesis in vitro.11,12 In addition, the CD151 protein was found to be involved in integrin-dependent neurite outgrowth, keratinocyte migration, neutrophil migration, and tumor metastasis.13-16 This implies that CD151 participates in transmembrane signaling pathways normally initiated by integrin ligation.
Like other members of the tetraspanin family, CD151 forms highly specific direct lateral interactions with other tetraspanin family members and transmembrane receptors including α3, α6, α7, and αIIb integrins.12,17 Several studies have suggested that CD151 may not modulate integrin-dependent cell adhesion, but markedly affects integrin-dependent cell spreading and morphogenesis when cells are grown on a Matrigel basement membrane.17,18 These studies indicate that CD151 is capable of modulating the signaling properties of integrins. The role of CD151 in platelet function is unclear. Previous studies have implied a strong dependence between CD151 and Fcγ receptor IIa (FcγRIIa) in platelet activation using Mab 14A2.H1, antihuman CD151 antibody.19 Mab 14A2.H1 was shown to recognize a restricted repertoire of CD151 excluding certain integrin complexes.20 More recent studies in human platelets have reported that CD151 functions independent of platelet FcγRIIa.21 Apart from CD151, platelets express several tetraspanins including CD9, CD63, and TSSC6.22-25 Of these, CD9 has probably been the only one whose platelet function has been examined in detail, however, the results are not conclusive. Several reports indicate that CD9 is functionally linked with the low-affinity immunoglobulin G (IgG) receptor, FcγRIIa, in human platelets,22,24 while others suggest that CD9 may regulate platelet function independent of FcγRIIa.25
In light of new evidence indicating that CD151 is independent of platelet FcγRIIa, we hypothesized that CD151 may play a role in regulating integrin αIIbβ3-mediated platelet function. In order to address this hypothesis, we studied the platelet biology of mice that are genetically deficient in CD151. Since murine platelets are devoid of the low-affinity IgG receptor, FcγRIIa, this provided an ideal system to define the functional role of CD151 in platelets. We aimed to determine if CD151 was both physically and functionally associated with the major platelet integrin αIIbβ3 in murine platelets. We dissected the functional importance of CD151 by examining platelet responses dependent upon integrin activation and postligand-binding events. These studies provide the first evidence that integrin αIIbβ3 and CD151 are physically and functionally associated in murine platelets. More importantly, it demonstrates that CD151 plays an essential role in platelet function by modulating the outside-in signaling properties of the major platelet integrin αIIbβ3.
Materials and methods
Antibodies
The following antibodies (Abs) were used in this study. Antihuman CD151 mAb 11B1.G4 has been previously described for detection of murine CD15126 ; antimouse CD9 mAb, antimouse integrin β3 mAb, fluorescein isothiocyanate (FITC)–conjugated antimouse P-selectin mAb, and FITC-conjugated antimouse CD3 mAb were purchased from BD Pharmingen (San Diego, CA). 1D4.5 (murine IgG2a) was used as an isotype control for 11B1, and all other antibodies are of rat origin. FITC-conjugated antimouse CD44 mAb was obtained from Beckman Coulter (Brea, CA). JON/A–phycoerythrin (PE) mAb was purchased from Cemfret Analytics (Wurzburg, Germany). Normal mouse IgG1 was obtained from Sigma Chemical (St Louis, MO). All human and mouse work was approved by the Austin Campus Human Research Ethics Committee and Animal Ethics Committee.
Mice
The construction of CD151-deficient mice on a C57BL/6 background has been recently described.27 These mice were housed in a pathogen-free facility at the Austin Research Institute Animal House Facility.
Preparation of washed platelets and PRP
Blood (700 μL) was taken into 100-μL 3.8% (wt/vol) trisodium citrate by cardiac puncture under anesthesia. Platelet-rich plasma (PRP) was obtained by centrifugation at 700g for 10 minutes at room temperature (RT). PRP was pooled from 2 to 3 mice for preparing platelet samples. In order to prepare washed platelets, PRP was centrifuged in the presence of prostaglandin E1 (PGE1, 50 ng/mL; Sigma Chemical) at 1500g for 10 minutes at room temperature without brake. Sedimented platelets were resuspended in Ringer citrate dextrose (RCD) buffer, pH 7.4 (108 mM NaCl, 38 mM KCl, 1.7 mM NaHCO3, 21.2 mM sodium citrate, 27.8 mM glucose, and 1.1 mM MgCl2 · 6 H2O, with pH adjusted to 7.4), counted, and diluted to 150 × 109/L.
Platelet aggregation studies
PRP derived from 2 to 3 wild-type C57BL/6 and CD151–/– mice was diluted in 53 μM Na2HPO4–12 μM KH2PO4 buffer to adjust platelet count to 100 × 109/L. Then, 250 μL diluted wild-type or CD151–/– PRP together with 100 μg/mL fibrinogen and 1 mM CaCl2 were placed into glass aggregometer cuvettes in the platelet aggregometer (Chronolog; Edward Keller, Hallam, Australia) with constant stirring (1000 rpm) at 37° C. Diluted platelet-poor plasma (PPP) 1:2 from wild-type and CD151–/– mice was used to set baseline for platelet aggregation runs. Once a baseline was obtained, different concentrations of various agonists (5 U/mL thrombin, 500 μM protease-activated receptor 4 (PAR-4) agonist peptide, 20 μg/mL collagen, 20 μg/mL Ca ionophore A23187, and 10 μM adenosine diphosphate [ADP]; Sigma) were used to initiate platelet aggregation profiles with both wild-type and CD151–/– PRP. For each platelet aggregation run, the slope and amplitude were determined.
Clot retraction assay
Clot retraction was essentially performed as previously described.28
Static platelet adhesion assays
Static platelet adhesion assays were performed using a modified method of Yuan et al.29 Whole mouse blood from wild-type and CD151–/– mice was labeled with 50 μg/mL 3,3′-dihexyloxacarbocyanine iodide (DiOC6) fluorescent dye in dimethyl sulphoxide (Molecular Probes, Eugene, OR) for 10 minutes at RT in the presence of PGE1 (50 ng/mL). Washed labeled platelets (100 × 109/L) were isolated and then incubated with the desired extracellular matrix over time (0-60 minutes) at 37° C to allow platelet adhesion and spreading to occur. Unbound platelets were washed away and adherent platelets were fixed with 3.7% (vol/vol) paraformaldehyde for 10 minutes at RT and viewed at × 100 magnification using a Leitz DMRBE fluorescent microscope (Leica, Hawthorn East, Australia). Images were captured using a Leica DC200 digital camera.
Platelet spreading on fibrinogen
Platelets (100 × 109/L) were added to 100 μg/mL fibrinogen-coated coverslips and incubated over time (0-60 minutes) at 37° C. Nonadherent platelets were removed and adherent platelets fixed with 3.7% (vol/vol) paraformaldehyde (ICN Biochemicals, Aurora, OH) for 30 minutes at RT, then permeabilized with 0.1% (vol/vol) Triton X-100 (Sigma) for 30 minutes at RT. Platelets were stained with tetramethylrhodamine isothiocyanate (TRITC)–phalloidin (2 μg/mL; Sigma) for 30 minutes at RT and were viewed at × 100 magnification using a Leitz DMRBE fluorescent microscope (Leica). Images were captured using a Leica DC200 digital camera.
For scanning electron microscopy (EM) analysis, the adherent platelets were fixed with 2.5% (vol/vol) glutaraldehyde (Sigma) in 0.1 M sodium cacodylate buffer, pH 7.3 (Sigma) containing 2% (wt/vol) sucrose for 15 minutes at RT. The fixed platelets were washed and then processed for dehydration, gold labeling, and capturing of scanning EM images using a Philips 515 scanning electron microscope (5 kV; Philips, Andover, MA).
Integrin β3 expression
For surface expression of integrin β3, 100 μL diluted PRP (platelet count adjusted to 150 × 109/L) from wild-type and CD151–/– mice was suspended in 500 μL RCD buffer, pH 6.5, and then labeled with 50 μg/mL FITC-conjugated antimouse β3 antibody (BD Pharmingen) for 30 minutes at RT in the dark. Platelets were fixed in fluorescence-activated cell sorter (FACS) Fix (16 g/L glucose, 40% [vol/vol] formaldehyde, and 0.1% [wt/vol] azide prepared in phosphate-buffered saline, pH 7.4) for 30 minutes at RT in the dark before samples were washed twice at 2000g for 5 minutes at RT. Platelet pellets were resuspended in 0.5 mL RCD buffer, pH 6.5, containing 0.2% (wt/vol) bovine serum albumin (BSA). Flow cytometry (FACS) analysis was performed using a FACSCalibur flow cytometer (Becton Dickinson, North Ryde, Australia) formatted for a platelet-specific FITC protocol with forward scatter (FSC), side scatter (SSC), and fluorescent parameters in log scale. Analysis of flow profile was performed using CellQuest Version 1.2.2 software (Becton Dickinson, San Jose, CA). The main platelet population was selected and gated before acquisition of data.
FITC-fibrinogen and JON/A-PE mAb binding
FITC-fibrinogen labeling and binding assays were performed essentially as described.30 JON/A-PE mAb (recognizes the active conformation of murine integrin αIIbβ3; 1:50 dilution) binding was analyzed by flow cytometry.
Platelet alpha granule secretion monitored by P-selectin exposure
Washed platelets (50 μL, 150 × 109/L) derived from wild-type and CD151–/– mice were labeled with 10 μg/mL FITC-conjugated antibody to P-selectin (BD Pharmingen) and stimulated with human thrombin (0-10 U/mL) and PAR-4 agonist peptide (0-500 μM). Samples were centrifuged at 2000g for 5 minutes at RT and platelet pellet was resuspended in 0.5 mL RCD buffer, pH 6.5, containing 0.2% (wt/vol) BSA prior to analysis by flow cytometry.
Platelet dense granule secretion monitored by serotonin release
Dense granule secretion was performed essentially as described.31
Calcium mobilization and inositol 1,4,5 triphosphate (IP3) measurement
Calcium mobilization and IP3 levels were performed essentially as described.32
Immunoprecipitation and Western blot studies
Statistics
The statistical significance of differences between means was evaluated using Student t test for paired samples, and P values of less than .05 (*) and .005 (**) were considered significant. Results are expressed as mean ± standard error of the mean (SEM).
Results
Physical association of integrin αIIbβ3 complex with CD151 in human and murine platelets
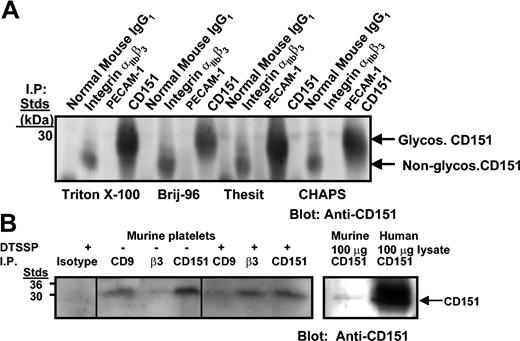
In order to examine a potential physical association of integrin αIIbβ3 complex with CD151 in human and murine platelets, immunoprecipitation studies were performed using antibodies directed against isotype control mAb, integrin αIIbβ3 mAb, platelet endothelial cell adhesion molecule 1 (PECAM-1) mAb, and CD151 (11B1 mAb) in the presence of either 1% Triton X-100 or with 1% Brij-96, 1% Thesit, and 1% CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonate). The only exception was with murine platelets, where chemical cross-linking with DTSSP was performed. Following immunoprecipitation, protein complexes were resolved on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under nonreducing conditions, and the presence of CD151 was detected by Western blotting. As shown in Figure 1A, glycosylated (30 kDa) and predominantly nonglycosylated (28 kDa) forms of CD151 were detectable only in human integrin αIIbβ3 (P2) and antihuman CD151 immunoprecipitates. As shown in Figure 1B, unglycosylated CD151 was detectable in murine integrin αIIbβ3 immunoprecipitates and in murine CD9 and CD151 immunoprecipitates, but not in the isotype control or PECAM-1 immunoprecipitates. This CD151–integrin αIIbβ3 association was stabilized by cross-linking with DTSSP in the presence of 1% (vol/vol) Triton X-100. As a positive marker of CD151, 11B1 immunoprecipitates of resting murine and human platelets were included (Figure 1B, right panel). These results are consistent with a type I direct interaction.
Coimmunoprecipitation of CD151 with integrin αIIbβ3 from human and murine platelet lysates. (A) Platelet lysates (1.5 mg) solubilized with indicated detergents were immunoprecipitated with normal IgG1, integrin complex–specific αIIbβ3 Ab, P2, PECAM-1.2, and 11B1 (anti-CD151). Samples were resolved on 12.5% SDS-PAGE under nonreducing conditions and Western blotted for CD151 with 11B1 Ab. (B) Wild-type mouse platelets (150 × 109/L) were chemically cross-linked with 2 mM DTSSP and then solubilized with 1% Triton X-100 and Triton-soluble fractions immunoprecipitated with isotype control, CD9, integrin αIIbβ3 complex–specific mAb, and 11B1 (CD151) (left). Murine and human platelet lysates (100 μg) blotted with 11B1 Ab are included as positive markers of CD151 recognition (right). Samples were resolved on 12.5% SDS-PAGE under nonreducing conditions and Western blotted for CD151 with biotinylated 11B1 Ab followed by horseradish peroxidase (HRP)–streptavidin. Glycos indicates glycosylated.
Coimmunoprecipitation of CD151 with integrin αIIbβ3 from human and murine platelet lysates. (A) Platelet lysates (1.5 mg) solubilized with indicated detergents were immunoprecipitated with normal IgG1, integrin complex–specific αIIbβ3 Ab, P2, PECAM-1.2, and 11B1 (anti-CD151). Samples were resolved on 12.5% SDS-PAGE under nonreducing conditions and Western blotted for CD151 with 11B1 Ab. (B) Wild-type mouse platelets (150 × 109/L) were chemically cross-linked with 2 mM DTSSP and then solubilized with 1% Triton X-100 and Triton-soluble fractions immunoprecipitated with isotype control, CD9, integrin αIIbβ3 complex–specific mAb, and 11B1 (CD151) (left). Murine and human platelet lysates (100 μg) blotted with 11B1 Ab are included as positive markers of CD151 recognition (right). Samples were resolved on 12.5% SDS-PAGE under nonreducing conditions and Western blotted for CD151 with biotinylated 11B1 Ab followed by horseradish peroxidase (HRP)–streptavidin. Glycos indicates glycosylated.
CD151 knock-out platelets have abnormalities in integrinαIIbβ3-mediated function
The initial characterization of the CD151 knock-out mice revealed that the mice are healthy and viable with normal Mendelian inheritance. These mice show normal development of hematopoiesis, including platelet production and a moderate in vivo bleeding defect associated with a tendency to rebleed.27 Based upon this in vivo bleeding defect, the CD151 knock-out mice have either an underlying endothelial or platelet defect contributing to the unstable hemostasis. In order to investigate the possibility that CD151 knock-out platelets have a functional platelet abnormality, we tested the kinetics of fibrin clot retraction of wild-type versus CD151–/– platelets. Clot retraction is an essential part of platelet thrombus consolidation and is dependent upon an active integrin αIIbβ3 complex. In this assay, wild-type and CD151–/– PRP (normalized platelet counts) were initiated to form clots at 37° Cby the addition of 2.5 U/mL thrombin. These clots were observed every 10 minutes over an hour period and digital images captured every 20 minutes. As shown in Figure 2A, a delay in the kinetics of clot retraction was observed in CD151 knock-out compared with wild-type platelets. Wild-type platelets started retracting at 20 minutes and were completely retracted in 60 minutes, while CD151-deficient platelets showed no sign of clot retraction at 20 minutes and were only partially retracted by the end of an hour. This defect in clot retraction was not attributed to reduced expression of integrin αIIbβ3 on the surface of CD151-deficient platelets as demonstrated by flow cytometry studies (Figure 2B). Therefore, our results suggest that CD151 is required for potentiating integrin αIIbβ3-mediated signaling events in murine platelets.
Delayed clot retraction in CD151-deficient platelets in the presence of normal integrin αIIbβ3 expression. (A) Photographs showing the kinetics of in vitro clot retraction using platelet-rich plasma (PRP) (normalized platelet counts) from wild-type and CD151–/– mice. Samples were treated with 10 nM thrombin. Red blood cells (5 μL) were added to enhance color contrast for photography. Each photograph is representative of 3 independent experiments. (B) Surface expression of integrin β3 on platelets was determined by staining with a buffer control, isotype control FITC-CD3 mAb, positive control FITC-CD44 mAb, FITC-CD9, and FITC-integrin β3 mAb for both wild-type and CD151–/– platelets. FITC-labeled samples were analyzed on a FACS Calibur analyzer. Results are representative of 3 independent experiments. The data represent the mean fluorescence intensity (MFI) ± SEM.
Delayed clot retraction in CD151-deficient platelets in the presence of normal integrin αIIbβ3 expression. (A) Photographs showing the kinetics of in vitro clot retraction using platelet-rich plasma (PRP) (normalized platelet counts) from wild-type and CD151–/– mice. Samples were treated with 10 nM thrombin. Red blood cells (5 μL) were added to enhance color contrast for photography. Each photograph is representative of 3 independent experiments. (B) Surface expression of integrin β3 on platelets was determined by staining with a buffer control, isotype control FITC-CD3 mAb, positive control FITC-CD44 mAb, FITC-CD9, and FITC-integrin β3 mAb for both wild-type and CD151–/– platelets. FITC-labeled samples were analyzed on a FACS Calibur analyzer. Results are representative of 3 independent experiments. The data represent the mean fluorescence intensity (MFI) ± SEM.
CD151–/– platelets display perturbation of platelet aggregation
Activation of platelets involves a sequence of events including cytoskeletal reorganization that induces platelet granule secretion, engagement and clustering of integrins to form platelet aggregates, and, eventually, formation of a stable hemostatic plug. As integrin αIIbβ3 is required for the process of platelet aggregation, we wanted to examine the effect of CD151 deficiency on platelet aggregation responses using a range of platelet agonists. For this purpose, we chose the classical G protein–coupled agonists, including PAR-4 agonist peptide (AYPGKF, 100-500 μM) and ADP (2.5-10 μM). In addition, we included type I acid soluble collagen (20-100 μg/mL) and calcium ionophore A23187 (2.5-20 μM) as agonists. PRP (platelet count adjusted to 100 × 109/L) was preincubated with 1 mM CaCl2 and 100 μg/mL human fibrinogen for 5 minutes prior to stimulation with various agonists. Representative platelet aggregation profiles of each agonist for wild-type and CD151 knock-out platelets are shown in Figure 3. CD151 deficiency led to an approximate 50% to 70% reduction of platelet aggregation in response to 500 μM PAR-4 agonist peptide, 20 μg/mL collagen, and 10 μM ADP. Ca ionophore A23187 (20 μg/mL) was less significantly affected in the absence of CD151, which is likely due to a different signaling mechanism to the other 3 agonists. Therefore, based upon these results, the CD151–/– platelets display reduced amplitude and slope of the platelet aggregation responses, indicating either an inside-out or outside-in integrin αIIbβ3 signaling defect or both.
CD151–/– platelets show reduced platelet aggregation responses. Aggregation responses of PRP (platelet count adjusted to 100 × 109/L) for wild-type and CD151–/– mice. (A) PAR-4 agonist peptide, 500 μM; (B) calcium ionophore A23187, 20 μg/mL; (C) collagen, 20 μg/mL; and (D) ADP, 10 μM. Data are representative of 4 experiments.
CD151–/– platelets show reduced platelet aggregation responses. Aggregation responses of PRP (platelet count adjusted to 100 × 109/L) for wild-type and CD151–/– mice. (A) PAR-4 agonist peptide, 500 μM; (B) calcium ionophore A23187, 20 μg/mL; (C) collagen, 20 μg/mL; and (D) ADP, 10 μM. Data are representative of 4 experiments.
CD151–/– platelets display normal static platelet adhesion
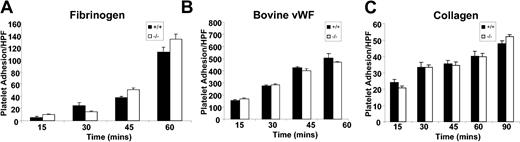
In order to assess whether deletion of CD151 modulates integrin αIIbβ3-mediated adhesive events, we examined the static platelet adhesion responses of DiOC6 fluorescently labeled wild-type and CD151–/– platelets to immobilized extracellular matrix (ECM) proteins including fibrinogen (100 μg/mL), bovine VWF (20 μg/mL) in the presence of botrocetin (1 μg/mL), and type I fibrillar collagen (20 μg/mL) over time (0-60 minutes). Following washing, fluorescently labeled platelets bound to various matrices were examined and quantitated for evidence of adhesion by fluorescence microscopy. As shown in Figure 4, wild-type and CD151–/– platelets showed similar kinetics of adhesion to fibrinogen under static conditions. This pattern of static adhesion for CD151–/– platelets was observed for immobilized bovine VWF and type I fibrillar collagen. Taken together, these static platelet adhesion profiles suggest that the absence of CD151 does not affect the platelet adhesive properties involving inactive integrin αIIbβ3 binding to the immobilized ligands, fibrinogen, or VWF, and collagen receptors (integrin α2β1 and glycoprotein VI [GPVI]) to the immobilized ligand, collagen.
Static platelet adhesion to extracellular matrices. Anticoagulated whole blood from CD151+/+ and CD151–/– mice was labeled with DiOC6 (50 μg/mL) and washed platelets were isolated. Labeled platelets/mL (1 × 109 platelets/μL) were then allowed to adhere to collagen-coated (2.5 mg/mL), fibrinogen-coated (100 μg/mL), or bovine VWF–coated (20 μg/mL) coverslips in the presence of botrocetin (1 μg/mL) for 15 to 60 minutes at 37° C. Adherent platelets were fixed at different time points and visualized using fluorescence microscopy. Quantitation of adherent CD151+/+ and CD151–/– platelets was determined by analysis of images acquired using × 100 objective. These data represent the mean ± SEM from 3 independent experiments. HPF indicates high power field.
Static platelet adhesion to extracellular matrices. Anticoagulated whole blood from CD151+/+ and CD151–/– mice was labeled with DiOC6 (50 μg/mL) and washed platelets were isolated. Labeled platelets/mL (1 × 109 platelets/μL) were then allowed to adhere to collagen-coated (2.5 mg/mL), fibrinogen-coated (100 μg/mL), or bovine VWF–coated (20 μg/mL) coverslips in the presence of botrocetin (1 μg/mL) for 15 to 60 minutes at 37° C. Adherent platelets were fixed at different time points and visualized using fluorescence microscopy. Quantitation of adherent CD151+/+ and CD151–/– platelets was determined by analysis of images acquired using × 100 objective. These data represent the mean ± SEM from 3 independent experiments. HPF indicates high power field.
Restricted cytoskeletal reorganization of CD151–/– platelets
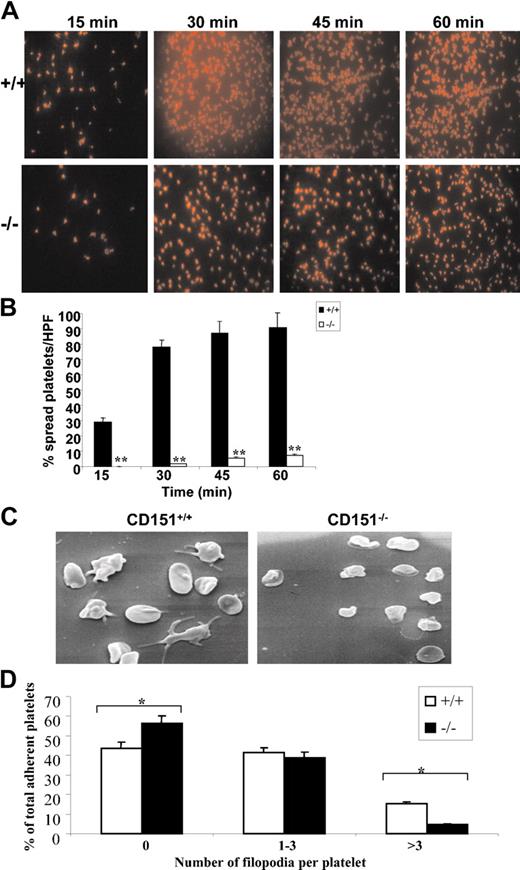
Once adherent to fibrinogen, platelets become activated and undergo substantial cytoskeletal remodeling, leading to platelet shape change and spreading. Outside-in integrin αIIbβ3 signaling has been demonstrated to play a major role in this process. We investigated the role of CD151 in modulating the platelet spreading using 2 different experimental systems to monitor the spreading of wild-type and CD151–/– platelets on fibrinogen. Firstly, the spreading of platelets on fibrinogen over time was investigated by staining of the actin cytoskeleton using rhodamine-labeled phalloidin. Secondly, the formation of platelet filopodia and spreading on fibrinogen over time was monitored using scanning EM.
As shown in Figure 5A-B, wild-type mouse platelets were seen to form a wide network of filopodia on fibrinogen over time with evidence of profound platelet spreading by 30 minutes. The majority of CD151–/– platelets underwent a far less pronounced change in morphology and maintained a more rounded morphology forming a smaller number of filopodia, and this was accompanied by at least a 10-fold reduction in spreading over time (91% versus 7% at time = 60 minutes, P < .005). Examination of platelet spreading on fibrinogen over time using scanning EM revealed a similar kinetic profile as observed in the rhodamine-labeled phalloidin staining. As shown in Figure 5C-D, CD151–/– platelets adhere as efficiently to fibrinogen as wild-type platelets, however, CD151–/– platelets exhibit a significant reduction in their capacity to extend filopodia. Specifically, CD151–/– platelets were characterized by a lack of filopodia, with fewer having in excess of 3 filopodia (P < .005; n = 4). These results indicate that CD151–/– platelets have restricted cytoskeletal reorganization when adhered to immobilized fibrinogen.
CD151–/– platelets have a defect in integrin-dependent cytoskeletal reorganization. (A) Washed platelets were placed on coverslips coated with fibrinogen in the presence of PGE1 for different time points at 37° C. Platelets were fixed, permeabilized, and stained for F-actin using rhodamine-conjugated phalloidin. Representative pictures of rhodamine-conjugated phalloidin-stained platelets spread on a fibrinogen surface for wild-type and CD151-deficient mice. The slides (panel A) were viewed under fluorescence microscopy using a Leitz DMRBE microscope (Leica, Hawthorn East, Victoria, Australia). Images were captured using a Leica DC200 digital camera. Magnification × 100. (B) Quantitation of platelet spreading per high-powered field was analyzed on confocal images from 8 random fields and expressed as a percentage of the total adherent platelets from CD151+/+ and CD151–/– platelets. **P < .005. (C) Washed CD151+/+ and CD151–/– platelets were allowed to adhere for 60 minutes at 37° C to a fibrinogen matrix. Adherent platelets were fixed and examined by scanning electron microscopy. Representative CD151+/+ and CD151–/– platelet scanning EM images are shown. (D) The number of filopodia per platelet were analyzed on scanning EM images from 8 random fields and expressed as a percentage of the total adherent platelets from CD151+/+ and CD151–/– platelets. *P < .005.
CD151–/– platelets have a defect in integrin-dependent cytoskeletal reorganization. (A) Washed platelets were placed on coverslips coated with fibrinogen in the presence of PGE1 for different time points at 37° C. Platelets were fixed, permeabilized, and stained for F-actin using rhodamine-conjugated phalloidin. Representative pictures of rhodamine-conjugated phalloidin-stained platelets spread on a fibrinogen surface for wild-type and CD151-deficient mice. The slides (panel A) were viewed under fluorescence microscopy using a Leitz DMRBE microscope (Leica, Hawthorn East, Victoria, Australia). Images were captured using a Leica DC200 digital camera. Magnification × 100. (B) Quantitation of platelet spreading per high-powered field was analyzed on confocal images from 8 random fields and expressed as a percentage of the total adherent platelets from CD151+/+ and CD151–/– platelets. **P < .005. (C) Washed CD151+/+ and CD151–/– platelets were allowed to adhere for 60 minutes at 37° C to a fibrinogen matrix. Adherent platelets were fixed and examined by scanning electron microscopy. Representative CD151+/+ and CD151–/– platelet scanning EM images are shown. (D) The number of filopodia per platelet were analyzed on scanning EM images from 8 random fields and expressed as a percentage of the total adherent platelets from CD151+/+ and CD151–/– platelets. *P < .005.
CD151–/– platelets show normal inside-out integrin αIIbβ3 signaling properties
As CD151–/– platelets have defects in cytoskeletal reorganization, clot retraction, and platelet aggregation, we wanted to test the possibility that platelet secretion may also be altered. In order to test this possibility, we examined the exposure of P-selectin and serotonin release as indicators of platelet alpha and dense granule secretion. As shown in Figure 6A-C, the surface expression of P-selectin on stimulated platelets and dense granule secretion derived from wild-type and CD151–/– platelets were equivalent over a range of thrombin (0-10 U/mL) and PAR-4 (0-500 μM) concentrations. Therefore, based upon these results, it would appear that CD151–/– platelets have normal alpha and dense granule secretion.
CD151–/– platelets have normal alpha and dense granule secretion. (A-B) Surface expression of P-selectin was determined for washed platelets stimulated by thrombin (A) or PAR-4 agonist peptide (B) at different concentrations and then stained with either a buffer control and FITC–P-selectin mAb for both wild-type and CD151+/+ platelets. FITC-labeled samples were analyzed on a FACS Calibur analyzer. Results are representative of 3 independent experiments. (C) Control CD151+/+ (▪) and CD151–/– (□) murine platelets were loaded with [3H]5-hydroxytryptamine (5-HT) and stimulated with thrombin (1 or 5 U/mL) or PAR-4 agonist peptide (250 or 500 μM) for 5 minutes. Platelets were pelleted by centrifugation at 13 900g (13 000) rpm for 5 minutes, and 5-HT secretion into the medium was determined by scintillation spectrometry. Results are expressed as a percentage of the total tissue content after substraction of release under basal conditions and are representative of 3 experiments. Fluor indicates fluorescence.
CD151–/– platelets have normal alpha and dense granule secretion. (A-B) Surface expression of P-selectin was determined for washed platelets stimulated by thrombin (A) or PAR-4 agonist peptide (B) at different concentrations and then stained with either a buffer control and FITC–P-selectin mAb for both wild-type and CD151+/+ platelets. FITC-labeled samples were analyzed on a FACS Calibur analyzer. Results are representative of 3 independent experiments. (C) Control CD151+/+ (▪) and CD151–/– (□) murine platelets were loaded with [3H]5-hydroxytryptamine (5-HT) and stimulated with thrombin (1 or 5 U/mL) or PAR-4 agonist peptide (250 or 500 μM) for 5 minutes. Platelets were pelleted by centrifugation at 13 900g (13 000) rpm for 5 minutes, and 5-HT secretion into the medium was determined by scintillation spectrometry. Results are expressed as a percentage of the total tissue content after substraction of release under basal conditions and are representative of 3 experiments. Fluor indicates fluorescence.
In the assessment of CD151–/– platelet aggregation responses, it was noticed that the primary phase of platelet aggregation (an event dependent on inside-out integrin αIIbβ3 signaling) was essentially intact, while the secondary phase of platelet aggregation (an event dependent on outside-in integrin αIIbβ3 signaling) was severely affected. Agonists that act through either G protein–coupled or nonreceptor tyrosine kinases are the main platelet activators used to assess the inside-out integrin αIIbβ3 signaling properties. For this purpose, we chose thrombin as a potent agonist of G protein–coupled receptors in murine platelets and phorbol 12-myristate 13-acetate ester (PMA), ADP, and ADP + epinephrine that have been previously shown to induce the binding of soluble FITC-fibrinogen by integrin αIIbβ3 on platelets. In order to test integrin αIIbβ3 activation on CD151–/– platelets, we examined the ability of wild-type and CD151–/– platelets to bind to soluble FITC-fibrinogen under resting conditions and following thrombin stimulation, PMA, ADP, and ADP + epinephrine. The binding of soluble FITC-fibrinogen was assessed by flow cytometry. As shown in Figure 7A-F, agonist-induced stimulation of wild-type and CD151–/– platelets demonstrated equivalent ability to bind soluble FITC-fibrinogen, to bind JON/A mAb, and to increase cytosolic-free calcium and IP3 levels of wild-type versus CD151–/– platelets. These results indicate that CD151–/– platelets display normal inside-out integrin αIIbβ3 signaling properties.
CD151–/– platelets show normal inside-out integrin αIIbβ3-mediated signaling. (A) Flow cytometric analysis of FITC-conjugated fibrinogen binding to platelets stimulated with thrombin (1 U/mL), PMA (20 μM), ADP (10 μM), and ADP (10 μM) + Epinephrine (20 μM) or unstimulated (control). Triplicate samples were analyzed. Graph shows compilation of mean fluorescence intensity ± SEM from wild-type and CD151–/– mice. Data are representative of 3 independent runs tested. (B) Flow cytometric analysis of JON/A-PE mAb binding to platelets stimulated with 0 to 1.0 U/mL thrombin and 100 to 500 μM PAR-4 agonist peptide or unstimulated (control). Triplicate samples were analyzed. Graph shows compilation of mean fluorescence intensity ± SEM from wild-type and CD151–/– mice. Data are representative of 3 independent runs tested. (C-E) Fura-2–loaded washed wild-type or CD151–/– platelets were stimulated with the indicated agonists (thrombin, 1 U/mL; PAR-4 agonist peptide [AYPGFK], 250 μM; and collagen-related peptide [CRP], 20 μg/mL), and cytoplasmic-free calcium was determined by measuring fluorescence emission spectra following excitation by 340- and 380-nm wavelengths. Arrow indicates addition of agonist. Data are representative of 3 independent experiments and are presented as a 340:380-nm ratio. (F) Intracellular IP3 levels are presented for wild-type (□) and CD151–/– (▪) platelets at 5 and 10 seconds following stimulation with thrombin. Data are presented as the mean ± SEM from 3 independent experiments.
CD151–/– platelets show normal inside-out integrin αIIbβ3-mediated signaling. (A) Flow cytometric analysis of FITC-conjugated fibrinogen binding to platelets stimulated with thrombin (1 U/mL), PMA (20 μM), ADP (10 μM), and ADP (10 μM) + Epinephrine (20 μM) or unstimulated (control). Triplicate samples were analyzed. Graph shows compilation of mean fluorescence intensity ± SEM from wild-type and CD151–/– mice. Data are representative of 3 independent runs tested. (B) Flow cytometric analysis of JON/A-PE mAb binding to platelets stimulated with 0 to 1.0 U/mL thrombin and 100 to 500 μM PAR-4 agonist peptide or unstimulated (control). Triplicate samples were analyzed. Graph shows compilation of mean fluorescence intensity ± SEM from wild-type and CD151–/– mice. Data are representative of 3 independent runs tested. (C-E) Fura-2–loaded washed wild-type or CD151–/– platelets were stimulated with the indicated agonists (thrombin, 1 U/mL; PAR-4 agonist peptide [AYPGFK], 250 μM; and collagen-related peptide [CRP], 20 μg/mL), and cytoplasmic-free calcium was determined by measuring fluorescence emission spectra following excitation by 340- and 380-nm wavelengths. Arrow indicates addition of agonist. Data are representative of 3 independent experiments and are presented as a 340:380-nm ratio. (F) Intracellular IP3 levels are presented for wild-type (□) and CD151–/– (▪) platelets at 5 and 10 seconds following stimulation with thrombin. Data are presented as the mean ± SEM from 3 independent experiments.
Discussion
This study provides the first report to demonstrate that the tetraspanin superfamily member CD151 is functionally as well as physically associated with the major platelet integrin αIIbβ3. We demonstrate a physical association of integrin αIIbβ3 with CD151 in resting murine and human platelets. As no naturally occurring examples of CD151 deficiency are available in humans, the availability of the CD151 knock-out mouse model has provided us with a unique opportunity to study the functional importance of CD151 in platelet biology. Our studies demonstrate that CD151-deficient platelets have a defect in postoccupancy events of integrin αIIbβ3 as indicated by delayed kinetics in clot retraction, reduced platelet aggregation responses, and reduced platelet spreading on fibrinogen. Finally, CD151-deficient platelets showed normal levels of integrin αIIbβ3 expression, normal alpha and dense granule secretion, and normal ability to bind to soluble FITC-fibrinogen, to bind JON/A mAb, and to cytosolic-free calcium and IP3 levels upon agonist stimulation. Taken together, these results support the identification of an outside-in integrin αIIbβ3 signaling defect in CD151-deficient platelets.
In this study, we show that the physical proximity of CD151 and integrin αIIbβ3 in platelets is likely to be of fundamental importance to mediate a close functional relationship between receptor signaling pathways. Tetraspanin web interactions have been subclassified into 3 different levels of biochemical interaction based upon the stability of the physical interaction with detergents of varying stringencies.33 Based upon these criteria, our results support the concept that the physical association of integrin αIIbβ3 with CD151 in platelets is a first-level direct interaction. This interaction is resistant to disruption by strong hydrophobic detergents including Triton X-100.
Previous studies have focused on the role of CD151 in other cell types particularly involving transfected cell systems. A major focus of the CD151 studies has involved examining the integrin α3β1, where it retains a stable association with approximately 90% of cellular α3β1 integrins. CD151 would appear to be important in the modulation of extracellular matrix–driven cell migration, neurite outgrowth, and cell spreading but not α3 or α6 integrin–mediated cell adhesion. CD151 has been reported to exert an influence on α6β1 integrin–dependent morphogenesis when fibroblasts and endothelial cells are grown on Matrigel. Tetraspanins are thought to modulate integrin-dependent signaling leading to alteration of cytoskeletal reorganization. Based upon these earlier studies, it has been proposed that tetraspanins are likely to influence the outside-in signaling properties of integrins. However, many of the molecular details remain elusive and have awaited studies in murine models where tetraspanin genes are deleted either singly or in combination.
Based upon this study, CD151 appears to be important in modulating the outside-in integrin αIIbβ3 signaling properties in platelets; it would suggest that CD151 participates in transmembrane signaling pathways. The N- and C-termini of CD151 are believed to be intracellular. These short amino acid sequences do not contain any enzymatic activity to confer autophosphorylation properties of their cytoplasmic regions. Therefore, it is likely that either phosphorylation or nonphosphorylation events may mediate the recruitment of signaling molecules or cytoskeletal proteins. At this stage, there is very little known about the signaling properties of CD151 and tetraspanin family members in general. Recent studies have suggested that CD151 can be coimmunoprecipitated with protein kinase C (PKC) and phosphatidylinositol 4-kinase.34,35 These studies provide evidence that integrins α3β1 and α6β1 that are strongly associated with tetraspanins are also found in association with protein kinase C. In these PKC-TM4SF integrin complexes, integrin α3 and α6 tails are phosphorylated in a PKC-dependent manner.34 Whether these are direct or indirect interactions with either the N- or C-terminus of CD151 is currently not known, although it has been proposed that PKC may be involved in a direct interaction with TM4SF members. At present, there is no evidence that PKC-CD151 integrin complexes exist in platelets. Recent studies have highlighted that the C-terminal of CD151 (SLKLEHY) is important for modulating integrin α6β1 adhesion strengthening by affecting integrin clustering.36 This finding is supported by the observation that the short C-terminal cytoplasmic domain of CD151 is required in α6β1 integrin–mediated spreading, migration, and cellular cable formation on Matrigel.18
One of interesting aspects of the CD151 KO platelet phenotype is that it does not correlate with features of the complete β3 knock-out mice, which display cardinal features of naturally occurring Glanzmann thrombasthenia platelets. Platelets derived from complete β3 knock-out mice have profound defects with absent platelet aggregation, inability to bind fibrinogen, absent fibrin-mediated clot retraction, and prolonged bleeding times.4 Despite having normal platelet production, these mice have a profound platelet defect that results in spontaneous bleeding complications predominantly involving cutaneous and gastrointestinal systems. The phenotype observed in integrin β3 knock-out mice is more severe than the CD151 KO phenotype reported in this study. The CD151 mice are viable and healthy without spontaneous bleeding complications.27 Indeed, our findings more closely resemble a mouse model that expresses integrin αIIbβ3 in which the tyrosines in the integrin cytoplasmic domain have been mutated to phenylalanine (Y747759F).37 Like the CD151 KO mice, these mice were found to have selectively impaired outside-in integrin αIIbβ3 signaling with normal integrin expression, but with defective platelet aggregation, delayed clot retraction responses in vitro, and an in vivo bleeding defect, which is characterized by a tendency to rebleed. One of the contrasting features in the diYF integrin mutant platelets is evidence of disaggregation in the secondary phase of platelet aggregation using G protein–coupled agonists, ADP, and thrombin.
No naturally occurring examples of this diYF integrin β3 mutant have been described. However, examination of the variant group of Glanzmann thrombasthenia patients has revealed several mutations in the β3 cytoplasmic domain that give rise to bidirectional integrin αIIbβ3 signaling defects. These include Ser752 → Pro β3 and Arg724 → Ter β3 mutations. Platelets from a thrombasthenic patient having a Ser752 → Pro mutation within the cytoplasmic domain of β3 fail to bind soluble fibrinogen after stimulation with either ADP or thrombin, indicating abnormal inside-out β3 signaling. Subsequent studies on the effects of this integrin β3 cytoplasmic domain mutation on cytoskeletal interactions and signal transduction have primarily been performed using transfected cells. The introduction of the Ser752 → Pro β3 mutant into Chinese hamster ovary (CHO) cells together with a constitutively active chimeric integrin α-subunit abolished ligand binding and reduced αIIbβ3-mediated cell spreading on immobilized fibrinogen, clot retraction, and focal adhesion.38 These studies highlight a bidirectional integrin αIIbβ3 signaling defect, indicating that integrin activation and postoccupancy events of integrin αIIbβ3 are affected in this Ser752 → Pro β3 mutant.
By examination of CD151-deficient platelets, we observed evidence of platelet aggregation responses with reduced amplitude and slope with a range of agonists. This feature is often associated with a defect in inside-out as well as outside-in integrin αIIbβ3 signaling. On occasion, we found evidence of disaggregation in the secondary phase of only PAR-4–induced platelet aggregation, but not ADP- or collagen-induced platelet aggregation (data not shown). Based upon repetition of numerous platelet aggregation assays, we have concluded that platelet disaggregation is not a general feature of CD151-deficient platelets. In fact, a recent report of an outside-in integrin αIIbβ3 signaling defect in calpain-deficient murine platelets has reported reduced platelet aggregation responses to thrombin, ADP, and collagen, but with no evidence of platelet disaggregation.28 We explored the possibility that CD151–/– platelets may have an underlying bidirectional integrin αIIbβ3 signaling defect. Based upon normal agonist-induced binding of soluble FITC-fibrinogen, JON/A binding, cytosolic-free calcium, and IP3 levels, it would appear that CD151–/– platelets do not have an inside-out integrin αIIbβ3 signaling defect. Taken together, these results support the notion that CD151 plays an important role in platelet function by acting as a modulator of outside-in integrin αIIbβ3 signaling in platelets.
In conclusion, in this study we have demonstrated a physical and functional association of the major platelet integrin αIIbβ3 with the tetraspanin family member CD151 in platelets. Furthermore, we show that absence of murine CD151 leads to an outside-in integrin αIIbβ3 signaling defect in platelets. Future studies will examine the signaling properties of CD151, cross-talk between CD151 and integrin signaling pathways, and the role of CD151 in regulating platelet thrombus formation both in vitro and in vivo.
Prepublished online as Blood First Edition Paper, June 29, 2004; DOI 10.1182/blood-2003-12-4430.
Supported by grants from the National Heart Foundation of Australia and the National Health and Medical Research Council (NHMRC) of Australia (D.E.J.). D.E.J. is a recipient of an NHMRC Senior Research Fellowship, and L.K.A. is a recipient of an NHMRC Principal Research Fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Dr YuPing Yuan for supplying the bovine VWF used in this study and for technical advice in platelet adhesion assays. We thank Dr Anna Friedhuber for performing the scanning electron microscopy analysis.



![Figure 6. CD151–/– platelets have normal alpha and dense granule secretion. (A-B) Surface expression of P-selectin was determined for washed platelets stimulated by thrombin (A) or PAR-4 agonist peptide (B) at different concentrations and then stained with either a buffer control and FITC–P-selectin mAb for both wild-type and CD151+/+ platelets. FITC-labeled samples were analyzed on a FACS Calibur analyzer. Results are representative of 3 independent experiments. (C) Control CD151+/+ (▪) and CD151–/– (□) murine platelets were loaded with [3H]5-hydroxytryptamine (5-HT) and stimulated with thrombin (1 or 5 U/mL) or PAR-4 agonist peptide (250 or 500 μM) for 5 minutes. Platelets were pelleted by centrifugation at 13 900g (13 000) rpm for 5 minutes, and 5-HT secretion into the medium was determined by scintillation spectrometry. Results are expressed as a percentage of the total tissue content after substraction of release under basal conditions and are representative of 3 experiments. Fluor indicates fluorescence.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/8/10.1182_blood-2003-12-4430/6/m_zh80200467920006.jpeg?Expires=1767750044&Signature=GEKYxZO3YMVAUIP94fIjlqXBDbP6q2tVdQLr9iNyuxCp8q5TcRhhusnO00pl~arynd6aMpwRLLjGJq8Y2czxIaI1bHFCsR~d-eiEcjBTdH4OOFC82fEupFDW-jUzE-v2JoSyMegRiT-xDDI7EGGxAzgOFGS5l9QMNpGXyrg~jVZIA3FIlYpeST0Wi1ziuvtbcACHFgadBqimMm6h18p23fSmq8LvDzjOud1IoJsUepD3c2d953pZSpAp~OSd6xbFiFbSRlr4-OMtv6cj5in-mrcvJEI-6FNlYuJObiAFJeedDCjgdY6bQqUzThcBZch5c2FykLFuRpvVoU2jDr~gjQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. CD151–/– platelets show normal inside-out integrin αIIbβ3-mediated signaling. (A) Flow cytometric analysis of FITC-conjugated fibrinogen binding to platelets stimulated with thrombin (1 U/mL), PMA (20 μM), ADP (10 μM), and ADP (10 μM) + Epinephrine (20 μM) or unstimulated (control). Triplicate samples were analyzed. Graph shows compilation of mean fluorescence intensity ± SEM from wild-type and CD151–/– mice. Data are representative of 3 independent runs tested. (B) Flow cytometric analysis of JON/A-PE mAb binding to platelets stimulated with 0 to 1.0 U/mL thrombin and 100 to 500 μM PAR-4 agonist peptide or unstimulated (control). Triplicate samples were analyzed. Graph shows compilation of mean fluorescence intensity ± SEM from wild-type and CD151–/– mice. Data are representative of 3 independent runs tested. (C-E) Fura-2–loaded washed wild-type or CD151–/– platelets were stimulated with the indicated agonists (thrombin, 1 U/mL; PAR-4 agonist peptide [AYPGFK], 250 μM; and collagen-related peptide [CRP], 20 μg/mL), and cytoplasmic-free calcium was determined by measuring fluorescence emission spectra following excitation by 340- and 380-nm wavelengths. Arrow indicates addition of agonist. Data are representative of 3 independent experiments and are presented as a 340:380-nm ratio. (F) Intracellular IP3 levels are presented for wild-type (□) and CD151–/– (▪) platelets at 5 and 10 seconds following stimulation with thrombin. Data are presented as the mean ± SEM from 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/8/10.1182_blood-2003-12-4430/6/m_zh80200467920007.jpeg?Expires=1767750044&Signature=h4n60rSlI8x0HQr~liDVxwK713uc~kgJrXXUl1lzt1Bh2RlpsHDIZAYvHP7RmhbYHAxkAJLc6~g38ivRXIukNwZbo1J2iiOCBztypbXAamUL20miompIBlufYKPPVvLX2U2FES9HGqy7C5q6nMftIkkL3IdSr~rrEpoIeDU9HrsczZ1kSZ3N9Zx1R8x84y7IkuX4O8rrFB30-GNMG~qXcKxSoSRxIEM9Zy571e6yCJWXoijV1p8QP7tJWnDVIsgfVJrqVCMTnXbMMh2LXVCZsXmmuWs19b6vnx7-MfDCeqIe0Saxwvi6L4Y0xji30hc5mMtmxZgJePO3kX0OMJHBUg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal