Abstract
Multiple myeloma (MM) expands in the bone marrow and causes devastating bone destruction by enhancing osteoclastic bone resorption in its vicinity, suggesting a close interaction between MM cells and osteoclasts (OCs). Here, we show that peripheral blood mononuclear cell-derived OCs enhanced growth and survival of primary MM cells as well as MM cell lines more potently than stromal cells, and that OCs protected MM cells from apoptosis induced by serum depletion or doxorubicin. OCs produced osteopontin (OPN) and interleukin 6 (IL-6), and adhesion of MM cells to OCs increased IL-6 production from OCs. In addition, IL-6 and OPN in combination enhanced MM cell growth and survival. However, the effects of OCs on MM cell growth and survival were only partially suppressed by a simultaneous addition of anti–IL-6 and anti-OPN antibodies and were completely abrogated by inhibition of cellular contact between MM cells and OCs. These results demonstrate that OCs enhance MM cell growth and survival through a cell-cell contact-mediated mechanism that is partially dependent on IL-6 and OPN. It is suggested that interactions of MM cells with OCs augment MM growth and survival and, thereby, form a vicious cycle, leading to extensive bone destruction and MM cell expansion.
Introduction
Multiple myeloma (MM) almost exclusively develops in the bone marrow and generates devastating bone destruction by osteoclasts (OCs) recruited around MM cells. A marked stimulation of osteoclastic bone resorption causes debilitating clinical symptoms, including intractable bone pain, disabling multiple fractures, and hypercalcemia. The severity of bone disease correlates with the tumor burden and is one of the major parameters in widely used Durie and Salmon clinical staging system. It is of note that the aggressive features of MM bone lesions have significantly contributed to its poor prognosis despite the recent development of intensive chemotherapeutic regimens.1,2 Therefore, elucidation of the molecular mechanism of bone destruction and tumor progression is essential for the development of effective therapies to improve survival as well as quality of life of patients with MM.
Interactions between receptor activator of nuclear factor-kappaB (RANK) expressed on the surface of the OC lineage cells and RANK ligand expressed on stromal cells play a key role in the development and activation of OCs, whereas osteoprotegerin, a decoy receptor for RANK ligand secreted from stromal cells, inhibits RANK ligand–RANK signaling.3-8 MM cells stimulate osteoclastogenesis by triggering a coordinated increase in RANK ligand and decrease in osteoprotegerin in bone marrow (BM) stromal cells.9-11 We and others have demonstrated that osteoclastogenic CC chemokines macrophage inflammatory protein 1α (MIP-1α) and MIP-1β are secreted from most of MM cells and play a critical role in the development of MM bone lesions.12-18 These chemokines act on MM cells in an autocrine/paracrine fashion and enhance MM cell adhesion to stromal cells through activation of integrins, including very late antigen-4 (VLA-4). The interaction between MM and stromal cells then induces RANK ligand expression by stromal cells, leading to OC differentiation and activation.12
Almost exclusive development of MM in the BM suggests that the BM microenvironment supports MM cell growth and survival. Among cell components in the BM, roles of stromal cells in MM cell growth and survival have been extensively studied. When cocultured with MM cells, stromal cells are stimulated to produce interleukin 6 (IL-6), which promotes proliferation of MM cells and prevents them from apoptosis induced by anticancer agents.19-21 Other than stromal cells, OCs induced by MM cells are among major cellular components of the BM microenvironment. Administration of inhibitors of osteoclast activity, including bisphosphonates, RANK-Fc, and osteoprotegerin, not only prevented MM-induced bone destruction but also interfered with tumor progression in animal models of MM.9,22-25 Repeated administration of bisphosphonates has also been reported to reduce the tumor burden without chemotherapy in a portion of patients with MM.26 These observations raise a possibility that an interaction between OCs and MM cells may play an important role in MM expansion in the BM. However, interactions between OCs and MM cells have been poorly understood because of difficulties in isolation and formation of OCs in vitro. We used a culture system to form OCs from human peripheral blood mononuclear cells (PBMCs) in vitro with soluble RANK ligand and macrophage colony-stimulating factor (M-CSF)27,28 and were able to examine interactions of MM cells with functionally mature human OCs in the absence of stromal cells. The results demonstrate that OCs potently enhance MM cell growth and survival in a cell-cell contact-dependent manner in part by way of elaboration of osteopontin (OPN) and IL-6 by OCs.
Materials and methods
Chemicals
The following reagents were purchased from the indicated manufacturers: recombinant human (rh) IL-6, M-CSF, mouse immunoglobulin G1 (IgG1), goat IgG, and mouse anti–human neutralizing IL-6 monoclonal antibody (MoAb), and mouse anti–human blocking MoAbs against CD11a, VLA-4, intercellular adhesion molecule-1 (ICAM-1), ICAM-3, and vascular cell adhesion molecule-1 (VCAM-1), goat anti–human neutralizing polyclonal antibodies against MIP-1α, insulin growth factor 1 (IGF-1), IL-3, hematopoietic growth factor (HGF), vascular endothelial growth factor (VEGF), and stem cell factor, and rh IgG1 Fc and rh CD56 Fc from R&D System (Minneapolis, MN); fluorescein isothiocyanate (FITC)–conjugated mouse anti–human αvβ3 integrin, VLA-4, VLA-5, and CD44 MoAbs, and mouse anti–human blocking MoAbs against αvβ3 integrin (23C6), CD11b, and CD18 from Pharmingen (San Diego, CA); mouse anti–human VLA-5 blocking MoAb from Chemicon (Temecula, CA), mouse anti–human CD3, CD4, CD5, CD8, CD11b, CD14, CD16, CD19, and CD33 MoAbs from Nichirei (Tokyo, Japan); mouse anti–human IgG and λ light chain MoAbs and biotin-conjugated goat anti–human IgG and λ from Zimed (San Francisco, CA); rhRANK ligand from Pepro Tech EC (London, England); purified human fibronectin from Chemicon; purified human and rhOPN from Sangi (Otaru, Japan) and Biogenesis (Poole, England), respectively. Neutralizing mouse anti–human OPN MoAb was a kind gift from Dr Masaki Noda (Tokyo Medical and Dental University, Tokyo, Japan). Minodronate (YM529) was provided by Yamanouchi Pharmaceutical (Tokyo, Japan).
Cells and cultures
Cell lines. Human MM cell lines, U266 and RPMI8226, were obtained from the American Type Culture Collection (Rockville, MD). A human IL-6–dependent MM cell line, OPC, was established in our laboratory which secretes monoclonal IgGλ.12 A mouse preosteoclast cell line, C7,3 was a generous gift of Dr Shin-ichi Hayashi (Tottori University, Tottori, Japan).
Isolation of mononuclear cells. PBMCs and BM mononuclear cells were isolated by Ficoll-Hypaque density gradient centrifugation (Pharmacia LKB Biotechnology, Uppsala, Sweden) from heparinized blood drawn from healthy volunteers and patients with MM after an informed consent had been obtained and used immediately. All procedures involving human specimens were performed according to the protocol approved by the institutional review board for human protection. Adherent cells were prepared according to the adherence technique as previously described.12
Generation of human OC-like multinucleated cells. OCs were generated in vitro according to the previously described procedures with a slight modification.27,28 Adherent cells were prepared from PBMCs and cultured in 24-well plates in Eagle minimal essential medium alpha modification (alpha-MEM; Life Technologies, Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS; Whittaker Bioproducts, Walkersville, MA), 50 ng/mL soluble RANK ligand, and 500 U/mL M-CSF. Media were replenished twice a week. In some experiments adherent cells were cultured in 75-cm2 tissue culture flasks for 2 weeks, then harvested with use of 0.05% trypsin/0.53 mM EDTA (ethylenediaminetetraacetic acid; GIBCO BRL, Gaithersburg, MD), replated onto 24-well plates at 1 × 104 cells/well or the indicated cell concentrations, and further cultured with soluble RANK ligand and M-CSF.
Purification of rabbit OCs. Collagen gel was prepared 1 day before preparation of the unfractionated bone cells according to the previously described procedures.29 Unfractionated bone cells were dissociated by vortexing from minced long bones of 5-day-old white rabbits. Cells were seeded onto the collagen gel culture dishes at 50 million cells/dish. After 20 hours of culture, cells were washed and treated with phosphate buffered saline (PBS) containing 0.001% pronase E (Sigma) and 0.02% EDTA for 10 minutes at 37° C. After washing with PBS, cells were treated with 0.01% bacterial collagenase (Sigma) in PBS for 10 minutes at room temperature. Subsequently, the culture dish was digested for 10 minutes at 37° C with 5 mL 0.1% collagenase. The released cells were collected and washed. OCs thus obtained were seeded in 96-well plates at 5 × 104 cells/well and cultured for 96 hours in alpha-MEM containing 3% FBS.
Pit formation assay. Bone slices from calf femur were placed onto each well of 96-well culture plates containing alpha-MEM with 10% FBS. Adherent cells isolated from PBMCs or purified rabbit OCs were inoculated on the bone slices and cultured. After incubation for the desired times, cells were removed with a rubber policeman. Pits formed on bone slices were stained with acid hematoxylin (Sigma), and the excavation areas were determined under a light microscope with a mesh glass installed in the ocular lens by counting the number of mesh squares covering the pits to evaluate osteoclastic bone resorption.
BM stromal cell cultures. Adherent fractions of BM mononuclear cells were cultured in alpha-MEM supplemented with 12.5% FBS, 12.5% horse serum (Whittaker Bioproducts), 50 U/mL penicillin, and 50 μg/mL streptomycin (GIBCO BRL, Rockville, MD). Marrow stromal cells were serially passed at confluency, using 0.05% trypsin/0.53 mM EDTA (GIBCO BRL), to obtain a homogeneous population of spindle-shaped cells. Cells were further passed at 2 × 105 cells/mL on 24-well culture plates. After cells were expanded, culture medium was changed to alpha-MEM containing 10% FBS.
Purification of primary MM cells. MM cell-rich fractions were prepared from BM mononuclear cells by negative selection with use of Dynabeads M-450 goat anti–mouse IgG (Dynal, Great Neck, NY) and a cocktail of MoAbs, including anti-CD3, CD4, CD8, CD19, CD11b, CD14, CD16, and CD33 MoAb, as previously described.12,30 Highly purified MM cells were subsequently prepared by a positive selection with use of magnetic beads coated with a human myeloma cell-specific anti-HM1.24 MoAb raised in our laboratory.30,31 Purity of thus obtained myeloma cells was more than 95%.
Cocultures and collection of conditioned media. For coculture experiments, we seeded MM cells onto OCs or stromal cells precultured on 24-well plates. Cells were cultured in alpha-MEM with 10% FBS, or 1% FBS in some experiments, and supplemented with cytokines and antibodies as indicated in each experiment. The cells were visualized with a phase-contrast microscopy (Olympus IX70; Tokyo, Japan), using LCPlanFl 20 × objective lens. Images were recorded with an Olympus CCD camera (SC35) and Viewfinder Lite software (Pixera, Los Gatos, CA), and digitally processed using Photoshop software (Adobe, San Jose, CA). Viable cells were counted by trypan blue staining after pipetting MM cells to detach from the anchorage cells. Conditioned media were harvested after 2 days in some experiments. Nonadhesive cocultures were performed by using a 0.45-μm pore-sized transmembrane filter (Intercell TP, Kurabo, Osaka, Japan).
Cytokine and immunoglobulin measurements
Human and mouse IL-6 levels were measured by using TiterZyme IL-6 Enzyme Immunoassay (PerSeptive Diagnostic, Cambridge, MA) and Quantikine mIL-6 Enzyme Immunoassay kits (R&D Systems), respectively, according to the manufacturers' instructions. Human OPN levels were measured by using Human Osteopontin Assay kit-IBL (Immuno-Biological Laboratories, Gunma, Japan).
Human immunoglobulin levels in culture supernatants were measured by using an enzyme-linked immunosorbent assay (ELISA) with some modifications of our previously described method.32 In brief, round-bottomed 96-well ELISA plates were coated with either 5 μg/mL mouse anti–human IgG or λ light chain MoAb and blocked with 1% bovine serum albumin (Sigma). After washing, diluted culture supernatants or human immunoglobulin standards were added to wells in the plates and incubated for 1 hour. After washing, biotin-conjugated goat anti–human IgG or λ light chain polyclonal antibodies were added. An avidin-biotin peroxidase system (Vectastain ABC kit; VECTOR, Burlingame, CA) was used to detect the second-stage polyclonal antibodies.
Flow cytometry
Cell preparation and staining for flow cytometry were performed as described previously.30 Approximately 106 cells were incubated with saturating concentrations of FITC-conjugated MoAbs on ice for 1 hour. Cellular viability and apoptosis were determined by flow cytometric analysis of annexin V binding and propidium iodide (PI) uptake (MEB-CYTO Apoptosis Kit; Medical and Biological Laboratories, Nagoya, Japan) according to the manufacturer's instructions. Samples were analyzed by EPICS-Profile (Coulter Electronics, Hialeah, FL).
OPN coating and cell adhesion assays
rhOPN was applied to 24-well culture plates at 10 μg/mL in Ca/Mg-free PBS and incubated at 4° C overnight. Nonspecific binding sites were subsequently blocked with 3% human serum albumin (Green-Cross, Osaka, Japan) in Ca/Mg-free PBS for 2 hours at 37° C. MM cells were labeled with 10 μg/mL fluorescent dye (BCECF-am; Dojindo, Kumamoto, Japan) in alpha-MEM for 2 hours at 37° C. For adhesion assays 1 × 106 MM cells were resuspended in alpha-MEM, plated onto prewashed rhOPN-coated plates, and incubated at 4° C for 30 minutes. They were then rapidly warmed up to 37° C and further incubated for 30 minutes. After washing gently 4 times at room temperature, fluorescence intensity of lysed adherent cells was measured as previously described.33
Statistical analysis
Statistical significance was determined by one-way analysis of variance (ANOVA) with Scheffé post hoc tests. The minimal level of significance was P = .05.
Results
PBMC-OCs supported primary MM cell survival
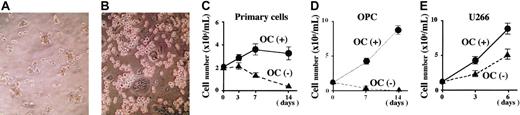
Because a large number of pure OCs were generated and readily available by the above-mentioned method, they were used to investigate potential roles of OCs in MM growth and survival. To avoid undesirable allogeneic reactions, we first performed coculture experiments by using PBMC-OCs from a patient with MM, along with MM cells derived from the same patient. Purified MM cells are known to be highly dependent on the BM microenvironment and to die soon when they are isolated and cultured even with an excess amount of a major MM growth factor IL-6. As expected, purified MM cells mostly died when cultured alone at day 14 (Figure 1A). In sharp contrast, many MM cells were still alive at day 14 when cocultured with PBMC-OCs (Figure 1B). Similar results were obtained when purified MM cells were cocultured with PBMC-OCs derived from healthy individuals (Figure 1C). The supportive effect of PBMC-OCs on MM cell survival was confirmed and reproduced with purified MM cells from 8 patients at different clinical stages (data not shown).
Effects of PBMC-OCs on growth and survival of primary MM cells and MM cell lines. Scanning microscopic images of primary MM cells cultured alone (A) or cocultured with OCs generated from PBMCs from the same patient with MM (B) for 14 days in alpha-MEM containing 10% FBS. Primary MM cells (2 × 105/mL) isolated from a patient with MM (C), an IL-6–dependent MM cell line, OPC (D), or a factor-independent MM cell line, U266 (E), were cultured in quadruplicate in the presence or absence of OCs (1 × 104/mL) derived from healthy individuals. At the indicated time points, viable cell number was determined by trypan blue staining under microscopy. Data were expressed as means ± SD.
Effects of PBMC-OCs on growth and survival of primary MM cells and MM cell lines. Scanning microscopic images of primary MM cells cultured alone (A) or cocultured with OCs generated from PBMCs from the same patient with MM (B) for 14 days in alpha-MEM containing 10% FBS. Primary MM cells (2 × 105/mL) isolated from a patient with MM (C), an IL-6–dependent MM cell line, OPC (D), or a factor-independent MM cell line, U266 (E), were cultured in quadruplicate in the presence or absence of OCs (1 × 104/mL) derived from healthy individuals. At the indicated time points, viable cell number was determined by trypan blue staining under microscopy. Data were expressed as means ± SD.
The effect of PBMC-OCs on survival and/or growth was also observed in MM cell lines, OPC and U266. IL-6–dependent OPC cells continued to proliferate without exogenous IL-6 when cocultured with PBMC-OCs, whereas they mostly died within 7 days in the absence of IL-6 (Figure 1D). Although IL-6–independent U266 cells grew alone, cocultures with PBMC-OCs further stimulated their proliferation (Figure 1E). These results demonstrate that OCs promote not only survival but also growth of MM cells.
The experiments in Figure 1 were performed with a high MM cell-to-OC ratio (20:1). However, the effect of OCs on the growth and survival of MM cells was also observed in the presence of a much lower number of OCs, and the presence of 200, 1000, and 5000 cells/mL OCs could increase the number of 2 × 105 cells/mL OPC cells at day 7 by 39%, 71%, and 207% from the baseline, respectively.
MM cells supported OC survival
When osteoclasts were cocultured with primary MM cells, apparently all OCs (1 × 104 cells/mL) remained alive even after 14 days without addition of sRANK ligand and M-CSF. In contrast, OCs decreased in number (< 103 cells/mL) and detached cells increased when OCs were cultured alone or cocultured in the presence of a filter separating them from MM cells. Thus, the effect of cell-cell contact appears to be bidirectional.
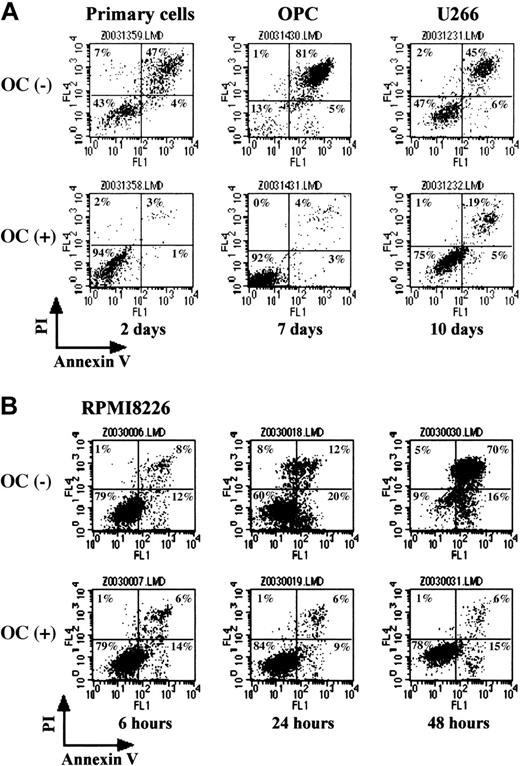
PBMC-OCs rescued MM cell death in a serum-depleted culture
To further examine supportive effects of PBMC-OCs on MM cell survival, MM cells were cultured in the presence or absence of PBMC-OCs in a medium containing 1% FBS, and the percentage of intact and apoptotic cells was determined by flow cytometry with double staining for annexin V and PI. In the absence of PBMC-OCs, the number of both annexin V– and PI-negative intact cells decreased to 43% after culturing purified MM cells for 2 days (Figure 2A). In contrast, 94% of MM cells were intact when cocultured with PBMC-OCs for the same period. Similarly, only 13% of OPC cells remained intact after 7 days without PBMC-OCs, whereas 92% of OPC cells were intact in cocultures with PBMC-OCs (Figure 2A). Although U266 cells were relatively resistant to serum withdrawal, they underwent apoptosis at day 10 (47% for intact cells), whereas PBMC-OCs rescued the cells from apoptosis (75% for intact cells) (Figure 2A). RPMI8226 cells are prone to die by serum depletion, and intact cell fractions decreased rapidly in the absence of PBMC-OCs. When RPMI8226 cells were cocultured with PBMC-OCs, there was a marked reduction in the number of annexin V and PI double-positive apoptotic cells (Figure 2B). Thus, OCs can protect MM cells from apoptosis induced by serum depletion.
PBMC-OCs protected MM cells from apoptosis. MM cells were cultured in alpha-MEM containing 1% FBS in the presence or absence of PBMC-OCs (1 × 104/mL). Primary MM cells, OPC cells, and U266 cells were harvested after gently pipetting at the indicated time points (A). RPMI8226 cells were harvested sequentially (B). Annexin V–FITC and PI binding was measured by flow cytometry. Intact cells are located in the lower left quadrant of dot plots.
PBMC-OCs protected MM cells from apoptosis. MM cells were cultured in alpha-MEM containing 1% FBS in the presence or absence of PBMC-OCs (1 × 104/mL). Primary MM cells, OPC cells, and U266 cells were harvested after gently pipetting at the indicated time points (A). RPMI8226 cells were harvested sequentially (B). Annexin V–FITC and PI binding was measured by flow cytometry. Intact cells are located in the lower left quadrant of dot plots.
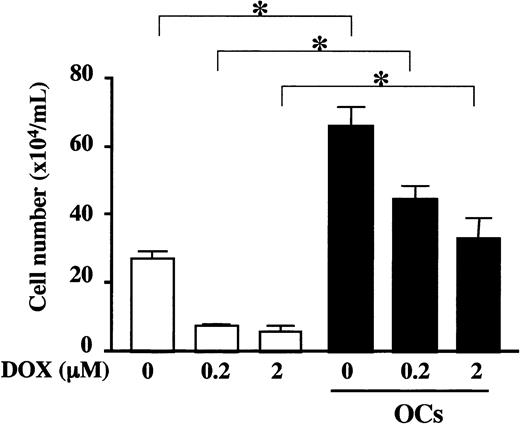
PBMC-OCs counteracted cytotoxic effects of doxorubicin
Because MM cells become refractory to chemotherapy as systemic bone destruction advances, we next examined whether OCs have any protective role against cytotoxic effects of an anticancer agent, doxorubicin (DOX). A DOX-sensitive MM cell line, RPMI8226, was exposed to DOX, washed, and plated onto wells with or without PBMC-OCs. As shown in Figure 3, DOX treatment dose-dependently reduced the number of viable RPMI8226 cells in the absence of PBMC-OCs. In contrast, cocultures with PBMC-OCs markedly increased the number of viable cells even after the exposure to DOX, and pretreatment with 2 μM DOX reduced the cell number by only 50% to a level comparable to that without DOX in the absence of OCs. These results demonstrate that OCs can counteract cytotoxic effects of DOX.
PBMC-OCs enhanced growth of RPMI8226 cells after exposure to DOX. RPMI8226 cells were exposed to 0, 0.2, and 2.0 μM DOX for 2 hours and washed, followed by plating out at 2 × 105/mL in alpha-MEM containing 10% FBS in quadruplicate into 24-well culture plates in the presence (▪) or absence (□) of PBMC-OCs (1 × 104/mL). Viable cell number was counted by trypan blue staining at day 4. Data are expressed as means ± SD. *Significantly different by one-way ANOVA with Scheffe post hoc tests, P < .05.
PBMC-OCs enhanced growth of RPMI8226 cells after exposure to DOX. RPMI8226 cells were exposed to 0, 0.2, and 2.0 μM DOX for 2 hours and washed, followed by plating out at 2 × 105/mL in alpha-MEM containing 10% FBS in quadruplicate into 24-well culture plates in the presence (▪) or absence (□) of PBMC-OCs (1 × 104/mL). Viable cell number was counted by trypan blue staining at day 4. Data are expressed as means ± SD. *Significantly different by one-way ANOVA with Scheffe post hoc tests, P < .05.
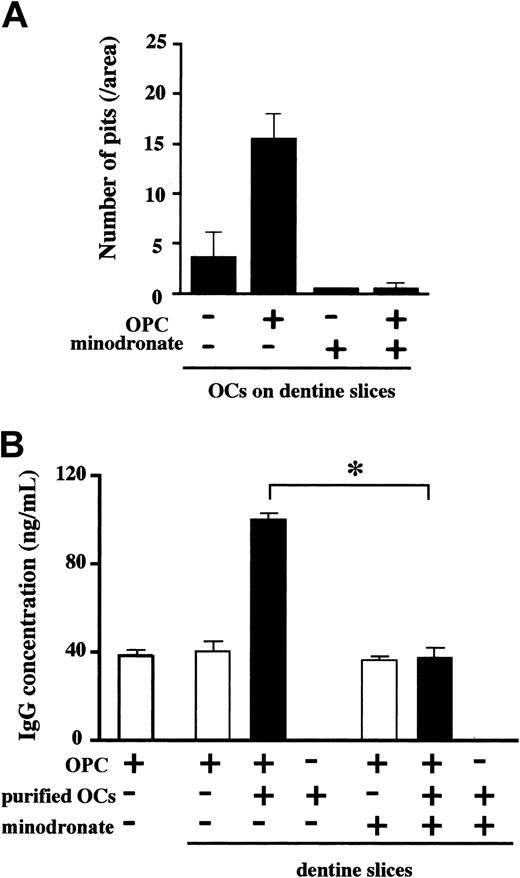
A bisphosphonate inhibited growth and survival of MM cells enhanced by OCs
To further clarify the role of OCs in MM cell growth and survival, we examined the effect of minodronate, a bisphosphonate that potently inhibits osteoclastic bone resorption,34 on MM cell growth and survival in cocultures with OCs isolated from rabbit bone on dentine slices. In the absence of minodronate, the number of resorption pits on dentine slices increased when the OCs were cocultured with a human IgG-producing MM cell line, OPC, for 4 days (Figure 4A). Human IgG concentration in conditioned media, a marker for OPC cell number and function, increased substantially when OPC cells were cocultured with purified rabbit OCs (Figure 4B). Addition of 0.1 μM minodronate into the cocultures almost completely inhibited pit formation (Figure 4A). Minodronate treatment at 0.1 μM also abolished the stimulatory effect of OCs on IgG production from OPC cells almost completely (Figure 4B). Because the same concentration of minodronate in itself showed no significant effect on OPC cell viability nor did it affect OPC cell survival in the presence of stromal cells (data not shown), suppressive effect of 0.1 μM minodronate on OPC cell growth and viability appeared to be mediated by its inhibitory effect on OCs.
A bisphosphonate inhibited OC-mediated MM cell growth on dentine slices. OCs were purified from rabbit bone cells and plated out on dentine slices in 96-well culture plates. Subsequently, human myeloma OPC cells (2 × 104/mL) and minodronate at 0.1 μM were added to the indicated wells in quadruplicate. After 4 days, the number of resorption pits on dentine slices was counted (A). Isolated OCs were cocultured with OPC cells on dentine slices in quadruplicate, minodronate was added at 0.1 μM into the indicated wells, culture supernatants were harvested at day 4, and IgG concentrations were measured by ELISA (B). Data are expressed as means ± SD. *Significantly different by one-way ANOVA with Scheffe post hoc tests, P < .05.
A bisphosphonate inhibited OC-mediated MM cell growth on dentine slices. OCs were purified from rabbit bone cells and plated out on dentine slices in 96-well culture plates. Subsequently, human myeloma OPC cells (2 × 104/mL) and minodronate at 0.1 μM were added to the indicated wells in quadruplicate. After 4 days, the number of resorption pits on dentine slices was counted (A). Isolated OCs were cocultured with OPC cells on dentine slices in quadruplicate, minodronate was added at 0.1 μM into the indicated wells, culture supernatants were harvested at day 4, and IgG concentrations were measured by ELISA (B). Data are expressed as means ± SD. *Significantly different by one-way ANOVA with Scheffe post hoc tests, P < .05.
OCs supported MM cells more potently than stromal cells
We next compared the capacity of OCs to enhance MM cell growth and survival with that of BM stromal cells obtained from patients. OPC and U266 cells were cocultured with PBMC-OCs or BM stromal cells. Although the number of OPC and U266 cells as well as the amount of M-proteins increased when these cells were cocultured with PBMC-OCs or BM stromal cells, the effect of OCs was much greater than that of 10 times higher number of stromal cells (Table 1). Addition of 1 ng/mL IL-6 and 1 μg/mL osteopontin to the cocultures with stromal cells did not further enhance MM cell growth (data not shown).
MM cell number and immunoglobulin concentrations in culture supernatants
Culture supernatants . | Mean cell number ± SD, 104 cells/mL . | Mean immunoglobulin concentrations ± SD, ng/mL . |
|---|---|---|
| OPC | 3 ± 1 | 140 ± 20 |
| OPC + PBMC-OCs | 30 ± 5* | 610 ± 70* |
| OPC + stromal cells | 18 ± 4 | 240 ± 30 |
| U266 | 14 ± 2 | 104 ± 10 |
| U266 + PBMC-OCs | 38 ± 3* | 260 ± 40* |
| U266 + stromal cells | 20 ± 2 | 147 ± 14 |
Culture supernatants . | Mean cell number ± SD, 104 cells/mL . | Mean immunoglobulin concentrations ± SD, ng/mL . |
|---|---|---|
| OPC | 3 ± 1 | 140 ± 20 |
| OPC + PBMC-OCs | 30 ± 5* | 610 ± 70* |
| OPC + stromal cells | 18 ± 4 | 240 ± 30 |
| U266 | 14 ± 2 | 104 ± 10 |
| U266 + PBMC-OCs | 38 ± 3* | 260 ± 40* |
| U266 + stromal cells | 20 ± 2 | 147 ± 14 |
OPC cells (2 × 105/mL) and U266 cells (1 × 105mL) were cultured alone, or cocultured with PBMC-OCs (1 × 104/mL) or primary bone marrow stromal cells (1 × 105/mL) in alpha-MEM containing 1% FBS in 24-well culture plates. Cells were cultured in quadruplicate. Viable MM cell number was counted at day 4 by trypan blue staining. At the same time, culture supernatants were harvested, and IgG or Igλ concentrations were measured by ELISA. Because OPC cells secrete IgG and U266 cells secrete Igλ, respective immunoglobulin concentrations were measured. The data were expressed as means ± SD.
Significantly different from the group with OPC or U266 cells alone, P < .05, and from the group with OPC or U266 cells in the presence of stromal cells, P < .05.
OCs enhanced MM cell growth and survival mainly by way of cell-cell contact
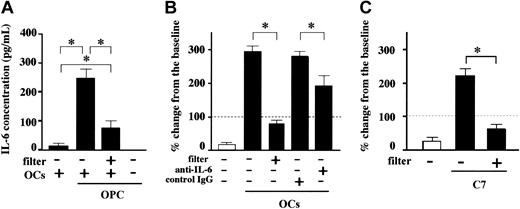
To clarify the mechanism of enhancement of MM cell growth and survival by OCs, we first examined IL-6 levels in culture supernatants. IL-6 is secreted by OCs and is known to be a major growth and survival factor of MM cells. As shown in Figure 5A, PBMC-OCs alone secreted only a minimal amount of IL-6, and MM cells did not secrete IL-6. However, when OCs were cocultured with MM cells, IL-6 levels increased substantially. Prevention of cell-cell contact between OCs and MM cells by membrane filters markedly reduced but did not abrogate the enhancement, suggesting a soluble factor(s) to cause this enhancement. However, neutralizing antibodies against MIP-1α, IL-3, VEGF, and HGF did not show any effect, and the mechanism for the enhanced IL-6 production without cell-cell contact remains unclear.
Cell-cell contact between MM cells and OCs enhanced MM cell growth. OPC cells (2 × 105/mL) were cultured in the presence or absence of PBMC-OCs (1 × 104/mL) in 24-well culture plates in quadruplicate for 7 days. Cell-cell contact was prevented by membrane filters in the indicated wells. (A) Culture supernatants were harvested at day 2, and IL-6 concentrations were measured by ELISA. (B) Either anti–IL-6 neutralizing antibody or control mouse IgG was added at 20 μg/mL to the indicated wells. (C) OPC cells (2 × 105/mL) were cocultured with a mouse preosteoclastic cell line, C7 (1 × 104/mL) for 7 days. Viable cell number was counted by trypan blue staining. Horizontal dashed lines indicate baseline. The data are expressed as means ± SD of the percentage of change from the baseline. *Significantly different by one-way ANOVA with Scheffe post hoc tests, P < .05.
Cell-cell contact between MM cells and OCs enhanced MM cell growth. OPC cells (2 × 105/mL) were cultured in the presence or absence of PBMC-OCs (1 × 104/mL) in 24-well culture plates in quadruplicate for 7 days. Cell-cell contact was prevented by membrane filters in the indicated wells. (A) Culture supernatants were harvested at day 2, and IL-6 concentrations were measured by ELISA. (B) Either anti–IL-6 neutralizing antibody or control mouse IgG was added at 20 μg/mL to the indicated wells. (C) OPC cells (2 × 105/mL) were cocultured with a mouse preosteoclastic cell line, C7 (1 × 104/mL) for 7 days. Viable cell number was counted by trypan blue staining. Horizontal dashed lines indicate baseline. The data are expressed as means ± SD of the percentage of change from the baseline. *Significantly different by one-way ANOVA with Scheffe post hoc tests, P < .05.
To clarify the origin of enhanced IL-6 production, human OPC cells were cocultured with a mouse preosteoclastic cell line, C7, and human and murine IL-6 levels were differentially measured by ELISA. After 2 days of culture, levels of murine IL-6 in culture medium were very high (480 ± 45 pg/mL), whereas no human IL-6 was detected. Thus, OCs but not MM cells secrete IL-6 in a cell-cell contact-dependent manner.
OPC is an IL-6–dependent cell line, and their cell number decreased to less than 20% of the baseline after 7 days of culture without OCs (Figure 5B). In contrast, when OPC cells were cultured for 7 days in the presence of OCs, the number of OPC cells increased to about 300% of the baseline (Figure 5B). To clarify whether the increase in OPC cell number in the presence of OCs was due to the increase in IL-6 production from OCs, the effect of an anti–IL-6 neutralizing antibody was examined. However, the increase in OPC cell number in the presence of OCs was only partially suppressed by an anti–IL-6 antibody, whereas it was almost completely abrogated by blockade of cell-cell contact by membrane filters (Figure 5B).
The importance of cell-cell contact with OCs for MM cell growth and survival was further corroborated by coculturing OPC cells with mouse preosteoclastic C7 cells. As shown in Figure 5C, cell-cell contact with C7 cells potently increased the number of OPC cells, whereas prevention of cell-cell contact by membrane filters again abrogated the effect of OCs on the number of OPC cells. Because murine IL-6 cannot act on human cells,35 and because OPC cells do not secrete detectable levels of human IL-6, these results clearly demonstrate a major role of cell-cell contact in the enhancement of MM cell growth and survival by OCs.
OPN and IL-6 partially contributed to MM cell growth and survival
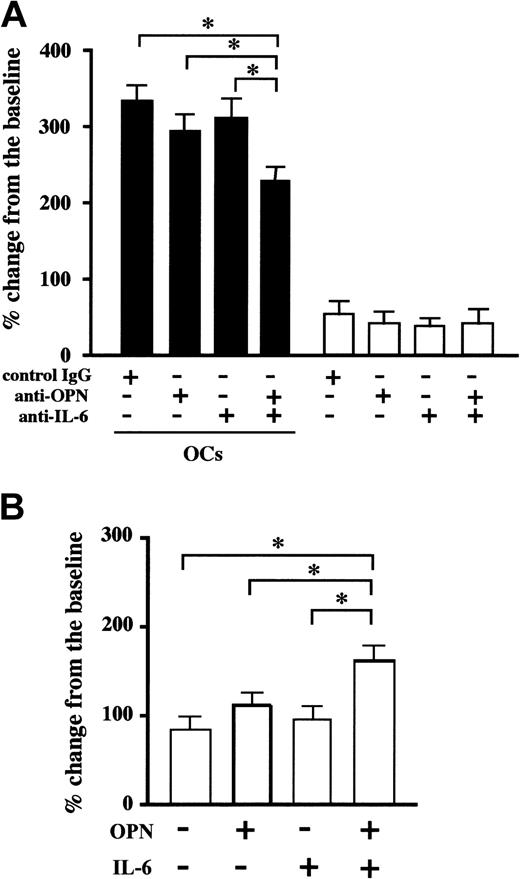
OPN is a noncollagenous matrix glycoprotein secreted from OCs as well as from osteoblasts. OPN interacts with various types of cells by way of binding to adhesion molecules, including αvβ3 integrin and CD44. Similar to cancer metastasis to bone in which OPN promotes tumor progression and decreases survival rate, plasma levels of OPN have been demonstrated to be elevated in patients with MM.36 PBMC-OCs constitutively secreted a large amount of OPN, and the levels of OPN produced by PBMC-OCs were about 140-fold higher than those from BM stromal cells (6880 ± 250 versus 48 ± 4 ng/105 cells for 3 days). On the basis of these observations, we asked whether OPN plays any role in the cell-cell contact-mediated enhancement of MM cell growth and survival. To reduce the effect of OPN contained in serum, cells were cultured in medium supplemented with 1% FBS. Under these conditions, U266 cells and PBMC-OCs stayed alive for more than 4 days. Although addition of neutralizing antibodies against OPN or IL-6 alone did not significantly suppress the number of U266 cells, anti-OPN and anti–IL-6 antibodies added in combination significantly suppressed the increase in the number of MM cells by OCs at day 4 (Figure 6A). Consistent with the above-mentioned observations, the number of U266 cells increased significantly when U266 cells were cultured on OPN-coated plates in the presence of exogenous IL-6, although OPN or IL-6 alone had no significant effect (Figure 6B). We also found that IL-6 pretreatment enhanced U266 cell adhesion to OPN-coated plates (32% ± 3% versus 8% ± 1% of the input) but did not alter surface expression levels of OPN receptors, including VLA-4, αvβ3 integrin, and CD44 (data not shown). These results suggest that IL-6 acts on MM cells to activate OPN receptors without affecting their expression levels and to enable them to respond to OPN more efficiently. In addition to OPN, we examined MM cell-matrix interactions by adding 1 and 5 μg/mL fibronectin to U266 and OPC cell cultures or by coating dishes with fibronectin. However, only a slight and insignificant increase in MM cell number was observed under our experimental conditions (data not shown).
OPN and IL-6 cooperatively acted to enhance MM cell growth. U266 cells (2 × 105/mL) were cultured in alpha-MEM containing 1% FBS in the presence (▪) or absence (□) of PBMC-OCs (1 × 104/mL) in 24-well culture plates in quadruplicate. Neutralizing antibodies against OPN and IL-6 alone or in combination, or control IgG were added at 20 μg/mL to the indicated wells (A). U266 cells were resuspended at 2 × 105/mL in alpha-MEM containing 1% FBS and cultured in wells coated with either rhOPN or human serum albumin in 24-well culture plates. rhIL-6 (10 ng/mL) was added to the indicated wells (B). Viable cell number was counted by trypan blue staining at day 4. The data are expressed as means ± SD. *Significantly different by one-way ANOVA with Scheffe post hoc tests, P < .05.
OPN and IL-6 cooperatively acted to enhance MM cell growth. U266 cells (2 × 105/mL) were cultured in alpha-MEM containing 1% FBS in the presence (▪) or absence (□) of PBMC-OCs (1 × 104/mL) in 24-well culture plates in quadruplicate. Neutralizing antibodies against OPN and IL-6 alone or in combination, or control IgG were added at 20 μg/mL to the indicated wells (A). U266 cells were resuspended at 2 × 105/mL in alpha-MEM containing 1% FBS and cultured in wells coated with either rhOPN or human serum albumin in 24-well culture plates. rhIL-6 (10 ng/mL) was added to the indicated wells (B). Viable cell number was counted by trypan blue staining at day 4. The data are expressed as means ± SD. *Significantly different by one-way ANOVA with Scheffe post hoc tests, P < .05.
Blockade of VLA-4 or αvβ3 integrin suppressed MM cell growth enhancement by OCs
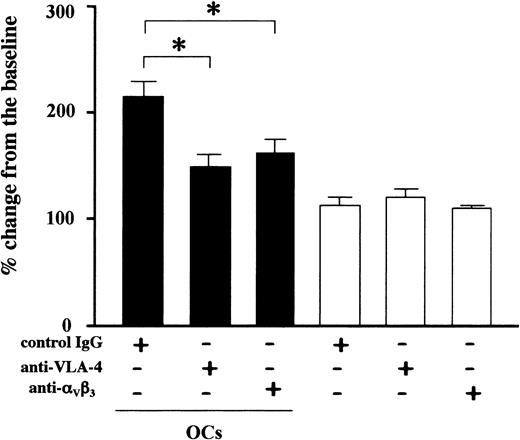
In an effort to determine the adhesion molecules involved in the adhesive interactions between OCs and MM cells, we examined effects of a battery of blocking antibodies and inhibitors against adhesion molecules and their ligands considered to be expressed on MM cell and/or OCs, including VLA-4, VLA-5, αvβ3 integrin, CD11a, CD11b, CD18, ICAM-1, ICAM-3, VCAM-1, and CD56. Among these inhibitors only antibodies against VLA-4 and αvβ3 integrin significantly suppressed the contact-mediated U266 cell growth enhancement by OCs (Figure 7). These results are consistent with the assumption that OCs enhance MM cell growth and survival through cell-cell contact that involves VLA-4 and αvβ3 integrin.
Blockade of VLA-4 or αvβ3 integrin suppressed MM cell growth enhancement by OCs. U266 cells (2 × 105/mL) were cultured in alpha-MEM containing 1% FBS in the presence (closed bars) or absence (open bars) of PBMC-OCs (1 × 104/mL) in 24-well culture plates in quadruplicate. Neutralizing antibodies against VLA-4 or αvβ3 integrin or control IgG were added at 20 μg/mL to the indicated wells. Viable cell number was counted by trypan blue staining at day 4. The data are expressed as means ± SD. *Significantly different by one-way ANOVA with Scheffe post hoc tests, P < .05.
Blockade of VLA-4 or αvβ3 integrin suppressed MM cell growth enhancement by OCs. U266 cells (2 × 105/mL) were cultured in alpha-MEM containing 1% FBS in the presence (closed bars) or absence (open bars) of PBMC-OCs (1 × 104/mL) in 24-well culture plates in quadruplicate. Neutralizing antibodies against VLA-4 or αvβ3 integrin or control IgG were added at 20 μg/mL to the indicated wells. Viable cell number was counted by trypan blue staining at day 4. The data are expressed as means ± SD. *Significantly different by one-way ANOVA with Scheffe post hoc tests, P < .05.
Discussion
Growth and survival of MM cells are highly dependent on the BM microenvironment except in terminal stages of the disease. In the BM microenvironment, MM cells enhance OC formation and function mainly through interactions with stromal cells to develop lytic bone lesions.37 BM stromal cells are also shown to enhance IL-6 production under adhesion to MM cells and have been considered as predominant cells to support MM cell growth and survival.20,21 In the present study, we demonstrate that OCs have potent growth-promoting and antiapoptotic effects on MM cells (Figures 1 and 2), and that the effects of OCs on MM cell growth and survival were much more potent than those of primary BM stromal cells (Table 1). The effect was observed even with very small numbers of OCs. Because OCs are formed around MM cells by way of cell-cell interactions, OCs thus formed may play an important role in creating beneficial environment for MM cell growth and survival as a feeder for MM cells. Thus, in addition to the growth advantage MM cells have acquired through genetic alterations and clonal selections,38,39 OCs endow MM cells with an additional growth and survival potential.
The present study demonstrated that cell-cell contact with OCs not only enhanced the growth of a DOX-sensitive MM cell line, RPMI8226, but also caused marked resistance of RPMI8226 cells to cytotoxic effects of DOX. In fact, the number of RPMI8226 cells treated with the highest dose of DOX in the presence of OCs was almost comparable to that without DOX in the absence of OCs (Figure 3). These observations are in agreement with clinical findings that MM at advanced stages with extensive osteolytic lesions shows refractoriness to chemotherapies and suggest that increased OC number and/or activity contributes to aggressiveness or drug resistance of MM cells. These results also suggest that there is a vicious cycle between MM cells and OCs in the BM microenvironment, in which enhancement of osteoclastic bone resorption not only causes destructive bone lesions but also develops drug resistance to further aggravate MM expansion. The observation that inhibition of OC activity by a bisphosphonate also suppressed immunoglobulin production from MM cells (Figure 4) further supports the notion that MM cell-OC interaction plays an important role in MM expansion and aggravation.
In regard to the mechanism of the OC-mediated enhancement of MM cell growth, cell-cell contact between MM cells and OCs appears to play an important role. Although cell-cell contact between MM cells and OCs markedly enhanced IL-6 production from OCs, addition of an anti–IL-6 neutralizing antibody only partially suppressed OC-mediated MM cell growth (Figure 5A-B). Furthermore, although nonhuman IL-6 cannot act on human cells,35 cell-cell contact-dependent proliferation of human MM cell line, OPC, was similarly enhanced by a murine preosteoclastic cell line, C7 (Figure 5C). These results demonstrate that cell-cell contact-mediated enhancement of MM cell growth and survival does not depend on the elevation of IL-6 production by OCs. Consistent with our present observations, Yaccoby et al40 recently reported that interactions between primary MM cells and OCs enhance MM cell survival, which is dependent on their physical contact as well as on IL-6 from OCs. Because MM cells become resistant to chemotherapies and acquire potential to grow under cell-cell contact with OCs, cell-cell interaction between MM cells and OCs may provide MM cells with even stronger potential for growth and survival independent of IL-6.
It has been reported that OPN-null mice develop much less bone metastases when inoculated with breast cancer cells,41 and that serum OPN levels correlate with survival and metastasis to bone in hormone-refractory prostate carcinoma patients.42 Thus, OPN is regarded as a candidate of metastasis gene to be a target of new cancer therapies.43 In addition to its actions on tumor cells, OPN is regarded as an angiogenic44,45 and immunoregulatory factor,46,47 as well as a factor to stimulate bone resorption44,48,49 and to inhibit bone mineralization.50 In the case of MM, cell surface OPN receptors such as VLA-4, αvβ3 integrin, and CD44 are expressed in MM cells,51 and cross-linking of OPN receptors has been reported to enhance the growth of U266 as well as primary MM cells.16,52 The present study demonstrated that OPN promoted MM cell growth in combination with IL-6, although cooperative action of OPN and IL-6 only partially explained the effects of OCs on MM cell growth and survival (Figure 6). Thus, production of OPN along with IL-6 from OCs that are recruited around MM cells may make a significant contribution to the progression of MM with its destructive bone lesions.
Finally, we demonstrated that VLA-4– and αvβ3 integrin-mediated adhesion is responsible for the contact-mediated MM cell growth enhancement by OCs. Interestingly, an antibody against VCAM-1, a ligand for VLA-4 known to be constitutively expressed on stromal cells, showed no significant effects. The results suggest that OCs may not express high levels of VCAM-1. Because OPN is a common ligand for VLA-4 and αvβ3 integrin and because OCs express abundant OPN, OPN may play a role in the enhancement of MM cell growth and survival mediated by these integrins. The present observations imply that these integrins may be new therapeutic targets against MM, although their precise roles in MM cell growth and survival remain unclear.
Prepublished online as Blood First Edition Paper, June 8, 2004; DOI 10.1182/blood-2003-11-3839.
Supported by The Award in Aki's Memory from The International Myeloma Foundation Japan (M.A.), a Grant-in-Aid for Scientific Research on Priority Areas (no. 12137207, to T.M.), and Grants-in-Aid for Scientific Research (nos. 13557082 and 14370329, to T.M.) and from the Ministry of Education, Culture, Science and Sports of Japan (no. 15591010, to M.A.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ms Momoko Nitta and Hiroe Amou for their expert technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal