Abstract
P-selectin glycoprotein ligand 1 (PSGL-1, CD162) and integrin αMβ2 (Mac-1, CD11bCD18) are leukocyte adhesion molecules essential for innate immunity and inflammation. The interaction of PSGL-1 with P-selectin (CD62P) mediates tethering, rolling, and weak adhesion of leukocytes, during which they become sufficiently activated in situ by locally released or displayed cytokines and chemoattractants for integrin-mediated firm adhesion. However, communication between P-selectin and the integrin, whether P-selectin can trigger β2-integrin activation, remains controversial. We found that P-selectin immunoglobulin chimera and PSGL-1 monoclonal antibodies (mAbs) increased adhesion of human neutrophils to immobilized, but not soluble, fibrinogen. This intermediate state of neutrophil adhesion was defined by moderate clustering of integrin αMβ2, no increase in CBRM1/5 (a mAb specific for the activation epitope on the αM subunit) recognition, and no increase in surface expression of αMβ2, whereas phorbol myristate acetate (PMA) induced extensive changes in these 3 parameters. Furthermore, platelet-activating factor or interleukin 8 acted in concert with P-selectin for further enhancing the activation of αMβ2. We thus propose a model in which P-selectin induces an intermediate state of integrin activation and then cooperates with other extracellular stimuli to support maximal adhesion of human neutrophils.
Introduction
The recruitment of leukocytes to the site of inflammation entails a cascade of cellular adhesive events, which include tethering (initial attachment), rolling, firm adhesion, and transendothelial migration of the responding cells.1,2 Members of the selectin family of cell adhesion molecules expressed on the endothelial surface interact with their cognate glycoprotein ligands on leukocytes to mediate the tethering and rolling phase of leukocyte recruitment; that is, the weak adhesive interactions.3,4 Members of the β2 subfamily of leukocyte integrins, as well as other integrin subfamilies, recognize their cognate ligands to then mediate the firm adhesion (arrest) of leukocytes. The initial weak adhesion brings leukocytes into proximity of cytokines/chemoattractants displayed on or released from the activated endothelium, such as interleukin 8 (IL-8) and platelet-activating factor (PAF). These transduce signals through their G protein–coupled receptors that activate the integrins to induce the firm leukocyte adhesion to the endothelium.1,5 Such integrin-mediated firm adhesion can occur through direct ligand engagement or indirect, bridging mechanisms. For example, activated β2 integrins can bind various members of intercellular cell adhesion molecule (ICAM) family on endothelial cells to support leukocyte adhesion and transmigration.6,7 As an example of the bridging mechanism, integrin αMβ2 can recognize fibrinogen or fibrin deposited on the endothelial surface or at the sites of inflammation to promote the accumulation of leukocytes.8-11 Fibrinogen engagement by activated αMβ2 is also one of the several mechanisms that contribute to formation of platelet-leukocyte conjugates,12,13 which are diagnostic of thrombotic events in vivo.
P-selectin, a member of the selectin family, is stored on the membranes of platelet α granules and endothelial Weibel-Palade bodies. On inflammatory and thrombogenic challenges, P-selectin rapidly translocates to the surface of these cells and contributes to the weak adhesion of leukocytes to stimulated endothelial cells and the heterotypic aggregation of activated platelets to leukocytes.14,15 A principal leukocyte ligand for P-selectin is P-selectin glycoprotein ligand 1 (PSGL-1), a disulfide bond-linked homodimer, with each subunit having a molecular weight of about 120 kDa.16-18 It is expressed on a variety of human leukocytes, including neutrophils, monocytes, certain subsets of T lymphocytes, eosinophils, and basophils. On neutrophils, PSGL-1 localizes to the tips of the microvilli.16,19,20 Recent studies of the PSGL-1 knockout mice have demonstrated that PSGL-1 is critically required for neutrophil rolling and migration mediated by P-selectin.21
The β2 subfamily of integrin, often referred to as the leukocyte integrins, consists of 4 members, in which 4 distinct α subunits combine with a common β2 subunit. αMβ2 is a major integrin on neutrophils and is notorious for its capacity to recognize many different ligands, such as blood coagulation proteins fibrinogen and factor X, ICAM-1, the complement pathway product C3bi as well as several extracellular matrix proteins.22 Ligand recognition by αMβ2 is influenced by the activation state of the receptor. Integrin activation is induced by either a conformational change within each receptor, which increases apparent affinity for ligand, or integrin clustering, which enhances avidity for ligand.23,24 At the extreme, activation of an integrin allows it to engage soluble ligands, whereas binding to the unactivated integrin cannot be demonstrated. Such activation is induced physiologically by ligation of an agonist receptor, which initiates an intracellular pathway that ultimately transmits a signal to the cytoplasmic tails of the integrin. This signal, an inside-out signaling, is then transmitted across the membrane to initiate the conformational or clustering events that are associated with integrin activation. Such activation has been demonstrated in response to several cytokines and chemoattractants.25,26 An additional mechanism for control of αMβ2 functions on neutrophils is a change in its cell surface expression. Neutrophils contain a large pool of intracellular αMβ2, which can become surface expressed on neutrophil stimulation leading to secretion.27
Recent studies suggest that P-selectin binding to its counterreceptor PSGL-1 promotes αMβ2-dependent homotypic neutrophil aggregation and neutrophil-platelet conjugation28,29 and α4β1-dependent adhesion of monocytes to vascular cell adhesion molecule 1 (VCAM-1),30 responses typically dependent on integrin activation. Hidari et al31 observed that engagement of PSGL-1 enhances tyrosine phosphorylation, activates mitogen-activated protein (MAP) kinases (ERK-1 and ERK-2) through MEK (MAP kinase kinase), and stimulates IL-8 secretion in neutrophils. Although these events are typically associated with integrin activation, functional activation of β2 integrins in human neutrophils was not detected.31 An earlier report also concluded that PSGL-1 ligation was not sufficient to activate the β2 integrins on human neutrophils.32 In addition, Blanks et al33 reported that P-selectin induced β2 integrin-mediated cell attachment to ICAM-1 by mouse but not human neutrophils. Thus, the relationship between PSGL-1 ligation and αMβ2 activation remains uncertain. In the present study, we demonstrate that the binding of P-selectin to PSGL-1 results in a moderate clustering and a partial activation of αMβ2, thus enhancing adhesion and binding of human neutrophils to immobilized, but not to soluble, fibrinogen and the αMβ2 activation-specific monoclonal antibody (mAb) CBRM1/5.34 PAF or IL-8 acts in concert with P-selectin for further activation of αMβ2. In contrast to phorbol myristate acetate (PMA), no changes in the level of αMβ2 are observed. Hence, our data suggest that PSGL-1 engagement by P-selectin defines an intermediate state of αMβ2 activation, which can be further activated by extracellular stimuli for maximal adhesion of human neutrophils to cognate αMβ2 ligands.
Materials and methods
Proteins and antibodies
Recombinant human P-selectin immunoglobulin chimera (P-sel Ig) was prepared as previously described.18,.35 It was characterized as a homodimer,36 with each monomer having 2 P-selectins (the lectin domain, the epidermal growth factor–like domain, and the first 2 complement-like repeats). Recombinant soluble human P-selectin (sP-selectin), which was previously characterized as a monomer,37 was purchased from R&D Systems (Minneapolis, MN). Recombinant human IL-8 was purchased from Calbiochem (La Jolla, CA). The endotoxin levels in these reagents were less than 1 endotoxin unit (EU) per microgram recombinant protein determined by the limulus amoebocyte lysate (LAL) from Cape Cod. Plasminogen-depleted human fibrinogen was from Enzyme Research Laboratories (South Bend, IN). G1 (a leukocyte adhesion-blocking IgG1 mAb to P-selectin) and PS1 (a leukocyte adhesion-nonblocking IgG1 mAb to P-selectin) were prepared and characterized as before.18,38 KPL-1 (a leukocyte adhesion-blocking IgG1 mAb to PSGL-1) was purchased from BD PharMingen (San Diego, CA). PL1 (a leukocyte adhesion-blocking IgG1 mAb to PSGL-1) and PL2 (a leukocyte adhesion-nonblocking IgG1 mAb to PSGL-1) were kindly provided by Dr Rodger P. McEver (W. K. Warren Medical Research Institute, University of Oklahoma Health Sciences Center, Oklahoma City). F(ab′)2 fragments of G1, PS1, and PL1 and Fab fragment of PL1 were prepared using ImmunoPure F(ab′)2 and Fab preparation kits (Pierce, Rockford, IL). IB4 (a leukocyte adhesion-blocking mAb to β2 subunit), 44a (a leukocyte adhesion-blocking mAb to αM subunit), and OKM1 (a leukocyte adhesion-nonblocking mAb to αM subunit) were purchased from American Tissue Culture Collection (ATCC; Manassas, VA). CBRM1/5 (a mAb specific for an activation-dependent epitope on αM subunit34 ) was a generous gift from Dr Timothy A. Springer (Center for Blood Research, Harvard Medical School, Boston, MA). Alexa Fluor 488–conjugated goat antibody to mouse IgG (H+L; cross-absorbed) was purchased from Molecular Probes (Eugene, OR). Alexa Fluor 488–conjugated fibrinogen was prepared using Alexa Fluor 488 protein-labeling kit (Molecular Probes). Human and mouse IgG, PAF, and PMA were purchased from Sigma (St Louis, MO).
Neutrophil preparation
Fresh human blood was collected from healthy, adult volunteers with their informed consent into acid-citrate-dextrose (ACD; 38 mM citric acid, 75 mM trisodium citrate, and 100 mM dextrose; 1:7 ACD final concentration). After removal of platelet-rich plasma by centrifugation of the whole blood at 100g at 22° C for 15 minutes, the cell pellet was diluted with ice-cold phosphate-buffered saline (PBS), pH 7.4, to original blood volume and layered onto one-third volume of Ficoll-Paque Plus (Amersham Biosciences, Piscataway, NJ) and centrifuged at 2000g at 18° C for 12 minutes. The supernatant and interface were removed, and ice-cold PBS was added to restore the original volume. The cell suspension was then mixed with one-third volume of 6% dextran (Sigma) in PBS, and the erythrocytes were allowed to settle at 22° C for 30 minutes. The top layer was collected and was centrifuged at 500g at 4° C for 5 minutes. After hypotonic lysis of residual erythrocytes with cold H2O, neutrophils were resuspended in ice-cold PBS at about 2 × 107/mL for immediate use. The purity of neutrophils was routinely more than 95% and viability was greater than 96% measured by trypan blue exclusion. The endotoxin levels in all buffers used were less than 0.03 EU/mL determined by the LAL method. Approval for these studies was obtained from the Cleveland Clinic Foundation institutional review board. Informed consent was provided according to the Declaration of Helsinki.
Adhesion assay
Fibrinogen (20 μg/mL) in PBS was immobilized on the 96-well tissue culture plates (0.1 mL/well; Costar, Cambridge, MA) at 4° C overnight. The wells were postcoated with 0.5% polyvinyl alcohol (Sigma) at 22° C for 1 hour and washed twice with PBS. Neutrophils were resuspended at 2 × 106/mL in Hanks balanced salt solution, pH 7.4, 1.2 mM CaCl2, and 0.8 mM MgCl2 supplemented with 1% bovine serum albumin (HBSS/Ca/Mg/BSA). The cells were incubated with various testing agents, such as 10 μg/mL human IgG, P-sel Ig chimera, sP-selectin, mouse IgG, KPL-1 mAb, PL1 mAb, PL2 mAb, PL1 Fab, PL1 F(ab′)2, 300 nM PAF, 0.3 μg/mL IL-8, or 8 nM PMA, for 2 minutes and then added to the coated wells (0.1 mL/well). For antibody inhibition experiments, 10 μg P-sel Ig chimera was preincubated with 20 μg G1 or PS1 F(ab′)2 in 30 μL HBSS/Ca/Mg/BSA at 22° C for 15 minutes. Alternatively, neutrophils were preincubated with 15 μg/mL mouse IgG, mAb 44a, and mAb IB4. After incubation at 37° C for 25 minutes in a 5% CO2 humidified atmosphere, nonadherent cells were carefully removed by washing 3 times with PBS. The adherent cells were quantified using a myeloperoxidase (MPO) assay as described previously.38 MPO activity was converted to neutrophil numbers using a standard curve that was generated by measurements of enzyme activity derived from a series dilution of known numbers of neutrophils.
Flow cytometric assay
Neutrophils, resuspended at 2 × 106/mL in HBSS/Ca/Mg/BSA, were incubated with 10 μg/mL human IgG, P-sel Ig chimera, 300 nM PAF, 300 ng/mL IL-8, or 8 nM PMA at 22° C for 2 minutes followed by 3 μg/mL mAb IB4 or CBRM1/5 and an Alexa Fluor 488–conjugated goat antibody to mouse IgG (cross-absorbed; 1:1000 dilution) at 37° C for 25 minutes in a 5% CO2-humidified atmosphere. Alternatively, neutrophils were incubated with 60 μg/mL Alexa Fluor 488–conjugated fibrinogen. The cells were centrifuged through a cushion of fetal calf serum and resuspended in 1% paraformaldehyde in PBS. Cell-bound antibodies or fibrinogen were detected by fluorescent-activated cell sorting (FACScan; Becton-Dickinson, San Jose, CA).
Confocal microscopy
Freshly isolated human neutrophils were incubated with buffer alone, 10 μg/mL human IgG, P-sel Ig chimera, 300 ng/mL IL-8, 8 nM PMA, 300 nM PAF, or 300 nM PAF plus 10 μg/mL P-sel Ig chimera in HBSS/Ca/Mg/BSA at 37° C for 25 minutes. The cells were fixed with 4% paraformaldehyde for 15 minutes at 22° C. After washing 3 times with PBS, samples were incubated with IB4 or OKM-1 mAb (10 μg/mL) in PBS/BSA (PBS supplemented with 1% BSA) and an Alexa Fluor 488–conjugated goat antibody to mouse IgG (cross-absorbed; 1:1000 dilution) at 22° C for 30 minutes. Following washing with PBS, the cells were mixed with the same volume of Vectashield mounting medium (Vector Laboratories, Burlingame, CA) containing DAPI (4′,6-diamidino-2-phenylindole) and dropped onto positively charged slides followed by mounting coverslips. The samples were then sealed with nail polish and observed under a confocal laser scanning microscope (Leica TCS SP2; Heidelberg, Germany) using HCX PL APO lens at 63 × 8 magnification, numerical aperture at 1.4, and Leica Confocal Software version 2. The relative fluorescence intensity was calculated using ImagePro Plus (version 4.5; Media Cybernetics, Silver Spring, MD).
Results
P-selectin increases αMβ2-mediated adhesion of neutrophils to fibrinogen
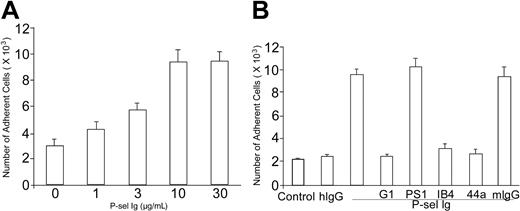
To test the effect of P-selectin on αMβ2 activation, we analyzed changes in the adhesion of human neutrophils to immobilized fibrinogen. Freshly isolated human neutrophils were added together with various concentrations of P-sel Ig chimera to fibrinogen-coated wells of tissue culture plates. After incubation at 37° C for 25 minutes, unbound cells were removed by washing with PBS, and bound neutrophils were quantified on the basis of their MPO activities. As shown in Figure 1A, P-sel Ig chimera enhanced neutrophil adhesion to fibrinogen in a concentration-dependent manner. A maximal effect was obtained at 10 μg/mL. Over the course of 12 such experiments, the increment in neutrophil adhesion to fibrinogen induced by this concentration of P-sel Ig chimera was increased by about 3-fold although there was considerable variability among donors.
P-selectin increases adhesion of neutrophils to fibrinogen. (A) Neutrophils were isolated from fresh human blood and added to 96-well tissue culture plates coated with fibrinogen. For the dose-response curve, neutrophils were incubated with indicated concentrations of P-selectin Ig chimera (P-sel Ig). (B) Neutrophils were incubated with human IgG (hIgG) or P-selectin Ig chimera (P-sel Ig), and added to wells coated without fibrinogen (control) or with fibrinogen. For antibody inhibition experiments, P-selectin Ig chimera (P-sel Ig), was preincubated with G1 F(ab′)2 (a leukocyte adhesion-blocking mAb to P-selectin) or PS1 F(ab′)2 (a leukocyte adhesion-nonblocking mAb to P-selectin) prior to addition of neutrophils. Alternatively, neutrophils were preincubated with IB4 (a leukocyte adhesion-blocking mAb to β2 subunit), 44a (a leukocyte adhesion-blocking mAb to αM subunit), or mouse IgG (mIgG), prior to addition to the fibrinogen-coated wells. After washing, the bound neutrophils were quantified by MPO activities. The numbers of bound neutrophils were calculated according to the standard curves of MPO activities measured using the known amounts of neutrophils. All results are expressed as the mean ± SD values of the adherent cells determined in triplicate measurements of more than 3 separate experiments.
P-selectin increases adhesion of neutrophils to fibrinogen. (A) Neutrophils were isolated from fresh human blood and added to 96-well tissue culture plates coated with fibrinogen. For the dose-response curve, neutrophils were incubated with indicated concentrations of P-selectin Ig chimera (P-sel Ig). (B) Neutrophils were incubated with human IgG (hIgG) or P-selectin Ig chimera (P-sel Ig), and added to wells coated without fibrinogen (control) or with fibrinogen. For antibody inhibition experiments, P-selectin Ig chimera (P-sel Ig), was preincubated with G1 F(ab′)2 (a leukocyte adhesion-blocking mAb to P-selectin) or PS1 F(ab′)2 (a leukocyte adhesion-nonblocking mAb to P-selectin) prior to addition of neutrophils. Alternatively, neutrophils were preincubated with IB4 (a leukocyte adhesion-blocking mAb to β2 subunit), 44a (a leukocyte adhesion-blocking mAb to αM subunit), or mouse IgG (mIgG), prior to addition to the fibrinogen-coated wells. After washing, the bound neutrophils were quantified by MPO activities. The numbers of bound neutrophils were calculated according to the standard curves of MPO activities measured using the known amounts of neutrophils. All results are expressed as the mean ± SD values of the adherent cells determined in triplicate measurements of more than 3 separate experiments.
To verify the specificity of this effect, that is, the roles of P-selectin and αMβ2, P-sel Ig chimera was added together with antibodies to their cell surface receptors. When P-sel Ig chimera was preincubated with F(ab′)2 fragments of G1 (as a function blocking IgG1 mAb to P-selectin) or PS1 (a nonblocking IgG1 mAb against P-selectin), the increment in neutrophil adhesion was blocked selectively by G1 (Figure 1B). Similarly, when neutrophils were preincubated with IB4 (a mAb to the integrin-β2 subunit that blocks leukocyte adhesion) or 44a (a blocking mAb to the integrin-αM subunit), the increment in neutrophil adhesion induced by P-sel Ig chimera was completely inhibited, whereas the mouse IgG had no detectable effect. This inhibition pattern indicates that (1) P-selectin specifically increases neutrophil adhesion and (2) the augmented adhesion is mediated by αMβ2.
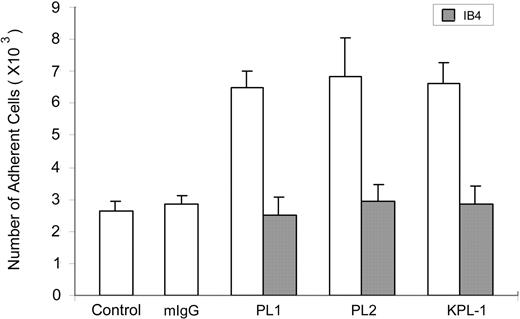
To evaluate the role of PSGL-1, the ligand for P-selectin, in the observed response, experiments were performed with anti–PSGL-1 mAbs. However, addition of KPL-1 and PL1, 2 blocking mAbs to PSGL-1, or PL2, a nonblocking PSGL-1 mAb, all increased neutrophil adhesion to fibrinogen by about 2-fold even in the absence of P-sel Ig chimera (Figure 2). This increment was blocked by IB4, confirming β2-integrin involvement. Control mouse IgG did not enhance cell adhesion (Figure 2). Thus, the mAb interaction with PSGL-1 is sufficient to induce functional up-regulation of αMβ2.
PSGL-1 antibodies enhance adhesion of neutrophils to fibrinogen. Washed neutrophils were incubated with 10 μg/mL mouse IgG (mIgG), KPL-1 (a leukocyte adhesion-blocking mAb to PSGL-1), PL1 (a leukocyte adhesion-blocking mAb to PSGL-1), or PL2 (a leukocyte adhesion-nonblocking mAb to PSGL-1) before they were added to the wells without fibrinogen coating (control) or the wells immobilized with fibrinogen. For inhibition experiments, neutrophils were preincubated with IB4 (a leukocyte adhesion-blocking mAb to β2 subunit). Other steps of the cell adhesion assay were carried out as described for Figure 1. All results are expressed as the mean ± SD values of the adherent cells determined in triplicate measurements of 3 separate experiments.
PSGL-1 antibodies enhance adhesion of neutrophils to fibrinogen. Washed neutrophils were incubated with 10 μg/mL mouse IgG (mIgG), KPL-1 (a leukocyte adhesion-blocking mAb to PSGL-1), PL1 (a leukocyte adhesion-blocking mAb to PSGL-1), or PL2 (a leukocyte adhesion-nonblocking mAb to PSGL-1) before they were added to the wells without fibrinogen coating (control) or the wells immobilized with fibrinogen. For inhibition experiments, neutrophils were preincubated with IB4 (a leukocyte adhesion-blocking mAb to β2 subunit). Other steps of the cell adhesion assay were carried out as described for Figure 1. All results are expressed as the mean ± SD values of the adherent cells determined in triplicate measurements of 3 separate experiments.
To exclude that the observed effects of P-sel Ig chimera were due to traces of endotoxin in the reagents, we tested the direct effect of lipopolysaccharide (LPS) on neutrophil adhesion to fibrinogen. Concentrations as high as 10 EU/mL LPS (> 10-fold higher than in the reagents) did not increase the adhesion of responding cells (data not shown).
Role of P-selectin multimerization in αMβ2 activation
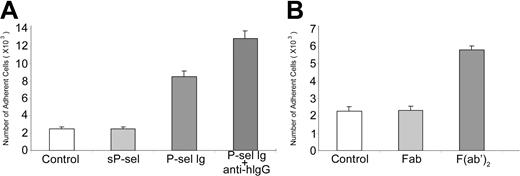
Because P-sel Ig chimera and PSGL-1 mAbs are dimers, they may cross-link PSGL-1. Because native P-selectin, isolated from human platelets and endothelial cells exists as dimeric and oligomeric forms even at the detergent concentrations well above the critical micelle concentration,39,40 such cross-linking of PSGL-1 has clear biologic relevance. To test whether cross-linking of PSGL-1 was important for αMβ2 activation, we examined the capacity of monomeric sP-selectin to activate the integrin. In contrast to the multimeric P-sel Ig chimera, monomeric sP-selectin (10 μg/mL) did not enhance neutrophil adhesion to immobilized fibrinogen (Figure 3A). Moreover, when the F(ab′)2 fragment of anti–human IgG antibody (Fc specific) was added together with P-sel Ig chimera, the combination induced more extensive adhesion than P-sel Ig chimera alone, consistent with a role of cross-linking of PSGL-1 in αMβ2 activation (Figure 3A). As controls, sP-selectin added with the anti–human IgG antibody failed to stimulate neutrophil adhesion to fibrinogen (data not shown). To further verify that PSGL-1 cross-linking is necessary for αMβ2 activation, we compared the activity of F(ab′)2 and Fab fragments of PL1 to stimulate neutrophil adhesion to fibrinogen. As shown in Figure 3B, the former but not the latter reagent induced cell adhesion. Together, these data suggest that engagement of PSGL-1 alone is not sufficient for αMβ2 activation, but engagement and cross-linking of PSGL-1 by dimers or higher multimers of P-selectin or mAbs to PSGL-1 will induce activation of the integrin.
PSGL-1 cross-linking up-regulates αMβ2. (A) Neutrophils were incubated with 10 μg/mL sP-selectin (sP-sel), P-selectin Ig chimera (P-sel Ig), and P-selectin Ig chimera cross-linked by F(ab′)2 fragment of anti–human IgG (Fc specific; anti-hIgG), respectively. The differences between sP-sel and control were statistically insignificant (P > .05), whereas the differences between P-sel Ig or P-sel Ig plus anti-hIgG and control were statistically significant (P < .01). (B) Neutrophils were treated with 10 μg/mL Fab or F(ab′)2 fragment of PL1. They were then transferred into the wells coated with fibrinogen. Other steps of the cell adhesion assay were same as for Figure 1. All results are expressed as the mean ± SD values of the adherent cells determined in triplicate measurements of 3 separate experiments. The differences between Fab and control were statistically insignificant (P > .05), whereas the differences between F(ab′)2 and control were statistically significant (P < .01).
PSGL-1 cross-linking up-regulates αMβ2. (A) Neutrophils were incubated with 10 μg/mL sP-selectin (sP-sel), P-selectin Ig chimera (P-sel Ig), and P-selectin Ig chimera cross-linked by F(ab′)2 fragment of anti–human IgG (Fc specific; anti-hIgG), respectively. The differences between sP-sel and control were statistically insignificant (P > .05), whereas the differences between P-sel Ig or P-sel Ig plus anti-hIgG and control were statistically significant (P < .01). (B) Neutrophils were treated with 10 μg/mL Fab or F(ab′)2 fragment of PL1. They were then transferred into the wells coated with fibrinogen. Other steps of the cell adhesion assay were same as for Figure 1. All results are expressed as the mean ± SD values of the adherent cells determined in triplicate measurements of 3 separate experiments. The differences between Fab and control were statistically insignificant (P > .05), whereas the differences between F(ab′)2 and control were statistically significant (P < .01).
Consequences of PSGL-1 ligation with P-selectin on αMβ2
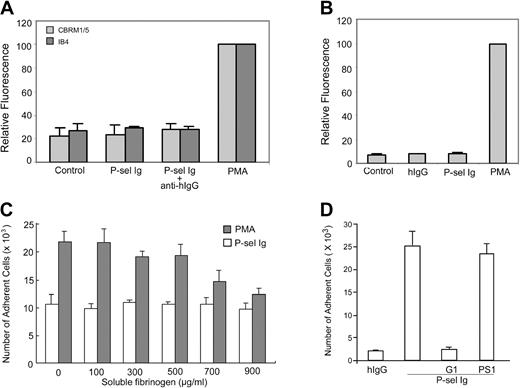
We next characterized the basis for the functional up-regulation of αMβ2 on treatment of neutrophils with P-sel Ig chimera. Enhanced adhesion to fibrinogen may arise from changes in expression levels or activation of the integrin by either affinity or avidity modulation. To assess whether P-sel Ig chimera increased the expression level of αMβ2, FACS analyses were performed on neutrophils with or without P-sel Ig chimera treatment using mAb IB4. This mAb to β2 subunit reports on the level of αMβ2. As shown in Figure 4A, P-sel Ig chimera failed to increase αMβ2 expression. Treatment of neutrophils with PMA did enhance mAb IB4 reactivity, providing a positive control. To assess changes in activation, mAb CBRM1/5 was used, which reacts with an activation-dependent epitope on the αM subunit. Whereas treatment of the cells with PMA resulted in up-regulation of the CBRM1/5 epitope in FACS analyses, stimulation with P-sel Ig chimera did not (Figure 4A). In view of these results, we also tested whether we could detect the binding of soluble fibrinogen to αMβ2 by FACS. As shown in Figure 4B, fibrinogen bound poorly to nonstimulated neutrophils or to neutrophils stimulated with P-sel Ig chimera. However, when the cells were stimulated with PMA, binding of soluble fibrinogen could be detected. Furthermore, in examining the adhesion of neutrophils to immobilized fibrinogen, we observed that soluble fibrinogen inhibited the interaction of PMA-stimulated, but not P-sel Ig chimera-stimulated, neutrophils (Figure 4C). Taken together, these data suggest that (1) nonstimulated neutrophils express αMβ2 in a state that it can neither adhere to fibrinogen nor bind it as a soluble ligand; (2) P-sel Ig chimera induces activation of αMβ2 to a state where it is competent to support neutrophil adhesion to immobilized, but not soluble, fibrinogen; and (3) PMA can lead to an αMβ2 activation state, in which the integrin can mediate fibrinogen recognition as both soluble and adhesive ligand.
Effects of PSGL-1 ligation on αMβ2. (A) Neutrophils were incubated with mAb IB4 or CBRM1/5 and Alexa Fluor 488–conjugated goat antibody to mouse IgG without (control) or with P-selectin Ig chimera (P-sel Ig) or PMA. They were then centrifuged through a cushion of FCS and fixed in 1% paraformaldehyde in PBS for flow cytometric analysis. Results are the representative of 3 independent experiments. (B) Neutrophils were incubated with Alexa Fluor 488–conjugated fibrinogen, in the absence (control) or presence of human IgG (hIgG), P-selectin Ig chimera (P-sel Ig), and PMA. The samples were processed as described in panel A. Results are the representative of 3 independent experiments. (C) Neutrophils were incubated with PMA or P-selectin Ig chimera (P-sel Ig) in the presence of an increasing concentration of soluble fibrinogen and then transferred to the wells coated with fibrinogen. The results of cell adhesion assays are expressed as the mean ± SD values of the adherent cells determined in triplicate measurements of 3 separate experiments. (D) Neutrophils were incubated with human IgG (hIgG) and P-selectin Ig chimera (P-sel Ig) in the presence of G1 or PS1 F(ab′)2 prior to adding them to the wells immobilized with moAb CBRM1/5. After washing, the bound neutrophils were quantified by MPO activities. All results are expressed as the mean ± SD values of the adherent cells determined in triplicate measurements of 3 separate experiments.
Effects of PSGL-1 ligation on αMβ2. (A) Neutrophils were incubated with mAb IB4 or CBRM1/5 and Alexa Fluor 488–conjugated goat antibody to mouse IgG without (control) or with P-selectin Ig chimera (P-sel Ig) or PMA. They were then centrifuged through a cushion of FCS and fixed in 1% paraformaldehyde in PBS for flow cytometric analysis. Results are the representative of 3 independent experiments. (B) Neutrophils were incubated with Alexa Fluor 488–conjugated fibrinogen, in the absence (control) or presence of human IgG (hIgG), P-selectin Ig chimera (P-sel Ig), and PMA. The samples were processed as described in panel A. Results are the representative of 3 independent experiments. (C) Neutrophils were incubated with PMA or P-selectin Ig chimera (P-sel Ig) in the presence of an increasing concentration of soluble fibrinogen and then transferred to the wells coated with fibrinogen. The results of cell adhesion assays are expressed as the mean ± SD values of the adherent cells determined in triplicate measurements of 3 separate experiments. (D) Neutrophils were incubated with human IgG (hIgG) and P-selectin Ig chimera (P-sel Ig) in the presence of G1 or PS1 F(ab′)2 prior to adding them to the wells immobilized with moAb CBRM1/5. After washing, the bound neutrophils were quantified by MPO activities. All results are expressed as the mean ± SD values of the adherent cells determined in triplicate measurements of 3 separate experiments.
To validate and generalize these observations, we tested whether P-sel Ig chimera could induce binding of neutrophils to immobilized mAb CBRM1/5. As shown in Figure 4D, stimulation of neutrophils with P-sel Ig chimera supported binding to CBRM1/5. This interaction was inhibited by the blocking P-selectin mAb, G1, but not by the nonblocking mAb, PS1. Thus, P-sel Ig chimera can induce αMβ2 activation to a state where insoluble but not soluble activation-dependent ligands can be recognized.
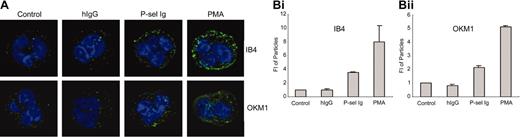
To further characterize the activation state of αMβ2 induced by P-sel Ig chimera, we assessed the distribution of the integrin on the cell surface. Nontreated neutrophils or neutrophils exposed to human IgG showed a weak rim and punctuate staining with either mAb IB4 to the β2 subunit or mAb OKM1 to the αM subunit. The image intensity was adjusted to a point where the rim staining was not detectable but the punctuate staining was. Under this condition, mAb IB4 (Figure 5A,Bi) and mAb OKM1 (Figure 5A,Bii) showed only a weak punctuate staining pattern. In contrast, PMA stimulation induced considerably brighter and larger patches of staining (Figure 5A). With P-sel Ig chimera stimulation, an intermediate staining was observed (Figure 5A). The patches were large and more abundant than those in control cells. However, they tended to be fewer and less intense than those treated with PMA (Figure 5A-B). These data are consistent with the induction of a variable degree of clustering of αMβ2 on the P-sel Ig chimera and PMA-stimulated neutrophils.
P-selectin triggers αMβ2 clustering. (A) Neutrophils were treated without (control) or with human IgG (hIgG), P-selectin Ig chimera (P-sel Ig), and PMA. After fixation, samples were stained by mAb IB4 to β2 subunit or mAb OKM1 to αM subunit followed by Alexa Fluor 488–conjugated goat antibody to mouse IgG and confocal laser scanning microscopy. The data presented here are representative images from 3 independent experiments. (B) The mean ± SD values of fluorescence intensities of the particles are presented for mAb IB4-stained (i) and mAb OKM1-stained (ii) neutrophils. These were quantified by image analyses using the ImagePro Plus program. The differences between hIgG and control were statistically insignificant (P > .05), whereas the differences between P-sel Ig or PMA and control were statistically significant (P < .01).
P-selectin triggers αMβ2 clustering. (A) Neutrophils were treated without (control) or with human IgG (hIgG), P-selectin Ig chimera (P-sel Ig), and PMA. After fixation, samples were stained by mAb IB4 to β2 subunit or mAb OKM1 to αM subunit followed by Alexa Fluor 488–conjugated goat antibody to mouse IgG and confocal laser scanning microscopy. The data presented here are representative images from 3 independent experiments. (B) The mean ± SD values of fluorescence intensities of the particles are presented for mAb IB4-stained (i) and mAb OKM1-stained (ii) neutrophils. These were quantified by image analyses using the ImagePro Plus program. The differences between hIgG and control were statistically insignificant (P > .05), whereas the differences between P-sel Ig or PMA and control were statistically significant (P < .01).
Additive effects of P-selectin with PAF or IL-8 on αMβ2 activation
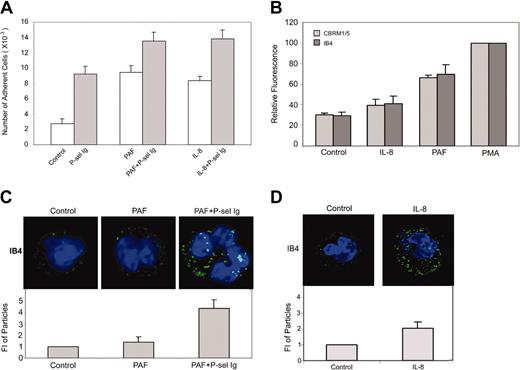
In the sequence of reactions that mediate leukocyte adhesion to endothelium, engagement of P-selectin occurs in a microenvironment rich in other neutrophil stimuli, notably IL-8 and PAF.30,40,41 Accordingly, we considered whether stimulation of neutrophil adhesion by P-selectin was influenced by these mediators. As shown in Figure 6A, similar to P-sel Ig chimera, PAF or IL-8 (concentrations inducing maximal effects are shown) increased adhesion of neutrophils to fibrinogen. The extent of the increment in adhesion induced by PAF or IL-8 was similar to that induced by P-sel Ig chimera. In combination with these agonists, P-sel Ig chimera further enhanced the extent of adhesion to fibrinogen. In contrast to P-sel Ig chimera, PAF did enhance the binding of mAb IB4, indicating an increase in integrin expression and did induce the activation epitope recognized by CBRM1/5 (Figure 6B). In contrast, IL-8 induced only a slight increment (∼10% compared to control) in the binding of either IB4 or CBRM1/5, indicating that IL-8 did not significantly alter integrin expression or conformation (Figure 6B). Addition of soluble fibrinogen partially inhibited the adhesion of the neutrophils induced by PAF alone or PAF plus P-sel Ig chimera but did not inhibit the adhesion supported by IL-8 alone or IL-8 plus P-sel Ig chimera (data not shown).
P-selectin and PAF/IL-8 cooperatively activate αMβ2. (A) Neutrophils were incubated in the absence (control) or presence of P-selectin Ig chimera (P-sel Ig), PAF, PAF plus P-selectin Ig chimera, IL-8, and IL-8 plus P-selectin Ig chimera. Samples were then transferred to the wells immobilized with fibrinogen and cell adhesion was measured. The results are expressed as the mean ± SD values of the adherent cells determined in triplicate measurements of 3 separate experiments. (B) Neutrophils were incubated without (control) or with PAF, IL-8, and PMA followed by staining with mAb IB4 or CBRM1/5 and Alexa Fluor 488–conjugated goat antibody to mouse IgG for flow cytometric analysis. Results were the representative of 3 independent experiments. (C-D) Neutrophils were treated without (control) or with PAF or PAF plus P-selectin Ig chimera (C), or IL-8 (D) and stained with mAb IB4 and Alexa Fluor 488–conjugated goat antibody to mouse IgG. They were then processed for confocal laser scanning microscopy. Neutrophils were stained with mAb IB4 for the representative images (top panels) and their intensities of fluorescent particles (means ± SDs; bottom panels). The differences between control and PAF were statistically insignificant (P > .05), whereas the differences between PAF plus P-sel Ig or IL-8 and control were statistically significant (P < .01).
P-selectin and PAF/IL-8 cooperatively activate αMβ2. (A) Neutrophils were incubated in the absence (control) or presence of P-selectin Ig chimera (P-sel Ig), PAF, PAF plus P-selectin Ig chimera, IL-8, and IL-8 plus P-selectin Ig chimera. Samples were then transferred to the wells immobilized with fibrinogen and cell adhesion was measured. The results are expressed as the mean ± SD values of the adherent cells determined in triplicate measurements of 3 separate experiments. (B) Neutrophils were incubated without (control) or with PAF, IL-8, and PMA followed by staining with mAb IB4 or CBRM1/5 and Alexa Fluor 488–conjugated goat antibody to mouse IgG for flow cytometric analysis. Results were the representative of 3 independent experiments. (C-D) Neutrophils were treated without (control) or with PAF or PAF plus P-selectin Ig chimera (C), or IL-8 (D) and stained with mAb IB4 and Alexa Fluor 488–conjugated goat antibody to mouse IgG. They were then processed for confocal laser scanning microscopy. Neutrophils were stained with mAb IB4 for the representative images (top panels) and their intensities of fluorescent particles (means ± SDs; bottom panels). The differences between control and PAF were statistically insignificant (P > .05), whereas the differences between PAF plus P-sel Ig or IL-8 and control were statistically significant (P < .01).
When changes in integrin distribution on the cell-surface of αMβ2 were evaluated by mAb IB4 immunofluorescence staining (Figure 6C-D), PAF alone failed to induce an apparent change in the cell surface distribution of the integrin compared to nonstimulated cells. However, treatment with a combination of PAF plus P-sel Ig chimera led to brighter and larger patches of αMβ2, comparable to those seen on the PMA-stimulated neutrophils (compare Figure 5A and Bi with Figure 6C). The staining induced by IL-8 alone was increased modestly compared to control cells but substantially less extensive than with PMA-treated cells (Figures 5A,Bi and 6D). Together, these data suggest that P-selectin can act in concert with other extracellular stimuli to induce still further activation of αMβ2.
Discussion
In this study, we have shown that ligation of PSGL-1 by P-selectin induces an intermediate adhesive state of neutrophil activation. This intermediate state is characterized by alterations in functions mediated by integrin αMβ2. In this intermediate state, αMβ2 surface expression is not changed, the integrin is partially clustered, it does not express the CBRM1/5 activation epitope, and it cannot bind soluble fibrinogen. The intermediate adhesive state can be further enhanced by biologically relevant stimuli, such as PAF or IL-8. On the basis of these findings, we propose a model of cooperative αMβ2 activation. This model has the following features: (1) the binding of P-selectin to PSGL-1 initiates an intracellular pathway, which ultimately transmits a signal to the cytoplasmic tails of αMβ2 for its activation; (2) the binding of extracellular stimuli, such as cytokines and chemoattractants, also activates αMβ2; (3) in contrast to strong pharmacologic stimuli, such as PMA, the physiologic stimuli examined in this manuscript, including P-selectin, PAF, and IL-8, only partially activate αMβ2, ie, induction of an intermediate state of αMβ2 activation; and (4) these stimuli can act in concert to induce full activation of αMβ2, which translates into maximal adhesion of human neutrophils to fibrinogen. Thus, the selectin-mediated reversible tethering not only renders leukocytes sufficient time to allow αMβ2 to be activated by cytokines and chemoattractants, but also cooperatively trigger the activation of αMβ2 at the adhesive zone in the capillary venules, resulting in firm adhesion of neutrophils to stimulated endothelial cells.
Considering the sequence of the events, that is, the selectin-mediated rolling occurs first and the integrin-mediated adhesion occurs afterward,1-4,14,15 selectins appear to play a role of “priming” leukocytes for optimal activation of the integrin. Whether this “priming” scenario acts as an essential prerequisite for integrin activation in vivo remains to be demonstrated. However, substantial evidence, including recent studies in PSGL-1 and P-selectin null mice,21;41 demonstrates the importance of this interaction to the inflammatory response. Data from mice42 and humans43 deficient in the β2 integrins also document the importance of these adhesion receptors to the inflammatory response. Furthermore, it has recently been shown that the inability to activate the β2 integrins leads to increased susceptibility to infections.44 These data are consistent with the role of the pathway that we have defined in leukocyte biology. Studies using PSGL-1 tailless or cytoplasmic domain mutants in knock-in mice may provide a definitive answer to the biologic importance of these cooperative signaling events.
The binding of P-selectin to PSGL-1 reportedly enhances tyrosine phosphorylation, activates mitogen-activated protein kinases (MAP kinases; ERK-1 and ERK-2) through MAP kinase kinase (MEK), and stimulates IL-8 secretion in human nuetrophils.31 In addition, neutrophil tethering mediated by E-selectin can activate β2 integrins through a MAP kinase signal transduction pathway.45 In contrast, cytokines and chemoattractants, such as PAF and IL-8, are synthesized by endothelial cells or displayed on their cell surface. They bind to their G protein–coupled receptors (GPCRs) and induce signaling that impinges on integrin cytoplasmic domains for activation of β2 integrins.46,47 Consistent with these concepts, our data that P-selectin–induced intermediate state of αMβ2 activation can be further activated by PAF or IL-8 also indicate that P-selectin and PAF/IL-8 may activate β2 integrins through distinct signaling pathways.
PSGL-1 is a homodimer covalently linked by a disulfide bond between a single extracellular cysteine in each subunit.17 The dimerization of PSGL-1 is functionally important because elimination of dimerization by mutation of the cysteine residue prevents the binding of P-selectin to PSGL-1–expressing cells48 and inhibits rolling of PSGL-1–expressing cells on immobilized P-selectin under flow.49 Consistent with an earlier observation PSGL-1 mAbs induce rapid phosphorylation,31 our data showing that PSGL-1 cross-linking by dimeric or higher oligomeric forms of P-selectin is obligatory for αMβ2 activation, indicate that the formation of PSGL-1 oligomers is essential for key signal transduction events. Such an oligomerization requirement is a common scheme in many signaling cascades in which ligand-induced dimerization or oligomerization is required (eg, various epidermal growth factors50 and GPCRs51 ). In this context, we should point out that native P-selectin, isolated from human platelets and endothelial cells exists as dimeric and oligomeric forms.39,40 Furthermore, using immunoelectron microscopy, we found that P-selectin molecules were not evenly distributed on the surface of human umbilical vein endothelial cells (HUVECs) following stimulation with tumor necrosis factor α (Y.-Q.M. and J.-G.G., unpublished observations, April 2002). Instead, the P-selectin molecules were clustered in sparse, but discrete patches, similar to the PSGL-1 patches we observed on neutrophils. Thus, the ligand for PSGL-1 on neutrophils may be presented in the multimeric state on the endothelium, which is requisite for αMβ2 activation.
Detailed structural studies of integrins at the atomic level have led to the suggestion that these adhesion receptors may exist in at least 3 conformational states: (1) an active or high-affinity state with an open conformation; (2) a transiently active, low-affinity state with an intermediate conformation; and (3) a resting, low or undetectable affinity state with a closed conformation.23,24 Both the open and intermediate conformations are competent for cell adhesion. However, the open conformation, but not the intermediate conformation, is critically required for the binding of soluble ligands. Accordingly, P-selectin–mediated adhesion of human neutrophils to immobilized, but not soluble, fibrinogen and mAb CBRM1/5 appears to suggest that P-selectin binding to PSGL-1 can only induce an intermediate state of neutrophil activation in which the functions of αMβ2 is partially but not fully enhanced. The finding that P-selectin does not trigger increased expression of αMβ2 but potentiates αMβ2 activation cooperatively with PAF or IL-1 is consistent with this proposition. As an alternative explanation, the capacity of P-selectin to evoke adhesion but not soluble ligand recognition may mainly result from the avidity modulation due to receptor clustering, instead of the affinity modulation due to receptor conformational change. These possibilities are not mutually exclusive because evidence indicates that integrins within clusters undergo conformational activation.52 The intermediate state of αMβ2 induced by P-selectin may preclude occupancy of the integrin by an overwhelmingly large excess of soluble and potentially competitive ligands in the circulation and instead limit occupancy to the target ligands presented on the surface of the endothelium.1,2,22 This intermediate state of neutrophil adhesion induced by P-selectin/PSGL-1 interaction may be analogous to the adhesion of cells to matricellular proteins, including thrombospondins, tenascins, and SPARC,53 which allow cells to transit either to a nonadhesive or firmly adhesive phenotype, depending on the additional chemical and biophysical signals within their surrounding environments. For leukocytes such transitions to firm adhesion (arrest) or de-adhesion may be a process that is essential for transmigration. P-selectin–mediated activation of αMβ2 to an intermediate rather than a fully activated state may act as a physiologically important gatekeeper, fine tuning the fate of leukocytes during their trafficking and recruitment in vivo.
Prepublished online as Blood First Edition Paper, June 24, 2004; DOI 10.1182/blood-2004-03-1108.
Supported by grants HL66197 and HL60169 from the National Institutes of Health (E.F.P.) and a start-up fund from University of Minnesota Medical School (J.-G.G.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank T. Xu and L. Zhang for their contribution of Figure 4C and Dr Judith A. Drazba and Mr Dmitry V. Leontiev for their assistance in the confocal microscopy experiments.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal