Abstract
Recent reports link Kaposi sarcoma-associated herpesvirus (KSHV) infection of bone marrow cells to bone marrow failure and lymphoproliferative syndromes. The identity of the infected marrow cells, however, remains unclear. Other work has demonstrated that circulating mononuclear cells can harbor KSHV where its detection predicts the onset and severity of Kaposi sarcoma. In either setting, bone marrow precursors may serve as viral reservoirs. Since mesenchymal stem cells (MSCs) in human bone marrow regulate the differentiation and proliferation of adjacent hematopoietic precursors, we investigated their potential role in KSHV infection. Our results indicate that primary MSCs are susceptible to both cell-free and cell-associated KSHV in culture. Moreover, infection persisted within nearly half of the cells for up to 6 weeks. Thus, MSCs possess a clear capacity to support KSHV infection and warrant further exploration into their potential role in KSHV-related human disease. (Blood. 2004;104:2736-2738)
Introduction
The etiologic agent of Kaposi sarcoma (KS), KS-associated herpesvirus (KSHV),1 infects a number of cell types within KS lesions, including endothelial cells, monocyte-derived cells, and characteristic “spindle cells” that help define these tumors.2,3 Within infected individuals, a variety of circulating bone marrow-derived cells also can harbor KSHV DNA. Some of these cells can transform to spindle-shaped cells displaying morphology and cell surface characteristics similar to KS spindle cells.4-6 Recent observations further implicate KSHV infection of bone marrow cells as a potential cause of pancytopenia, marrow engraftment failure, and hemophagocytic lymphohistiocytosis.7-10 However, the precise identity of the infected marrow cells and whether they may serve as reservoirs for subsequent viral dissemination and the clinical manifestations of KSHV infection remain unknown.
Human adult and fetal bone marrow contain a rare (0.01%-0.001%) but critical population of stroma-based cells—mesenchymal stem cells (MSCs)—that regulate the differentiation and proliferation of hematopoietic cells within bone marrow.11,12 Although the mechanisms by which MSCs exert these effects are unclear, in vitro and in vivo data indicate that certain cell lineages that they influence also can be targets for KSHV infection in human patients, including CD34+ progenitor cells.12
In this study, we provide the first report of direct KSHV infection of primary human bone marrow cells, demonstrating susceptibility of fetal-derived MSCs to KSHV infection in culture. Our results raise the possibility that MSCs may play a role in the development of KSHV-related pathology and suggest the potential of this cell culture system for studying the long-term effects of KSHV infection on relevant human hematopoietic precursor cells.
Study design
Isolation of mesenchymal stem cells
Isolation and enrichment of adherent stem cells from human fetal bone marrow was undertaken with commercially available reagents (RosetteSep and MesenCult enrichment media, StemCell Technologies, Vancouver, BC, Canada), following the manufacturer's recommendations. MSCs were identified by flow cytometry (FACS Calibur) using a panel of fluorochrome-conjugated monoclonal cell surface antibodies (Figure 1) from Caltag (Burlingame, CA).
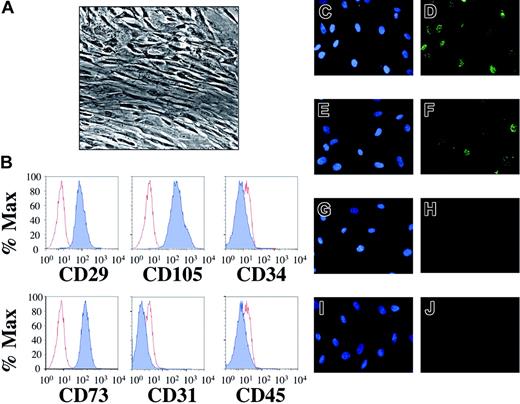
Mesenchymal stem cells isolated from human fetal bone marrow harbor latent KSHV. Following extraction from human fetal bone marrow, hematopoietic cells were incubated with a stem cell enrichment media and maintained in tissue culture under optimal conditions for mesenchymal stem cell growth (“Study design”). (A) Phase contrast microscopy (original magnification × 40, Nikon Eclipse TE2000-E, Improvision Software, Improvision, Lexington, MA) reveals confluent layers of elongated cells with reduced nuclear-cytoplasmic ratios. (B) Flow cytometric staining demonstrates that greater than 99% of the cells expressed cell surface CD29, CD73, and CD105 (dark histograms) compared to isotype controls (light histograms). In contrast, cell surface expression of CD31, CD34, and CD45 was absent. This staining pattern identified the population of cells isolated as MSCs (“Results and discussion”) and remained unchanged for at least 3 weeks after infection (not shown). MSCs were incubated with concentrated KSHV (C-D), induced BCBL-1 cells (E-F), UV-inactivated KSHV (G-H), or uninduced BCBL-1 cells (I-J). Forty-eight hours later, indirect IFA for the latency-associated nuclear antigen (LANA) revealed KSHV infection with either route of infection (D, F), giving rise to the punctate intranuclear staining pattern (green dots) characteristic of LANA expression. The controls (H, J) lacked LANA reactivity. Of note, infection with concentrated KSHV led to a higher rate of infection than did BCBL-1 coculture at the donor-to-target-cell ratios employed in this experiment (compare D to F). Omission of polybrene in cell-free experiments also led to infection but with approximately one third the efficiency (data not shown). Nuclei were counterstained with DAPI (4′6-diamino-2-phenylindole) (C, E, G, I).
Mesenchymal stem cells isolated from human fetal bone marrow harbor latent KSHV. Following extraction from human fetal bone marrow, hematopoietic cells were incubated with a stem cell enrichment media and maintained in tissue culture under optimal conditions for mesenchymal stem cell growth (“Study design”). (A) Phase contrast microscopy (original magnification × 40, Nikon Eclipse TE2000-E, Improvision Software, Improvision, Lexington, MA) reveals confluent layers of elongated cells with reduced nuclear-cytoplasmic ratios. (B) Flow cytometric staining demonstrates that greater than 99% of the cells expressed cell surface CD29, CD73, and CD105 (dark histograms) compared to isotype controls (light histograms). In contrast, cell surface expression of CD31, CD34, and CD45 was absent. This staining pattern identified the population of cells isolated as MSCs (“Results and discussion”) and remained unchanged for at least 3 weeks after infection (not shown). MSCs were incubated with concentrated KSHV (C-D), induced BCBL-1 cells (E-F), UV-inactivated KSHV (G-H), or uninduced BCBL-1 cells (I-J). Forty-eight hours later, indirect IFA for the latency-associated nuclear antigen (LANA) revealed KSHV infection with either route of infection (D, F), giving rise to the punctate intranuclear staining pattern (green dots) characteristic of LANA expression. The controls (H, J) lacked LANA reactivity. Of note, infection with concentrated KSHV led to a higher rate of infection than did BCBL-1 coculture at the donor-to-target-cell ratios employed in this experiment (compare D to F). Omission of polybrene in cell-free experiments also led to infection but with approximately one third the efficiency (data not shown). Nuclei were counterstained with DAPI (4′6-diamino-2-phenylindole) (C, E, G, I).
KSHV infection of MSCs
The KSHV-infected primary effusion lymphoma (PEL) cell line, BCBL-1, was chemically induced to support lytic KSHV replication, as described previously.13 MSCs were incubated for 2 hours with KSHV concentrated from BCBL-1 supernatants, or for 16 hours with induced BCBL-1 cells.14 KSHV-infected MSCs were identified with an immunofluorescence assay that detects expression of the KSHV latency-associated nuclear antigen (LANA) in the nuclei of fixed cells.14
Quantification of input KSHV
The concentration of encapsidated viral genomes from each viral preparation was determined using a fluorescently labeled KSHV DNA probe complementary to ORF73.14 Unless otherwise stated, all cell-free infections were carried out in the presence of 8 μg/mL polybrene (Sigma Aldrich, St Louis, MO). BCBL-1/MSC ratios in coculture experiments were adjusted after determining the concentration of viable (trypan blue excluding) cells from each group.
Results and discussion
To test whether MSCs could act as potential targets of KSHV infection within human bone marrow, we first enriched for this minor population by culturing marrow cells from mid-gestational fetal donors in selective media (Figure 1A; and “Study design”).15 After 4 days, an analysis revealed a surface profile indicative of MSCs, with CD34-CD31-CD45-CD29+CD73+CD105+ staining in more than 99% of cells (Figure 1B).12 We next attempted to infect MSCs with both cell-free (concentrated virus) and cell-associated (coculture) methods.14 Within 48 hours, both approaches resulted in marked infection of the cells as evidenced by expression of the viral latency-associated nuclear antigen, LANA (Figure 1D,F). MSCs exposed to either UV-inactivated KSHV or uninduced BCBL-1 showed no significant LANA reactivity (Figure 1H,J). Among its multiple functions, LANA tethers the viral episome to human chromosomes, and its distinct punctate intranuclear staining pattern serves as a marker of infection and the presence of these episomes.16-18
The proportion of cells infected with KSHV correlated with the amount of input virus (Figure 2A-B). The dependence on high virus-to-target-cell ratios to achieve nearly saturating rates of latent KSHV infection likely reflects the small proportion of viral particles (we estimate approximately 1 in 500) that are infectious after their concentration from the media of induced BCBL-1 cells.13,14
KSHV infection in MSCs correlates with the amount of viral input and persists for several weeks. (A) Coculture of lytically induced BCBL-1 cells with MSCs resulted in ratio-dependent expression of LANA, with maximal LANA reactivity of approximately 34%, 48 hours after infection at a BCBL-1/MSC ratio of 9:1. Error bars represent the SD of 4 individual fields of at least 100 cells. Higher BCBL-1/MSC ratios in otherwise identical experiments resulted in even higher infection rates (not shown). (B) Incubation of cell-free KSHV with MSCs resulted in concentration-dependent rates of LANA expression, with maximal infection (> 96%) 48 hours after exposure of the culture to approximately 9300 KSHV genome equivalents/cell. Error bars as per panel A. In parallel experiments, identical aliquots of input KSHV (and virus-to-cell ratios) achieved similar rates of LANA expression within T4 TIME cells (96% LANA reactivity), although LANA expression decreased more rapidly over time in these cells compared to MSCs (40% reactivity for MSC at 3 weeks versus 15% for TIME cells, data for TIME cells discussed in Tomescu et al14 ). (C) Time course following LANA IFA reactivity of MSCs infected with cell-free KSHV (with a genome equivalents to target cell ratio of 5900:1). Data reflect LANA reactivity (mean ± range) in 2 settings: (1) infection of MSCs from a single donor in 2 separate experiments using KSHV isolated from separate BCBL-1 stocks (dotted line); and (2) infection of MSCs isolated from 3 separate donors and infected with KSHV from the same viral stock (solid line). Note: after a 7- to 9-day lag in the growth of the MSC culture following infection, expansion of the population resumed with a constant doubling time of approximately 30 hours through week 6 (not shown).
KSHV infection in MSCs correlates with the amount of viral input and persists for several weeks. (A) Coculture of lytically induced BCBL-1 cells with MSCs resulted in ratio-dependent expression of LANA, with maximal LANA reactivity of approximately 34%, 48 hours after infection at a BCBL-1/MSC ratio of 9:1. Error bars represent the SD of 4 individual fields of at least 100 cells. Higher BCBL-1/MSC ratios in otherwise identical experiments resulted in even higher infection rates (not shown). (B) Incubation of cell-free KSHV with MSCs resulted in concentration-dependent rates of LANA expression, with maximal infection (> 96%) 48 hours after exposure of the culture to approximately 9300 KSHV genome equivalents/cell. Error bars as per panel A. In parallel experiments, identical aliquots of input KSHV (and virus-to-cell ratios) achieved similar rates of LANA expression within T4 TIME cells (96% LANA reactivity), although LANA expression decreased more rapidly over time in these cells compared to MSCs (40% reactivity for MSC at 3 weeks versus 15% for TIME cells, data for TIME cells discussed in Tomescu et al14 ). (C) Time course following LANA IFA reactivity of MSCs infected with cell-free KSHV (with a genome equivalents to target cell ratio of 5900:1). Data reflect LANA reactivity (mean ± range) in 2 settings: (1) infection of MSCs from a single donor in 2 separate experiments using KSHV isolated from separate BCBL-1 stocks (dotted line); and (2) infection of MSCs isolated from 3 separate donors and infected with KSHV from the same viral stock (solid line). Note: after a 7- to 9-day lag in the growth of the MSC culture following infection, expansion of the population resumed with a constant doubling time of approximately 30 hours through week 6 (not shown).
Among LANA-positive cells, spontaneous lytic reactivation was nearly absent (< 0.08% of cells). In contrast, after addition of 12-O-tetradecanoylphorbol-13-acetate (TPA) and sodium butyrate we observed expression of the immediate early lytic protein, RTA, in approximately 8% of cells (see the Supplemental Figure link at the top of the online article on the Blood website)—a treatment that induces viral reactivation in latently infected PEL cell lines.19 However, immunofluorescence (IFA) detected only rare (< 0.08%) cells expressing lytic proteins MIR-1, MIR-2, and MCP. These results suggest that these cells, under the conditions of our assay, are capable of at least the earliest steps of lytic reactivation but emphasize the inherent bias toward latency, a phenomenon observed with other de novo infected primary and immortalized cells, including telomerase-immortalized endothelial (TIME) cells.14,17,20 Finally, concentrated supernatants from infected MSCs were unable to transmit KSHV to naive cells (MSC, pDMVEC, or T4 TIME cells) and contained no detectable encapsidated viral DNA (not shown).14
To assess the stability of KSHV infection within MSCs, we tracked the proportion of LANA-positive cells over time (Figure 2C). Initial infection was evident in approximately 85%-90% of the MSC during the first week. By weeks 3 and 6, this proportion dropped to approximately 45% and 30%, respectively. By weeks 9 and 12 after infection, LANA reactivity fell progressively, but declining cell viability and growth rates confounded the interpretation of the data at these late time points. Nevertheless, a pattern of gradual loss of LANA reactivity in the setting of undetectable spontaneous viral production mirrors our own results with both primary and immortalized human endothelial cells14 and the observations of others with a variety of other cell types.17
Although the loss of LANA reactivity from initially infected MSCs could reflect a growth advantage of uninfected over infected cells, we observed no such advantage in uninfected compared to infected cultures. Further, recently published data in immortalized cell lines demonstrate that the progressive loss of transfected DNA plasmids containing the KSHV origin of replication in the setting of stable LANA expression was not due to growth advantages of untransfected cells but to another, as yet poorly understood, mechanism.17
In human patients, infected MSCs could potentially contribute to known KSHV-related pathology by directly or indirectly affecting known targets of KSHV, including CD34+, CD19+, and CD14+ cells.21 This might occur through horizontal transmission of virus within a potentially more permissive in vivo environment or through indirect influence on hematopoietic cells via paracrine mechanisms.22 Interestingly, subsets of CD14+ cells are capable of endothelial cell transformation21,23 and share common cell surface markers with spindle cells—the cellular hallmark of KS.24 Moreover, data suggesting that MSCs modulate neoangiogenesis within murine lymphoma tissue in immunocompromised mice11 and also localize to perivascular structures25 make even more attractive the idea that MSCs may play a direct role in KSHV pathogenesis.
The present study is the first to demonstrate the ability of KSHV to infect primary MSCs, bone marrow progenitor cells that play an essential role in hematopoiesis. Future exploration of in vivo KSHV infection of MSC in animal models, as well as infected human patients, should shed further light on the role that these critical bone marrow cells may play in KSHV-related disease.
Prepublished online as Blood First Edition Paper, July 6, 2004; DOI 10.1182/blood-2004-02-0693.
Supported in part by a Farrow Fellowship (C.H.P.), by the Elizabeth Glaser Pediatric AIDS Foundation (D.H.K.), and by the Pew Scholars Program in Biological Sciences (D.H.K.).
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Costin Tomescu for careful review of this manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal