Abstract
Cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells use the perforin/granzyme pathway as a major mechanism to kill pathogen-containing cells and tumor cells.1,2 Dysregulation of this pathway results in several human diseases, such as hemophagocytic lymphohistiocytosis. Here we characterize the single-cell expression pattern of granzymes A and B in human lymphocytes using a flow cytometry-based assay. We demonstrate that most circulating CD56+8- NK cells, and approximately half of circulating CD8+ T lymphocytes, coexpressed both granzymes A and B. In contrast, few circulating CD4+ T lymphocytes expressed granzymes A or B. Activation of CD8+ T lymphocytes with concanavalin A (ConA)/interleukin-2 (IL-2), and activation of CD4+ T lymphocytes with antibodies to CD3/CD28 or CD3/CD46 (to generate T regulatory [Tr1] cells), induced substantial expression of granzyme B, but not granzyme A. Naive CD4+CD45RA+ cells stimulated with antibodies to CD3/CD46 strongly expressed granzyme B, while CD3/CD28 stimulation was ineffective. Finally, we show that granzyme B-expressing CD4+ Tr1 cells are capable of killing target cells in a perforin-dependent, but major histocompatibility complex (MHC)/T-cell receptor (TCR)-independent, manner. Our results demonstrate discordant expression of granzymes A and B in human lymphocyte subsets and T regulatory cells, which suggests that different granzymes may play unique roles in immune system responses and regulation.
Introduction
The innate and adaptive immune systems include cytotoxic lymphocytes (natural killer [NK] and cytotoxic T lymphocyte [CTL] cells) that are important for responses to intracellular pathogens and tumor cells. Although there are several mechanisms by which these cells kill (eg, Fas/Fas ligand, tumor necrosis factor [TNF]/TNF receptor, and Toll receptors), the major mechanism is that of the perforin/granzyme (granule exocytosis) pathway.1-4 This pathway is critical for host mechanisms of defense, including viral clearance and tumor cell killing. Dysregulation of this pathway is associated with a number of human diseases, such as hemophagocytic lymphohistiocytosis, Griscelli syndrome, and X-linked lymphoproliferative disease.1,5-7
The granzymes are serine proteases that are packaged into the specialized cytotoxic granules of CTL and NK cells. It is believed that individual cells are armed with both perforin and granzymes, and that expression is limited to CD8+ T lymphocytes and NK cells.8 Although human CD4+ T lymphocyte clones have been shown to express perforin and granzymes, the functional significance of the granule exocytosis pathway in these cells has not yet been clearly established.9-14
Recent data from Kelso et al have suggested that individual cells may express different combinations of granzymes, implying an additional level of control in the process of cellular cytotoxicity.15 Most studies of granzyme expression have relied on Northern blots, RNA protection assays, and/or reverse-transcription-polymerase chain reaction (RT-PCR) analysis to define cellular expression patterns. In addition, the majority of these studies have analyzed bulk cell populations.8 There are 2 prior studies that have reported single-cell expression of either granzyme A or granzyme B in human CD8+ T-cell subsets using intracellular flow cytometry.16,17
In this report, we examine the dual expression patterns of both granzymes A and B using granzyme-specific monoclonal antibodies in an intracellular flow cytometry assay. We used this assay to characterize the expression patterns of granzymes A and B in resting and activated human peripheral blood mononuclear cells (PBMCs). Most CD56+8- NK cells, nearly all CD56+8+ NKT cells, and approximately half of the circulating CD8+ T lymphocytes were found to coexpress both granzymes A and B. While few resting CD4+ T lymphocytes expressed granzyme A or B, activation of CD4+ T lymphocytes with concanavalin A (ConA)/interleukin-2 (IL-2), or with antibodies directed against CD3/CD28 or CD3/CD46, induced high levels of granzyme B expression, but not granzyme A. Naive CD4+CD45RA+ T cells stimulated with antibodies to CD3/CD46 to generate adaptive Tr cells strongly expressed granzyme B, while stimulation with antibodies to CD3/CD28 was ineffective at driving granzyme B expression. In contrast, memory CD4+CD45RO+ T cells were found to express granzyme B with both modes of stimulation. Finally, we show that these activated granzyme B-expressing CD4+ T lymphocytes exhibit perforin-dependent cytotoxicity against allogeneic target cells in a major histocompatibility complex (MHC)/T-cell receptor (TCR)-independent manner. These results demonstrate a previously unrecognized, discordant expression pattern between human granzymes A and B, and suggest that different subsets of effector cells may use different granzymes to kill target cells. In addition, the strong expression of granzyme B in adaptive Tr1 regulatory cells suggests that the granule exocytosis pathway may be involved in regulating immune responses.
Materials and methods
Isolation and stimulation of human PBMCs and CD4+ T lymphocytes
Human peripheral blood samples were collected from healthy donors in accordance with protocols approved by the Washington University School of Medicine (WUSM) Human Studies Committee and Internal Review Board. All procedures followed were in accordance with institutional ethical standards and with the Helsinki Declaration of 1975, as revised in 2000. Peripheral blood mononuclear cells (PBMCs) were obtained using Ficoll-Paque (Amersham, Piscataway, NJ). For concanavalin A (ConA) stimulation of PBMCs, 1 × 107 cells were incubated for 3 days in K10 media containing 50 U/mL recombinant human IL-2 (rhIL-2, kind gift of Dr J. DiPersio) and 5 μg/mL ConA (Sigma, St Louis, MO). For isolation and preparation of adaptive CD4+ T regulatory (Tr1) cells, human PBMCs were positively selected with anti-CD4 antibodies (OKT4; American Type Culture Collection [ATCC], Manassas, VA), washed, labeled with goat anti-mouse magnetic beads, and then isolated over a magnetic column (Miltenyi Biotec, Bergisch Gladbach, Germany). CD4+ lymphocytes were routinely more than 96% pure as determined by flow cytometry. Purified CD4+ lymphocytes were cultured on plates coated with antibodies to CD3 (OKT3 or Hit3a; Pharmingen, San Diego, CA), and CD28 (28.2; Pharmingen) or CD46 (TRA-2-10) in complete RPMI containing 10 U/mL rhIL-2 as previously described.18 After 4 days of culture, cells were analyzed by flow cytometry for granzyme expression, cytotoxicity, IL-10 production, and surface marker expression to reconfirm the cell phenotype. Purification of naive (CD4+CD45RA+) and memory (CD4+CD45RO+) T cells was performed in a similar manner as described above.
Animals
Wild-type B6 mice were obtained from Taconic (Germantown, NY). Granzyme B knock-out mice expressed normal levels of all other known murine granzymes.8 All mice were bred and kept in pathogen-free housing in accordance with WUSM animal care guidelines, using protocols approved by the WUSM Animal Studies Committee.
Murine lymphokine activated killer (LAK) cell preparation
Mice used for splenocyte preparations were anesthetized according to institutional animal care guidelines. LAK preparation was as previously described.19 Only adherent LAK cells were analyzed for granzyme B expression by flow cytometry.
Intracellular granzyme staining and flow cytometry
Cells (1 × 106) were labeled with peridinin chlorophyll-alpha protein (Per-CP)-, phycoerythrin (PE)-, Biotin-, and/or fluorescein isothiocyanate (FITC)-conjugated antibodies against cell surface markers (anti-human CD4, CD8, CD16, CD25, CD56, MHC class II-DR, Fas, FasL [Becton Dickinson, Pharmingen]; anti-human MHC class I [A, B, C] [Leinco Technologies, St Louis, MO]). Samples were fixed and permeabilized (Becton Dickinson, Pharmingen), and then stained with primary conjugated anti-granzyme A antibody (CB9; Becton Dickinson-Pharmingen) diluted at 1:100 in staining buffer (Dulbecco phosphate buffer, 1% human albumin, 0.1 μg/μL human immunoglobulin), and/or primary conjugated antigranzyme B antibody (GB12; Caltag, Burlingame, CA) diluted at 1:400 in staining buffer. During all steps of staining, permeabilization, and washing, human immunoglobulins (0.1 μg/μL final concentration; Gammaguard; Baxter Healthcare, Deerfield, IL) and human albumin (1%; Aventis Behring, Bridgewater, NJ) were used to block nonspecific Fc receptor antibody binding. Samples were analyzed on a FACScan (Becton Dickinson). All fluorescence-activated cell-sorter (FACS) scans depicted are representative of 4 or more donors. Anti-granzyme A and anti-granzyme B antibodies were analyzed for antigen specificity by competitive assays using recombinant granzyme A (Kamiya Biomedical, Seattle, WA) and/or granzyme B proteins20 (10:1 molar ratio). Statistical analyses were performed by one-way analysis of variance (ANOVA) with Bonferroni posttest analysis, or Student t test, using GraphPad Prism version 3.0a for Macintosh (GraphPad Software, San Diego, CA).
Flow-based killing assay (FloKA)
A flow-based killing assay was developed to measure in vitro cellular cytotoxicity of CD4+ lymphocytes (unactivated or activated) in a manner previously described.21 Human cell lines (U937, K562, and BJAB) were washed with phosphate-buffered saline (PBS), resuspended at 1 × 106 cell/mL, and then labeled at 37°C for 15 minutes with 125 nM final concentration of 5- (and 6-) carboxyfluorescein diacetate succinimydyl ester (CFSE; Molecular Probes, Eugene, OR). Labeling reactions were stopped with complete RPMI media. Labeled target cells (1 × 105) were added to 96-well V-bottom tissue culture-treated plates (Corning, Corning, NY) along with indicated effector cells in complete RPMI media containing 50 U/mL rhIL-2. The effector-target (E/T) ratio was 20:1 for all time points shown. Immediately before analysis, 1 μg/mL (final concentration) 7-aminoactinomycin D (7-AAD; Calbiochem, La Jolla, CA) was added to each sample. 7-AAD incorporation is used as a surrogate marker for late cell death/apoptosis, intercalating with DNA in cells that have lost membrane integrity. To analyze the role of the perforin/granzyme pathway in killing, EGTA (ethylene glycol tetraacetic acid, 4 mM; Calbiochem) was added to inhibit calcium-dependent perforin polymerization. Similar inhibition of cytotoxicity was seen using the perforin inhibitor concanamycin A (CMA, 100 nM; Wako, Richmond, VA). However, CMA was found to be substantially toxic to both target and effector cells over time (data not shown). EGTA was not found to be toxic to either target or effector cells, thus it was used for the majority of cytotoxicity experiments to determine the role of perforin-induced cell death. For Fas ligand blocking experiments, 10 μg purified anti-human Fas ligand antibody (DX2; Becton Dickinson) was added to each sample of effector cells and incubated at room temperature for 15 to 30 minutes prior to adding target cells. All samples were briefly centrifuged prior to incubating at 37°C for the indicated times. FloKA assays were compared with standard chromium release assays and gave similar results (data not shown).21 All cytotoxicity assays were performed in duplicate or triplicate. Data presented are representative of 3 or more individual cytotoxicity experiments.
Results
Expression of granzymes A and B in resting versus ConA/IL-2-activated human PMBC subsets
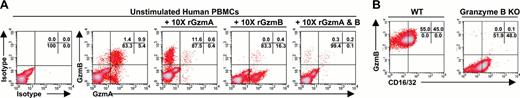
A flow-based assay was used to detect granzyme expression in single cells using monoclonal antibodies directed against human granzyme A or B. Cells were first stained with cell surface-specific antibodies, and then fixed, permeabilized, and stained with granzyme-specific antibodies. We first established antibody specificity by premixing the antibodies with recombinant granzymes before staining human PBMCs. A 10-fold molar excess of recombinant granzyme A and/or B effectively competed away the intracellular staining of each respective granzyme antibody (Figure 1A). The human granzyme B-specific antibody cross-reacted with murine granzyme B in lymphokine activated killer cells (LAKs). The flow cytometry signal was absent in granzyme B-deficient LAK cells (Figure 1B), further confirming the specificity of this antibody. The human granzyme A-specific antibody did not cross-react with murine granzyme A, so specificity could not be defined in the knock-out setting (data not shown).
Antibodies against human granzyme A or B show specificity in recombinant protein competition assays and in knock-out cells. (A) Competition assays with 10-fold molar excess of recombinant granzyme (rGzm) A and/or granzyme B demonstrate anti-granzyme A and B antibody specificities in unstimulated healthy human PBMCs. (B) Anti-granzyme B antibody specificity in wild-type (WT) murine LAKs versus granzyme B knock-out (KO) LAKs. Percentages of total cells for each quadrant are indicated in the upper right corner of each subpanel.
Antibodies against human granzyme A or B show specificity in recombinant protein competition assays and in knock-out cells. (A) Competition assays with 10-fold molar excess of recombinant granzyme (rGzm) A and/or granzyme B demonstrate anti-granzyme A and B antibody specificities in unstimulated healthy human PBMCs. (B) Anti-granzyme B antibody specificity in wild-type (WT) murine LAKs versus granzyme B knock-out (KO) LAKs. Percentages of total cells for each quadrant are indicated in the upper right corner of each subpanel.
We next defined granzyme expression patterns in different lymphocyte compartments. Analysis of resting human PBMCs revealed that nearly all CD56+ cells and approximately half of the circulating CD8+ T cells coexpressed both granzymes A and B (Figure 2A). Small percentages of circulating lymphocytes (primarily CD8+ T cells) expressed only granzyme A or granzyme B (Figure 2A). Minimal numbers of circulating CD4+ T cells and CD25+ lymphocytes expressed granzyme A or B (Figure 2A). Stimulation of human PBMCs with ConA/IL-2 substantially increased the percentage of CD4+, CD8+, and CD25+ lymphocytes expressing granzyme B (Figure 2A, 3A). In contrast, the percentage of granzyme A-expressing CD8+ lymphocytes decreased by nearly half upon stimulation with ConA/IL-2, while CD56+ cells showed essentially no change in their granzyme expression pattern (Figure 2A, 3A). Similar results were observed in 6 or more healthy donors (Figure 3A).22-26
Single-cell analysis for granzymes A and B demonstrates discordant expression in activated human PBMC subsets. (A) Representative FACS analysis for intracellular granzyme A and B expression in unactivated and activated (ConA/IL-2 for 5 days) healthy human PBMCs. Lineage-specific (CD4, CD8, and CD56) and nonlineage (CD25) surface markers are shown. (B) Representative FACS analysis for granzyme A and B expression in resting CD56+CD8+ T-cell and CD56+CD8- NK cell subsets from 2 different donors. Data are representative of 6 or more healthy donor samples with similar expression patterns. Percentages of total cells in each quadrant are indicated in the upper right corner of each subpanel.
Single-cell analysis for granzymes A and B demonstrates discordant expression in activated human PBMC subsets. (A) Representative FACS analysis for intracellular granzyme A and B expression in unactivated and activated (ConA/IL-2 for 5 days) healthy human PBMCs. Lineage-specific (CD4, CD8, and CD56) and nonlineage (CD25) surface markers are shown. (B) Representative FACS analysis for granzyme A and B expression in resting CD56+CD8+ T-cell and CD56+CD8- NK cell subsets from 2 different donors. Data are representative of 6 or more healthy donor samples with similar expression patterns. Percentages of total cells in each quadrant are indicated in the upper right corner of each subpanel.
Healthy donor summary of granzyme A and B expression in cellular subtypes. (A) Data summary of granzyme A and B expression in unstimulated and stimulated (ConA/IL-2) human CD4+, CD8+, and CD56+ lymphocyte subsets. (B) Data summary of granzyme A and B expression in resting CD56+CD8+ T-cell and CD56+CD8- NK cell subsets. Data are representative of 6 or more donor samples. P values are as shown. NS indicates not significant. Horizontal bars represent the mean of each data set.
Healthy donor summary of granzyme A and B expression in cellular subtypes. (A) Data summary of granzyme A and B expression in unstimulated and stimulated (ConA/IL-2) human CD4+, CD8+, and CD56+ lymphocyte subsets. (B) Data summary of granzyme A and B expression in resting CD56+CD8+ T-cell and CD56+CD8- NK cell subsets. Data are representative of 6 or more donor samples. P values are as shown. NS indicates not significant. Horizontal bars represent the mean of each data set.
The delineation between human NK and NKT cells has been based primarily on the surface expression of CD3, the α/α form of CD8, and CD56+. The majority of human NK cells express only CD56+, whereas human NKT cells also express CD3 and the α/α form of CD8.23,26 To further characterize the granzyme expression in CD56+ cell subsets (ie, CD56+8- NK cells and CD56+8+ NKT cells),25 we costained for surface CD56 and CD8 and examined the subsets for granzyme A and B expression. Our analysis showed significant variability in the percentages of CD56+8- NK cells and CD56+8+ NKT cells among donors. Several donors had high percentages of CD56+8+ NKT cells with low percentages of CD56+8- NK cells (Figure 2B, top panels), while other donors had low percentages of CD56+8+ NKT cells with high percentages of CD56+8- NK cells (Figure 2B, bottom panels). Other donors had intermediate percentages of each subset (data not shown). The majority of CD56+8+ NKT cells coexpressed granzymes A and B in all donors examined (Figures 2B, 3B). Although 2 donors with high percentages of CD56+8+ NKT cells had CD56+8- NK cells that lacked granzyme B expression (Figure 3B), most individuals had CD56+8- NK cells that coexpressed granzymes A and B (Figure 3B). Those individuals with CD56+8- NK cells that lacked granzyme B had high percentages of CD56+8+ NKT cells that expressed granzyme B (Figure 2B). A summary of the relative percentage of granzyme A and B expression in human peripheral blood subsets is summarized in Table 1.
Relative percentages of granzyme A- and B-expressing cells in human blood subsets
Cell type . | GzmA . | GzmB . |
|---|---|---|
| B cells | — | — |
| PMNs* | — | — |
| CD8+ cells | ||
| Circulating (CD8+CD56-) | ++ | ++ |
| Circulating (CD8+CD56+) | +++ | ++ |
| ConA/IL-2 | + | +++ |
| IL-2 | + | + |
| NK cells | ||
| Circulating (CD56+) | +++ | +++ |
| Circulating (CD56+8-) | +++ | ++/+++ |
| PBMCs (NK and CD8+) | ||
| Circulating | + | + |
| ConA/IL-2 | -/+ | +++ |
| CD4+ cells | ||
| Circulating | -/+ | -/+ |
| ConA/IL-2 | -/+ | +++ |
| IL-2 | -/+ | -/+ |
| CD3/CD28 | -/+ | ++/+++ |
| CD3/CD46 (Tr1) | -/+ | +++ |
| CD4+CD45RA+ (naive) | ||
| IL-2 | -/+ | -/+ |
| CD3/CD28 | -/+ | -/+ |
| CD3/CD46 | -/+ | +++ |
| CD4+CD45RO+ (memory) | ||
| IL-2 | -/+ | -/+ |
| CD3/CD28 | -/+ | +++ |
| CD3/CD46 | -/+ | +++ |
Cell type . | GzmA . | GzmB . |
|---|---|---|
| B cells | — | — |
| PMNs* | — | — |
| CD8+ cells | ||
| Circulating (CD8+CD56-) | ++ | ++ |
| Circulating (CD8+CD56+) | +++ | ++ |
| ConA/IL-2 | + | +++ |
| IL-2 | + | + |
| NK cells | ||
| Circulating (CD56+) | +++ | +++ |
| Circulating (CD56+8-) | +++ | ++/+++ |
| PBMCs (NK and CD8+) | ||
| Circulating | + | + |
| ConA/IL-2 | -/+ | +++ |
| CD4+ cells | ||
| Circulating | -/+ | -/+ |
| ConA/IL-2 | -/+ | +++ |
| IL-2 | -/+ | -/+ |
| CD3/CD28 | -/+ | ++/+++ |
| CD3/CD46 (Tr1) | -/+ | +++ |
| CD4+CD45RA+ (naive) | ||
| IL-2 | -/+ | -/+ |
| CD3/CD28 | -/+ | -/+ |
| CD3/CD46 | -/+ | +++ |
| CD4+CD45RO+ (memory) | ||
| IL-2 | -/+ | -/+ |
| CD3/CD28 | -/+ | +++ |
| CD3/CD46 | -/+ | +++ |
+/++/+++ indicates the relative percentage of cells demonstrating intracellular granzyme (Gzm) expression based on intracellular flow cytometry analysis. PMNs indicates polymorphonuclear cells; —, none detected; ++, 25% to 50%; +++, more than 75%; +, 10% to 25%; ++/+++, 50% to 75%; and -/+, less than 10%.
Grossman and Ley27
Induction of perforin and granzyme B expression in activated human CD4+ T lymphocytes
Given the substantial induction of granzyme B in the CD4+ T-cell compartment after stimulation with ConA/IL-2, we examined purified CD4+ T cells for their ability to express granzyme B following activation with more physiologic stimuli. We assessed 3 forms of activation, including IL-2 only, or IL-2 in conjunction with plate-bound antibodies directed against CD3/CD28 or CD3/CD46 receptors. We chose CD3/CD28 as a classic T-cell activation strategy, and CD3/CD46 as a means to generate adaptive CD4+ T regulatory (Tr1) cells. We have previously shown that coligation of the T-cell receptor and the complement regulatory protein CD46 (ie, membrane cofactor protein/MCP) results in the generation of a Tr1 cell phenotype. These cells produce IL-10 and suppress the proliferation of bystander T cells.18
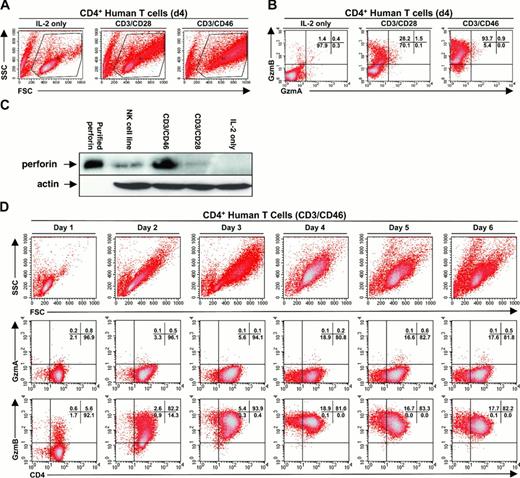
Activated CD4+ T lymphocytes displayed a progressive increase in forward scatter (size) and side scatter (granularity). CD3/CD46-activated Tr1 cells showed the greatest increase in side scatter when compared with IL-2 or CD3/CD28-activated cells (Figure 4A). The observed increase in granularity corresponded with an increase in the percentage of cells expressing granzyme B, but not granzyme A (Figure 4B). Western blots revealed that Tr1 cells contained more perforin than CD3/CD28-activated CD4+ T lymphocytes (Figure 4C). To further analyze the kinetics of granzyme B expression in CD4+ T lymphocytes, we examined CD3/CD46-activated CD4+ T lymphocytes over 6 days from the same donor. The results of this examination showed peak granzyme B expression at days 3 to 4 (Figure 4D, bottom panels), which corresponded with their maximum increase in cell size/granularity (Figure 4D, top panels). After day 4, the activated CD4+ T lymphocytes showed a decrease in their mean fluorescence intensity for granzyme B expression (Figure 4D, bottom panels), corresponding with their decrease in cell size/granularity (Figure 4D, top panels). No significant granzyme A expression was observed at any time (Figure 4D, middle panels).
Purified human CD4+ T lymphocytes demonstrate activation-dependent expression of granzyme B but not granzyme A. (A) Human CD4+ T lymphocytes exhibit a differential increase in side scatter (SSC)/granularity and forward scatter (FSC)/size when activated by IL-2, CD3/CD28, or CD3/CD46. (B) Granzyme B expression in human CD4+ T cells activated by IL-2, CD3/CD28, or CD3/CD46. (C) Western blot analysis for perforin expression (top panel) in purified human CD4+ T lymphocytes activated by IL-2, CD3/CD28, or CD3/CD46. Partially purified perforin is shown as a positive control. The Western blot was reprobed (bottom panel) with an antiactin antibody to assess for protein loading. (D) Representative FACS analysis demonstrating the kinetics of activation and granzyme expression in CD3/CD46-stimulated CD4+ T lymphocytes over 6 days. Data shown are representative of 3 or more independent donor samples. Percentages of total cells in each quadrant are indicated in the upper right corner of each subpanel in panels B and D.
Purified human CD4+ T lymphocytes demonstrate activation-dependent expression of granzyme B but not granzyme A. (A) Human CD4+ T lymphocytes exhibit a differential increase in side scatter (SSC)/granularity and forward scatter (FSC)/size when activated by IL-2, CD3/CD28, or CD3/CD46. (B) Granzyme B expression in human CD4+ T cells activated by IL-2, CD3/CD28, or CD3/CD46. (C) Western blot analysis for perforin expression (top panel) in purified human CD4+ T lymphocytes activated by IL-2, CD3/CD28, or CD3/CD46. Partially purified perforin is shown as a positive control. The Western blot was reprobed (bottom panel) with an antiactin antibody to assess for protein loading. (D) Representative FACS analysis demonstrating the kinetics of activation and granzyme expression in CD3/CD46-stimulated CD4+ T lymphocytes over 6 days. Data shown are representative of 3 or more independent donor samples. Percentages of total cells in each quadrant are indicated in the upper right corner of each subpanel in panels B and D.
Differential granzyme B expression between naive and memory CD4+ lymphocytes
The above data examined granzyme expression in bulk CD4+ T lymphocytes and showed the strongest expression of granzyme B in adaptive (ie, CD3/CD46 stimulated) Tr1 cells. Since CD4+ T lymphocytes activated through CD3/CD28 also expressed granzyme B, we set out to examine granzyme expression between naive (CD4+CD45RA+) and memory (CD4+CD45RO+) cells. We first flow-purified CD4+CD45RA+ naive and CD4+CD45RO+ memory T cells (Figure 5A), activated them using 3 different modes of stimulation (IL-2 only, CD3/CD28, and CD3/CD46), and then examined them for granzyme A and B expression. The results showed that memory T cells were more rapidly activated by CD3/CD28 or CD3/CD46, as defined by an earlier increase in cell size and granularity (Figure 5B). Stimulation of naive T cells with antibodies to CD3/CD46 induced a large percentage of cells expressing granzyme B at day 4, with a lesser amount at day 2 (Figure 5C). In contrast, CD3/CD28 stimulation was ineffective at inducing granzyme B expression in naive T cells at both time points. Activation of memory T cells with CD3/CD28 resulted in similar levels of granzyme B expression compared with CD3/CD46 stimulation at both time points (Figure 5C). At no time point was granzyme A expression observed in either naive or memory T cells (data not shown).
Differential activation and granzyme B expression in purified human naive and memory CD4+ T lymphocytes. (A) FACS plot demonstrating the gates used to flow-purify CD4+CD45RA+ naive and CD4+CD45RO+ memory T lymphocytes. (B) Representative FACS plots demonstrating the differential kinetics of activation (FSC and SSC) between naive and memory CD4+ T lymphocytes at days 2 and 4. Modes of activation are indicated above each column of FACS plots. (C) Summary of granzyme B expression between naive and memory CD4+ T lymphocytes at days 2 and 4. T-cell subsets and modes of activation are indicated below each column. Data shown are representative of 3 or more independent donor samples.
Differential activation and granzyme B expression in purified human naive and memory CD4+ T lymphocytes. (A) FACS plot demonstrating the gates used to flow-purify CD4+CD45RA+ naive and CD4+CD45RO+ memory T lymphocytes. (B) Representative FACS plots demonstrating the differential kinetics of activation (FSC and SSC) between naive and memory CD4+ T lymphocytes at days 2 and 4. Modes of activation are indicated above each column of FACS plots. (C) Summary of granzyme B expression between naive and memory CD4+ T lymphocytes at days 2 and 4. T-cell subsets and modes of activation are indicated below each column. Data shown are representative of 3 or more independent donor samples.
Target cell killing by activated CD4+ T lymphocytes expressing granzyme B and perforin
Although CD4+ T-cell clones have been shown to express perforin and granzymes, the functional importance of the granule exocytosis pathway in these cells is not clear.9-14 Since we observed significant expression of granzyme B in activated CD4+ T lymphocytes, we tested their ability to kill a variety of cell lines using an in vitro flow-based killing assay (FloKA; Figure 6A-B). Tr1 regulatory cells were capable of inducing 7-AAD positivity (a surrogate for cell death) in 3 human cell lines (ie, U937, K562, BJAB) (Figure 6C; BJAB data not shown). Cytotoxicity was abolished with the perforin inhibitors EGTA or concanamycin A (CMA) (Figure 6C-D). CMA was found to exhibit substantial independent toxicity against effector cells, and therefore we used EGTA for the rest of the experiment (data not shown). The amount of target cell killing correlated with the observed percentage of granzyme B-expressing cells (Figure 4B). IL-2-activated CD4+ T lymphocytes showed minimal killing, while Tr1 regulatory cells (ie, CD3/CD46 activated) exhibited maximal killing (Figure 6C). CD3/CD28 activation demonstrated an intermediate level of killing (data not shown). Supernatants from the activated CD4+ T lymphocytes failed to induce any cytotoxicity against target cells, arguing against a soluble factor (eg, TNFα) being responsible for the induced target cell death (data not shown).
Granzyme B-expressing Tr1 regulatory cells exhibit perforin-dependent cytotoxicity. (A) An example of the flow-based cytotoxicity assay (FloKA) using CFSE-labeled target cells, which can be specifically gated upon and analyzed for 7-AAD incorporation. (B) Representative example of cytotoxicity induced by Tr1 regulatory cells (ie, CD3/CD46-activated CD4+ T lymphocytes), demonstrating time-dependent 7-AAD incorporation in human U937 target cells. Cytotoxicity was blocked by EGTA (4 mM), which blocks calcium-dependent perforin polymerization. Percentage shown indicates the percentage of cells shown in each regional gate depicted. (C) Representative example of cytotoxicity by IL-2-activated CD4+ T lymphocytes versus Tr1 regulatory cells (ie, CD3/CD46 activated), against human target cells (U937 and K562) at 4 and 8 hours. Effector to target (E/T) ratios and EGTA controls are shown. (D) Inhibition of cytotoxicity by concanamycin A (CMA; 100 nM). Data are representative of at least 4 independent experiments showing similar results. Values shown are the mean ± SD; *P < .05; **P < .01; ***P < .001.
Granzyme B-expressing Tr1 regulatory cells exhibit perforin-dependent cytotoxicity. (A) An example of the flow-based cytotoxicity assay (FloKA) using CFSE-labeled target cells, which can be specifically gated upon and analyzed for 7-AAD incorporation. (B) Representative example of cytotoxicity induced by Tr1 regulatory cells (ie, CD3/CD46-activated CD4+ T lymphocytes), demonstrating time-dependent 7-AAD incorporation in human U937 target cells. Cytotoxicity was blocked by EGTA (4 mM), which blocks calcium-dependent perforin polymerization. Percentage shown indicates the percentage of cells shown in each regional gate depicted. (C) Representative example of cytotoxicity by IL-2-activated CD4+ T lymphocytes versus Tr1 regulatory cells (ie, CD3/CD46 activated), against human target cells (U937 and K562) at 4 and 8 hours. Effector to target (E/T) ratios and EGTA controls are shown. (D) Inhibition of cytotoxicity by concanamycin A (CMA; 100 nM). Data are representative of at least 4 independent experiments showing similar results. Values shown are the mean ± SD; *P < .05; **P < .01; ***P < .001.
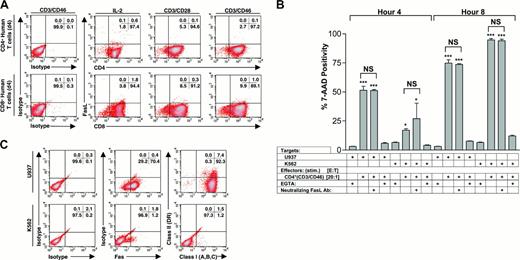
Although the target cell killing was completely inhibited by reagents that directly or indirectly inhibit the action of perforin (ie, CMA and EGTA), we also tested the role of the Fas/Fas ligand (FasL) pathway for the observed killing. We examined the expression of FasL on CD4+ and CD8+ effector T lymphocytes and found minimal expression with any of the modes of activation (ie, IL-2, CD3/CD28, and CD3/CD46; Figure 7A). In addition, a FasL blocking antibody failed to inhibit target cell death (Figure 7B). Furthermore, the different target cell lines varied considerably with respect to surface expression of MHC and Fas molecules. We found that the U937 cells expressed high levels of both Fas and class I (A, B, C) on their surface, while K562 cells expressed essentially no Fas, MHC class I (A, B, C), or MHC class II (DR) (Figure 7C), consistent with prior reports by Day et al.28 These data further suggest that Fas/FasL interaction is not required for the observed cell death, and implicate an MHC-independent mechanism of target cell recognition.
Fas/FasL and MHC class I are not required for Tr1-induced cytotoxicity. (A) FACS analysis for expression of FasL on CD4+ and CD8+ effector T lymphocytes. Methods of stimulation (over 4 days) are shown. (B) A FasL blocking antibody (clone DX2; 10 μg/200 μL) does not block the cytotoxicity of Tr1 regulatory cells against target cells (U937 and K562) at 4 and 8 hours. E/T ratios and EGTA controls for perforin-dependent cytotoxicity are shown. Data are representative of more than 3 independent experiments showing similar results. Values shown are the mean ± SD; *P < .05; **P < .01; ***P < .001. (C) FACS analysis for Fas, MHC class II (DR), and MHC class I (A, B, C) on U937 and K562 cells. Percentage of total cells in each quadrant is shown in the upper right corner of each subpanel in panels A and C.
Fas/FasL and MHC class I are not required for Tr1-induced cytotoxicity. (A) FACS analysis for expression of FasL on CD4+ and CD8+ effector T lymphocytes. Methods of stimulation (over 4 days) are shown. (B) A FasL blocking antibody (clone DX2; 10 μg/200 μL) does not block the cytotoxicity of Tr1 regulatory cells against target cells (U937 and K562) at 4 and 8 hours. E/T ratios and EGTA controls for perforin-dependent cytotoxicity are shown. Data are representative of more than 3 independent experiments showing similar results. Values shown are the mean ± SD; *P < .05; **P < .01; ***P < .001. (C) FACS analysis for Fas, MHC class II (DR), and MHC class I (A, B, C) on U937 and K562 cells. Percentage of total cells in each quadrant is shown in the upper right corner of each subpanel in panels A and C.
Discussion
Previous studies have attempted to examine the cellular expression patterns of granzymes in human lymphocyte populations, but these studies have been difficult because of the high degree of nucleotide similarity between the granzymes. In addition, most of these studies have not demonstrated granzyme expression patterns at the single-cell level. Several previous studies have reported single-cell expression of either granzyme A or granzyme B in human CD8+ T-cell subsets using intracellular flow cytometry.16,17 In the present study, we defined the specificity of 2 antibodies against human granzymes A and B in a flow-based assay using competitive recombinant protein and knock-out assays, allowing for single-cell expression analysis for both granzyme A and granzyme B in human lymphocyte subsets.
Our studies revealed that approximately half of circulating CD8+ T lymphocytes expressed either granzyme A or B, with the majority coexpressing both. Small numbers of CD8+ T lymphocytes expressed only granzyme A or only granzyme B, which is consistent with recently published data from Kelso et al using single-cell RT-PCR.15 In addition, using our flow-based system we were able to determine that activation increased the percentage of granzyme B-expressing CD8+ T lymphocytes, while the percentage of CD8+ T lymphocytes expressing granzyme A significantly decreased. We also demonstrated that there is considerable variability in the percentages of circulating CD56+CD8- NK cells and CD56+CD8+ T cells among donors. The majority of donors had intermediate or high percentages of CD56+CD8- NK cells that coexpressed granzyme A and B in the majority of their cells. There were 2 healthy donors that had low levels of circulating CD56+CD8- NK cells that expressed granzyme A, but not granzyme B, consistent with the findings by Sayers et al in a single donor.29 Interestingly, these donors had high levels of circulating CD56+CD8+ T cells that coexpressed granzymes A and B. The coexpression of granzymes A and B in CD56+CD8+ T cells was also observed in the majority of CD56+CD8+ T cells from all donors.
Analysis of resting CD4+ T lymphocytes showed essentially no expression of either granzyme A or B, consistent with previous reports. Activation of CD4+ T lymphocytes with IL-2 alone resulted in minimal granzyme B expression, whereas generation of Tr1 regulatory lymphocytes through activation of CD3/CD46 induced granzyme B expression in more than 90% of the cells. Activation of CD4+ T lymphocytes with “classical” CD3/CD28 stimulation resulted in an intermediate percentage of granzyme B-expressing cells. None of the activation strategies induced the expression of granzyme A.
When we examined purified naive and memory CD4+ T-cell subsets, we found that few naive CD4+ T cells expressed granzyme B after 2 days of stimulation with either CD3/CD28 or CD3/CD46, while an intermediate percentage of memory cells expressed granzyme B with both modes of stimulation (Figure 5B). After 4 days of stimulation with CD3/CD46, a large percentage of naive CD4+ T cells expressed granzyme B, while stimulation with CD3/CD28 resulted in few granzyme B-expressing cells (Figure 5B). In addition, CD3/CD46 stimulation of naive CD4+ T cells resulted in high levels of IL-10 production, whereas CD3/CD28 stimulation resulted in little IL-10 production. These data suggest that costimulation of naive CD4+ T cells with antibodies to CD46, but not CD28, is able to drive an adaptive Tr1 phenotype. However, both modes of stimulation are able to drive granzyme B expression in memory CD4+ T cells (Figure 5B).
With progressive immune activation, such as with complement-opsonized antigen-antibody immune complex formation and/or activation of the complement receptor CD46, CD4+ T lymphocytes may be induced to express granzyme B to control immune responses. Along these lines, we demonstrated that CD3/CD46-activated Tr1 cells expressed the highest level of granzyme B and were effective at killing unprimed allogeneic target cells in a perforin-dependent manner. Although we and others have shown that supernatants from Tr1 regulatory cells inhibit the proliferation of naive T lymphocytes through the production of IL-10, none of the previous experiments were specifically designed to determine the role of cytotoxicity.18,30,31 Our results suggest that the perforin/granzyme pathway may represent an additional cell-contact mechanism of immune regulation by Tr1 regulatory cells.
Certain cell types have developed mechanisms to protect themselves from the action of granzyme B, suggesting that inhibition of granzyme B appears to be important for both normal immune responses and to prevent immune pathology. For example, mature (but not immature) dendritic cells express the granzyme B inhibitor PI-9 (proteinase inhibitor-9), implying a potential need to protect properly stimulated antigen-presenting cells. In addition, immune-privileged sites such as the placenta, testis, ovary, and eye express high levels of PI-9.32 Perforin/granzyme-expressing adaptive T regulatory cells may also play an important role in autoimmunity, as suggested by an experimental autoimmune encephalomyelitis (EAE) model in which the absence of perforin (but not Fas/FasL) led to the most severe form of EAE in several murine strains.33 Our group has confirmed this observation using granzyme B knock-out mice, which exhibited a significantly more severe form of EAE than wild-type mice (J. Russell and T.J.L., unpublished observations, November 2003).34 These data, along with the findings mentioned here, suggest that the perforin/granzyme pathway may play a role in controlling normal immune responses.
The mechanism(s) by which granzyme B-expressing CD4+ T lymphocytes cells recognize and kill target cells is not yet clear. However, 2 lines of evidence suggest that it is an MHC/TCR-independent process, akin to NK cell target recognition. First, one of our target cell lines (ie, K562 cells) lacks MHC class I and class II expression.28 Secondly, the CD4+ T lymphocyte effector cells were not primed against the allogeneic target cells prior to the killing assays. Studies are currently under way to define the cellular targets for granzyme B-expressing CD4+ T lymphocytes, and the molecules required for target cell recognition.
After this paper was submitted for publication, a report by Sedelies et al demonstrated cross-reactivity of a commercially available anti-granzyme B monoclonal antibody (GB7) with granzyme H.35 However, additional characterization of the recognition epitope of GB7 and the granzyme B monoclonal antibody used in this study (GB12) demonstrated that the 2 antibodies recognize different nonoverlapping epitopes (Kummer et al36 ; M.-H. Jang, Cell Sciences, Canton, MA, written communication, 2004). The data presented here using the GB12 antibody are very similar to the observation by Sedelies et al, who detected an increase in granzyme B expression in activated CD4+ and CD8+ T cells using an anti-granzyme B monoclonal antibody that did not cross-react with recombinant human granzyme H.35 In addition, they demonstrated that expression of granzyme H decreases with activation in CD4+ and CD8+ T-cell subsets, in contrast to our observation of increased granzyme B expression in these cells.35 These data strongly suggest that the GB12 monoclonal antibody used in our study recognizes granzyme B and not granzyme H. Finally, previous studies in our laboratory have also shown that granzyme H is predominantly expressed in the NK/LAK compartments, with much lower levels of expression in T cells.37,38
The flow-based assays described in this paper will help to define subsets of human cytotoxic cells that may have unique roles in killing different target cells. Further definition of these subsets and their physiologic functions will be important in the understanding of a variety of pathologic disease states, as well as normal immune system regulation.
Note added in proof. Since this paper was submitted, Parakishvili et al39 have similarly demonstrated that CD4+/perforin+ T cells from B-CLL patients are able to kill autologous B-CLL cells ex vivo via a perforin-mediated mechanism.
Prepublished online as Blood First Edition Paper, July 6, 2004; DOI 10.1182/blood-2004-03-0859.
Supported by grants by the National Institutes of Health Training Programs (NICHHD 5T32H007499, W.J.G.; NIH 5T32-HD-43010, J.W.V.) and the National Institutes of Health (AI37618, J.P.A.; DK 49786, T.J.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank the Siteman Cancer Center Hi Speed Cell Sorter Core, as well as Talal Chatila, John Russell, Andy Bredemeyer, Michael Rettig, and Paula Revell for their support of this work and for comments on the manuscript.
The license of recombinant murine granzyme B is held by T.J.L. (Sigma). The authors have no other special/competing interests to disclose.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal