Abstract
β2-Integrin clustering on activation is a key event in leukocyte adhesion to the endothelium during the inflammatory response. In the search for molecular mechanisms leading to this clustering, we have identified low-density lipoprotein (LDL) receptor–related protein (LRP) as a new partner for β2-integrins at the leukocyte surface. Immobilized recombinant LRP fragments served as an adhesive surface for blood-derived leukocytes and the U937 cell line. This adhesion was decreased up to 95% in the presence of antibodies against β2-integrins, pointing to these integrins as potential partners for LRP. Using purified proteins, LRP indeed associated with the αMβ2 complex and the αM and αL I-domains (Kd, app ≈ 0.5 μM). Immunoprecipitation experiments and confocal microscopy revealed that endogenously expressed LRP and αLβ2 colocalized in monocytes and U937 cells. Furthermore, activation of U937 cells resulted in clustering of αLβ2 and LRP to similar regions at the cell surface, indicating potential cooperation between both proteins. This was confirmed by the lack of αLβ2 clustering in U937 cells treated by antisense oligonucleotides to down-regulate LRP. In addition, the absence of LRP resulted in complete abrogation of β2-integrin–dependent adhesion to endothelial cells in a perfusion system, demonstrating the presence of a previously unrecognized link between LRP and leukocyte function.
Introduction
Low-density lipoprotein (LDL) receptor–related protein (LRP), also known as α2-macroglobulin receptor or CD91,1 is a member of the LDL-receptor family. It consists of an 85-kDa intracellular and transmembrane domain that is noncovalently linked to a 515-kDa extracellular domain.2 The extracellular domain comprises 4 clusters of complement-type repeats, 2 of which (clusters II and IV) play a dominant role in ligand binding.3,4 At present, more than 30 structurally and functionally unrelated ligands have been identified for this receptor (for a review, see Herz and Strickland5 ), suggesting that LRP is involved in a diverse range of (patho)physiologic processes.
The intracellular domain of LRP harbors 2 NPXY-motifs and 1 YXXL motif; the latter is the main motif that controls internalization of LRP.6 In its function as an endocytic receptor, LRP mediates the cellular uptake of both circulating and membrane-associated proteins, which are subsequently degraded in lysosomes.7 Alternatively, ligands can be transcytosed8 or transported to the nucleus.9 Apart from its endocytic function, LRP has been shown to be involved in signaling pathways.10 In this respect, various intracellular adaptor proteins have been identified that interact with the cytoplasmic tail of LRP through its NPXY motif.11 Moreover, binding of adaptor proteins to the cytoplasmic tail inhibits the internalization of LRP.11 This allows LRP to form heterodimeric complexes with other receptors at the cell surface, such as N-methyl-d-aspartate receptor12 and platelet-derived growth factor receptor.13,14 In these cases, the presence of LRP is essential for appropriate function of the coreceptor.
LRP is expressed in a variety of cell types, including hepatocytes, fibroblasts, neurons, and smooth muscle cells.15 Among blood cells, LRP is expressed in leukocytes, including polymorpho-nuclear cells (PMNs) and monocytes, but not in erythrocytes or platelets.15,16 PMNs and monocytes are essential in the inflammatory process. Leukocytes circulate in blood in a resting state and become activated in response to inflammatory stimuli. Leukocyte activation may induce adhesion to the vascular wall and subsequent migration to inflammatory sites.
Firm adhesion and subsequent transmigration involves adhesion molecules, such as β2-integrins. β2-Integrins are specifically expressed in leukocytes, and 4 isotypes are known.17 β2-integrins consist of a common β2-chain (CD18), which is noncovalently linked to an α-chain, namely αL (CD11a), αM (CD11b), αX (CD11c), or αD (CD11d). The α-chains contain an I-domain, which harbors the main ligand-binding site.18-20 Although all β2-integrin isoforms interact with a wide subset of proteins, ligand recognition appears to be specific among the isoforms. The main ligands of β2-integrins expressed at the cell surface of other cell types are the intercellular adhesion molecules (ICAMs). At present, 5 ICAMs have been described with slightly different binding specificities.21 For instance, ICAM-1, -2, and -4 associate with αL and αM, whereas ICAM-3 and -5 are only recognized by αL.21-23 Furthermore, αL specifically binds to junction adhesion molecule-1,24 and αM binds to junction adhesion molecule-3 and glycoprotein Ibα.25-28 Other β2-integrin ligands are fibrinogen, collagen type I, iC3b, and neutrophil inhibitory factor.20,29,30
Whereas the role of β2-integrins in leukocyte function has been well studied, little is known about the role of LRP in this connection. The present study focused on the identification of leukocyte-surface proteins that associate with LRP. Our results show that LRP is able to interact with β2-integrins. Moreover, LRP appears to regulate αLβ2-integrin clustering and, as such, β2-integrin–mediated adhesion to endothelial cells. These observations identify a previously unrecognized link between LRP and the inflammatory system.
Materials and methods
Materials
Cell culture medium RPMI 1640, Dulbecco modified Eagle medium (DMEM)/F-12, penicillin, streptomycin, and l-glutamine were obtained from Gibco Life Technologies (Paisley, United Kingdom). Fetal bovine serum was from Cambrex Bio Science (Verviers, Belgium). Microtiter plates were from Costar (New York, NY) or Nunc (Roskilde, Denmark). The Biacore2000 system and required reagents were from Biacore AB (Uppsala, Sweden). Phorbol-12-myristate-13-acetate (PMA), methotrexate, P-nitrophenyl phosphate (PNP), polyvinylpyrrolidone-360 (PVP-360), and bovine serum albumin (BSA) fraction V were purchased from Sigma (St Louis, MO).
Antibodies and proteins
The following antibodies were used: anti-αL clone 38 (R&D Systems, Minneapolis, MN), anti-αM clone 44 (BD PharMingen, San Diego, CA) and M1/70 (R&D Systems), anti-β2 clone 68-5A5 (Cymbus, Hants, United Kingdom) and R2E7B,31 goat anti-LRP A-18 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-LRP clones α2-M-R-II2C7, α2-M-R-II4/8 and anti–LRP-fluorescein isothiocyanate (FITC) clone α2-M-R-I4C2 (Biomac, Leipzig, Germany), goat antimouse-FITC (Becton Dickinson, San Jose, CA), antiurokinase-type plasminogen activator receptor (uPAR) clone 62022 (R&D Systems), anti-α4 clone HP2/1 (Immunotech, Westbrook, ME), donkey antigoat-FITC and donkey antimouse–Texas RED (both from Jackson ImmunoResearch, West Grove, PA). Receptor-associated protein32 was purified as a glutathione-S-transferase fusion protein (GST-RAP), as described.33 Purified full-length LRP and stable cell lines expressing recombinant LRP fragments (LRP clusters II and IV) were kindly provided by Dr H. Pannekoek (Department of Biochemistry, University of Amsterdam, The Netherlands). Clusters II and IV were purified from the cell culture supernatant using a GST-RAP Sepharose column, as reported previously.4 The αMβ2 complex and the I-domains of the αM and αL subunit fused to GST were purified as described.34 Human multimeric vitronectin was purified as described.35
Cell lines and culture conditions
Human umbilical vein endothelial cells (HUVECs) were isolated according to the method of Jaffe et al,36 with some modifications.37 Only first and second passages were used for experiments. The monocytic line U93738 was obtained from the American Type Culture Collection (Manassas, VA) (CRL-1593.2) and was maintained in RPMI 1640, 10% fetal bovine serum, 50 U/mL penicillin, 50 μg/mL streptomycin, and 50 μM β-mercaptoethanol in a humidified CO2 (5%) incubator at 37°C. To down-regulate LRP protein expression, U937 cells (5.0 × 105 cells/mL) were incubated with phosphorothioate antisense oligodeoxynucleotides at a concentration of 20 μM for 2 days. Fresh oligonucleotides (10 μM) were added every 24 hours. The sequence of the antisense oligonucleotide was 5′-CGGCGGGGTCAGCAT-3′, which is complementary to the initiation site on LRP mRNA.39 As a control, oligonucleotides having the corresponding sense sequence 5′-ATGCTGACCCCGCCG-3′ were used.39 LRP expression was examined by confocal scanning fluorescence microscopy (see “Confocal scanning fluorescence microscopy”). PMNs were freshly isolated from blood obtained from healthy volunteers by Ficoll-Paque (Amersham-Pharmacia, Uppsala, Sweden) density centrifugation. Erythrocytes were removed from the granulocyte fraction by ice-cold erythrocyte lysis buffer (0.155 mM NH4Cl, 7.4 mM KHCO3, and 0.1 mM EDTA [ethylenediaminetetraacetic acid], pH 7.4). Peripheral blood monocytes were isolated from the mononuclear cell fraction using CD14 microbeads and AutoMACS (Miltenyi-Biotec, Bergisch-Gladbach, Germany). After isolation, cells were directly used for experiments. Cell purity was greater than 95% for PMNs and 90% for monocytes, as examined by CD15 and CD14 detection by flow cytometric analysis, respectively.
Static cell adhesion
In static cell adhesion experiments, LRP clusters II and IV (50 μg/mL) were immobilized in microtiter wells in Tris-buffered saline (pH 7.4) for 16 hours at 4°C. Alternatively, HUVECs were grown in microtiter wells until confluence. HUVECs were stimulated with tumor necrosis factor-α (TNF-α) (100 U/mL) for 4 hours and were subsequently fixed for 15 minutes with 4% paraformaldehyde. Wells were blocked with either 5% BSA (U937 cells) or 0.5% PVP-360 (PMNs/monocytes) for 1 hour at 37°C. Cells (2 × 106/mL) were washed twice with phosphate-buffered saline (PBS) and were activated with 100 nM PMA in DMEM/F-12 supplemented with 0.1% BSA and 1 mM MnCl2 for 15 minutes. Where indicated, cells were preincubated for 15 minutes with specific blocking antibodies (20 μg/mL) against different integrin subunits, or wells coated with LRP cluster II or IV were incubated with 50 μg/mL GST-RAP in the presence of 3 mM CaCl2 for 15 minutes before the addition of cell suspensions. Cells (1.5 × 105/well) were incubated in the microtiter plates for either 60 minutes at 37°C (U937 cells to clusters II and IV) or 30 minutes at room temperature (PMNs/monocytes to clusters II and IV; U937 cells to HUVECs). Non-bound cells were removed by gently washing wells with PBS. Adherent cells were determined by endogenous alkalic phosphatase activity using PNP as a substrate (3 mg/mL in 1% Triton-X100/50 mM acetic acid [pH 5]). Optical density was measured at 405 nm. Alternatively, cells were visualized using light microscopy (Leitz Diaplan; Leica, Rijswijk, The Netherlands) and computer-assisted analysis with OPTIMAS 6.0 software (DVS, Breda, The Netherlands).
Surface plasmon resonance analysis
Surface plasmon resonance (SPR) binding assays were performed using a Biacore2000 biosensor system. LRP was immobilized on a CM5-sensorchip at a density of 7.7 fmol/mm2 using the amine-coupling kit, as described by the manufacturer. A control channel was routinely activated and blocked in the absence of protein. Binding of GST/I-domain fusion proteins to LRP-coated channels was corrected for binding to noncoated channels (less than 5% of binding to coated channels). SPR analysis was performed in 100 mM NaCl, 0.005% Tween-20, 2 mM CaCl2, 2 mM MnCl2, 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) (pH 7.4) at 25°C with a flow rate of 5 μL/min. Regeneration of the sensor chip surface was performed by incubating with 0.1 M H3PO4 for 2 minutes at a flow rate of 5 μL/min. Data obtained from steady state SPR analysis were used for the calculation of the apparent affinity constants (Kd, app), as described.40
Immunoprecipitations
U937 cells (6 × 106) were stimulated for 15 minutes with PMA and were lysed in PBS containing 1% Nonidet-40 and 0.5% DOC at 4°C for 1 hour. Lysates were clarified by centrifugation and precleared by incubation with Protein G–Sepharose. The lysate was immunoprecipitated with anti-LRP or anti-αM antibodies (1 μg/mL) and Protein A– or Protein G–Sepharose, respectively, for 16 hours at 4°C. Immunocomplexes were pelleted, washed, and resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting. Detergent-resistant membrane fractions were isolated as described,41 and immunocomplexes were prepared similarly.
Confocal scanning fluorescence microscopy
U937 cells or blood monocytes were collected on object glasses by cytospin centrifugation (105 cells/spin). Cells were fixed in 4% paraformaldehyde/PBS and were blocked in 2% BSA/0.1% saponin/PBS. Localization of the αL subunit and LRP was detected using mouse anti-αL (1:10) and goat anti-LRP (1:20) antibodies, followed by incubation with donkey antimouse (1:100) and donkey antigoat (1:200) antibodies, which were labeled with Texas RED and FITC, respectively. Cells were mounted in mowiol containing 2.5% 1,4-diazabicyclo[2.2.2]octane. Cells were visualized using Leica DMIRB confocal scanning laser microscope equipped with a 63×/1.40 Plan APO objective lens and a TCS 4D system (Leica, Voorburg, The Netherlands).
Cell adhesion under flow conditions
HUVECs were coated on glass coverslips, grown until confluence, and stimulated with TNF-α (100 U/mL) for 4 hours before perfusion. Cells (2 × 106 cells/mL) were perfused over HUVECs for 10 minutes at a flow rate of 100 μL/min. Wall shear stress was calculated to be 0.8 dyne/cm2. During perfusion, the flow chamber42,43 was mounted on a microscope stage (Axiovert 25; Zeiss, Oberkochen, Germany), which was equipped with a black-and-white charge-coupled device videocamera (Sanyo, Osaka, Japan) and was coupled to a VHS videorecorder. Video images were evaluated for the number of adherent cells, with dedicated routines made in the image analysis software OPTIMAS 6. U937 cells (nontransfected and transfected with sense or antisense LRP-oligonucleotides) that were in contact with the surface appeared as bright white–centered cells after proper adjustment of the microscope during recording.
Statistical analysis
All data are expressed as mean ± SD, unless stated otherwise. Between-group variations were examined using the Student t test. A P value of less than .05 was considered statistically significant.
Results
Leukocytes adhere to immobilized LRP fragments
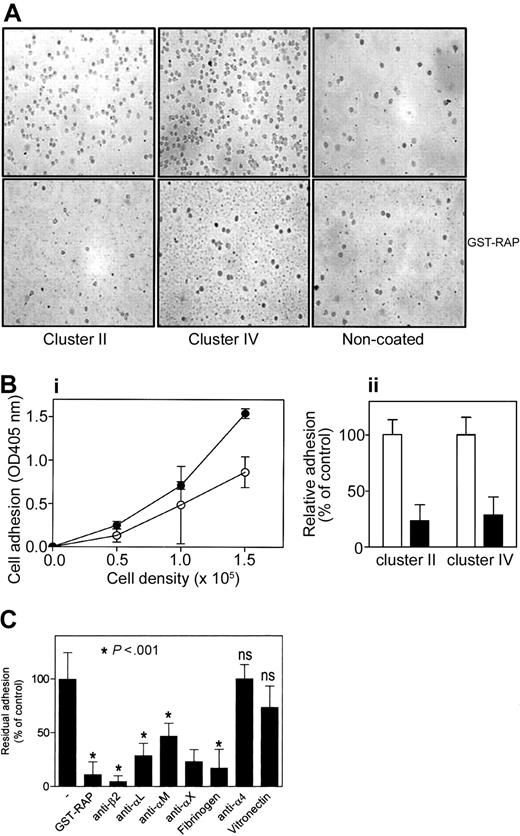
To investigate whether leukocytes express surface proteins that interact with LRP, adhesion of blood monocytes and PMNs—and of the monocytic line U937 to immobilized ligand-binding LRP fragments (ie, clusters II and IV)—was determined. Freshly isolated monocytes were stimulated with PMA and were added to wells coated with cluster II or IV. Each recombinant fragment appeared to provide an adhesive surface for monocytes, as visualized by light microscopy (Figure 1A). Dose-dependence was subsequently tested by measuring endogenous phosphatase activity of adhered cells, which revealed that adhesion to clusters II and IV was cell number dependent (Figure 1Bi). In addition, adhesion to these LRP fragments was decreased by 74% and 71% in the presence of GST-RAP, respectively (Figure 1Bii). Similar data were obtained for PMNs (data not shown) and U937 cells (Figure 1C), indicating that leukocytes express LRP-binding elements at the surface.
Adhesion of monocytes to LRP clusters II and IV. Freshly isolated monocytes were stimulated with 100 nM PMA for 15 minutes and added to immobilized LRP cluster II or IV for 30 minutes at room temperature in the presence or absence of LRP-antagonist GST-RAP (50 μg/mL). (A) Typical experiment visualized by light microscopy, in which 1.5 × 105 cells were added to each well. Original magnification, 400×. (Bi) To quantify cell adhesion, different amounts of cells (0-1.5 × 105 cells/well) were added. After incubation and subsequent washing, bound cells were lysed using 1% Triton-X100/50 mM acetic acid (pH 5.0), and endogenous alkalic phosphatase activity was determined using PNP as substrate. ○ indicates LRP cluster II; •, LRP cluster IV. (Bii) Relative adhesion in the presence (▪) or absence (□) of GST-RAP. Data are corrected for adhesion to uncoated wells (less than 20% of cluster IV coated wells) and represent mean ± SD of 3 experiments performed in duplicate. (C) PMA-stimulated U937 cells (1.5 × 106) were incubated with indicated antibodies (20 μg/mL), fibrinogen (50 μg/mL), or vitronectin (100 μg/mL) for 15 minutes, or wells were preincubated with GST-RAP (50 μg/mL) and added to immobilized IV for 60 minutes at 37°C. Adhered cells were detected as described in panel B. Presented is the percentage of adhesion relative to adhesion in the absence of antibodies or GST-RAP. Data represent the mean ± SD of 3 to 10 experiments performed in duplicate. ns indicates not significant (P > .05).
Adhesion of monocytes to LRP clusters II and IV. Freshly isolated monocytes were stimulated with 100 nM PMA for 15 minutes and added to immobilized LRP cluster II or IV for 30 minutes at room temperature in the presence or absence of LRP-antagonist GST-RAP (50 μg/mL). (A) Typical experiment visualized by light microscopy, in which 1.5 × 105 cells were added to each well. Original magnification, 400×. (Bi) To quantify cell adhesion, different amounts of cells (0-1.5 × 105 cells/well) were added. After incubation and subsequent washing, bound cells were lysed using 1% Triton-X100/50 mM acetic acid (pH 5.0), and endogenous alkalic phosphatase activity was determined using PNP as substrate. ○ indicates LRP cluster II; •, LRP cluster IV. (Bii) Relative adhesion in the presence (▪) or absence (□) of GST-RAP. Data are corrected for adhesion to uncoated wells (less than 20% of cluster IV coated wells) and represent mean ± SD of 3 experiments performed in duplicate. (C) PMA-stimulated U937 cells (1.5 × 106) were incubated with indicated antibodies (20 μg/mL), fibrinogen (50 μg/mL), or vitronectin (100 μg/mL) for 15 minutes, or wells were preincubated with GST-RAP (50 μg/mL) and added to immobilized IV for 60 minutes at 37°C. Adhered cells were detected as described in panel B. Presented is the percentage of adhesion relative to adhesion in the absence of antibodies or GST-RAP. Data represent the mean ± SD of 3 to 10 experiments performed in duplicate. ns indicates not significant (P > .05).
Binding of U937 cells to LRP fragments involves β2-integrins
Binding of leukocytes to LRP cluster IV was examined in more detail using U937 cells. First, GST-RAP was observed to diminish the adhesion of U937 cells to cluster IV by 89% (Figure 1C). Activated leukocytes are characterized by the presence of active adhesion molecules, such as α4β1, αVβ3, and β2-integrins. To assess the contribution of these integrins in the binding of U937 cells to LRP fragments, adhesion was examined in the presence of potential inhibitors. Anti–α4-integrin antibodies and the αVβ3-ligand vitronectin were unable to reduce adhesion (Figure 1C). In contrast, a β2-integrin–directed antibody inhibited the binding of stimulated U937 cells to cluster IV by 95% (Figure 1C). Given that β2-integrins consist of a heterodimeric complex with distinct α-subunits, we also examined the effect of antibodies directed against various α-subunits on cell adhesion. These antibodies decreased adhesion to cluster IV up to 77% (Figure 1C). Adhesion was also reduced in the presence of the β2-integrin ligand fibrinogen (Figure 1C). It should be noted that similar data were obtained when adhesion to cluster II was tested (data not shown). Thus, it appears that LRP provides a binding site for β2-integrins and that αLβ2, αMβ2, and αXβ2 are able to associate with LRP.
LRP comprises a binding site for β2-integrins
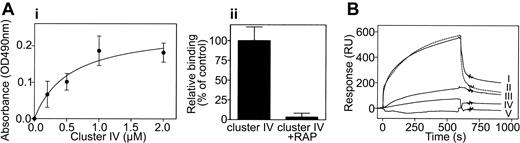
To assess the interaction between LRP and β2-integrins at the level of purified proteins, binding of LRP cluster IV to the αMβ2 complex was assessed in a qualitative manner using an immunosorbent assay. Various concentrations of LRP cluster IV (0-2 μM) were incubated with immobilized αMβ2 (2.5 μg/well), and bound cluster IV was subsequently determined using polyclonal anticluster IV antibodies. Cluster IV bound to immobilized αMβ2 in a dose-dependent and saturable manner, and half-maximum binding was observed at a concentration of 0.5 μM cluster IV (Figure 2Ai). Furthermore, binding of cluster IV to the immobilized complex could be blocked in the presence of GST-RAP (Figure 2Aii). In a second approach, we investigated whether the α-subunits of the αLβ2 or αMβ2 complex contribute to the interaction with LRP. Therefore, SPR analysis was performed using LRP and the recombinant I-domains of both subunits fused to GST. SPR analysis demonstrated that both I-domain/GST fusion proteins associate with immobilized full-length LRP in a reversible manner (Figure 2B, lines I and II), whereas GST alone did not bind to LRP (Figure 2B, line V). In addition, binding of the αM I-domain to LRP was inhibited in the presence of the αM ligand fibrinogen (Figure 2B, line III). Steady state analysis further indicated that the αL and αM I-domains both interact with LRP with an apparent affinity constant of 0.5 μM. These data demonstrate that β2-integrins directly interact with LRP and that binding is at least in part mediated by the α-subunits.
Complex formation between LRP and αMβ2. (Ai) Purified recombinant cluster IV (0-2.0 μM) was incubated with immobilized αMβ2 complex (2.5 μg/well) in Tris-buffered saline/3 mM CaCl2/1 mM MnCl2 (pH 7.4) for 2 hours at 37°C. Bound cluster IV was subsequently determined using peroxidase-labeled polyclonal antibodies directed against cluster IV. Data represent mean ± SEM of 4 experiments. (Aii) Binding of 200-nM cluster IV to immobilized αMβ2 in the presence or absence of a 10-fold excess of GST-RAP. (B) 500 nM purified recombinant I-domain of the αM- (line I) or the αL-subunit (line II) were perfused over LRP immobilized onto a CM5-sensor chip (7.7 fmol/mm2) in 100 mM NaCl, 0.005% Tween-20, 2 mM CaCl2, 2 mM MnCl2, 25 mM HEPES (pH 7.4) at a flow rate of 5 μL/min for 20 minutes at 25°C. Line III: Before perfusion, 500 nM αM was preincubated with a 5-fold molar excess of fibrinogen for 30 minutes. Line IV: 5 μM fibrinogen. Line V: 500 nM GST. Ligand solution was replaced with buffer 10 minutes after injection to initiate dissociation. Depicted are sensorgrams corrected for aspecific binding, which was less than 5% of binding to LRP-coated channels.
Complex formation between LRP and αMβ2. (Ai) Purified recombinant cluster IV (0-2.0 μM) was incubated with immobilized αMβ2 complex (2.5 μg/well) in Tris-buffered saline/3 mM CaCl2/1 mM MnCl2 (pH 7.4) for 2 hours at 37°C. Bound cluster IV was subsequently determined using peroxidase-labeled polyclonal antibodies directed against cluster IV. Data represent mean ± SEM of 4 experiments. (Aii) Binding of 200-nM cluster IV to immobilized αMβ2 in the presence or absence of a 10-fold excess of GST-RAP. (B) 500 nM purified recombinant I-domain of the αM- (line I) or the αL-subunit (line II) were perfused over LRP immobilized onto a CM5-sensor chip (7.7 fmol/mm2) in 100 mM NaCl, 0.005% Tween-20, 2 mM CaCl2, 2 mM MnCl2, 25 mM HEPES (pH 7.4) at a flow rate of 5 μL/min for 20 minutes at 25°C. Line III: Before perfusion, 500 nM αM was preincubated with a 5-fold molar excess of fibrinogen for 30 minutes. Line IV: 5 μM fibrinogen. Line V: 500 nM GST. Ligand solution was replaced with buffer 10 minutes after injection to initiate dissociation. Depicted are sensorgrams corrected for aspecific binding, which was less than 5% of binding to LRP-coated channels.
LRP and β2-integrins are targeted to detergent-insoluble membrane regions
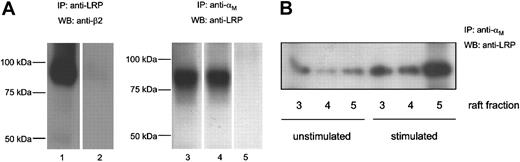
To address the possibility that both receptors associate in the cellular environment, complex formation was assessed in immunoprecipitation experiments using U937 cells. With nonstimulated U937 cells, the β2-subunit was readily immunoprecipitated using an antibody against LRP but not an isotype-matched control antibody (Figure 3A, lanes 1-2). In turn, an anti-αM antibody, but not a control antibody, was able to immunoprecipitate LRP (Figure 3A, lanes 3 and 5). LRP coprecipitated with an anti-αM–integrin antibody to the same extent when cells were stimulated with PMA for 1 hour (Figure 3A, lane 4). Because leukocyte stimulation is associated with the redistribution of β2-integrins at the cell surface to cholesterol-enriched regions,44 we further examined the presence of LRP/β2-integrin complexes in such membrane fractions of resting and stimulated U937 cells. In detergent-insoluble fractions of nonstimulated cells, some coprecipitation of LRP with β2-integrins was observed (Figure 3B, left 3 lanes). An increase in coprecipitated LRP was found on stimulation of U937 cells with PMA (Figure 3B, right 3 lanes), suggesting that the LRP/β2-integrin complex is targeted to cholesterol-enriched membrane regions.
Coimmunoprecipitation of LRP and β2-integrins. (A) U937 cells were lysed for 1 hour on ice and incubated with Protein A-Sepharose and anti-LRP clone α2-M-R-II2C7 (lane 1) or an isotype control (lane 2) at 4°C overnight. Beads were washed extensively and boiled to release bound proteins. Samples were analyzed by SDS-PAGE and Western blotting using anti-β2 (clone R2E7B) and peroxidase-conjugated rat antimouse. Unstimulated (lane 3) or stimulated (lane 4) cells were lysed and incubated with protein G-Sepharose and an anti-αM antibody (clone M1/70) (lanes 3 and 4) or an isotype control (lane 5). Precipitated proteins were analyzed by SDS-PAGE and Western blotting using anti-LRP antibody (clone α2-M-R-II4/8). (B) Lipid rafts were isolated from unstimulated or stimulated cells, as described elsewhere.41 Immunoprecipitations were performed as described in the legend of Figure 3A.
Coimmunoprecipitation of LRP and β2-integrins. (A) U937 cells were lysed for 1 hour on ice and incubated with Protein A-Sepharose and anti-LRP clone α2-M-R-II2C7 (lane 1) or an isotype control (lane 2) at 4°C overnight. Beads were washed extensively and boiled to release bound proteins. Samples were analyzed by SDS-PAGE and Western blotting using anti-β2 (clone R2E7B) and peroxidase-conjugated rat antimouse. Unstimulated (lane 3) or stimulated (lane 4) cells were lysed and incubated with protein G-Sepharose and an anti-αM antibody (clone M1/70) (lanes 3 and 4) or an isotype control (lane 5). Precipitated proteins were analyzed by SDS-PAGE and Western blotting using anti-LRP antibody (clone α2-M-R-II4/8). (B) Lipid rafts were isolated from unstimulated or stimulated cells, as described elsewhere.41 Immunoprecipitations were performed as described in the legend of Figure 3A.
Surface expression of αLβ2 is independent of LRP
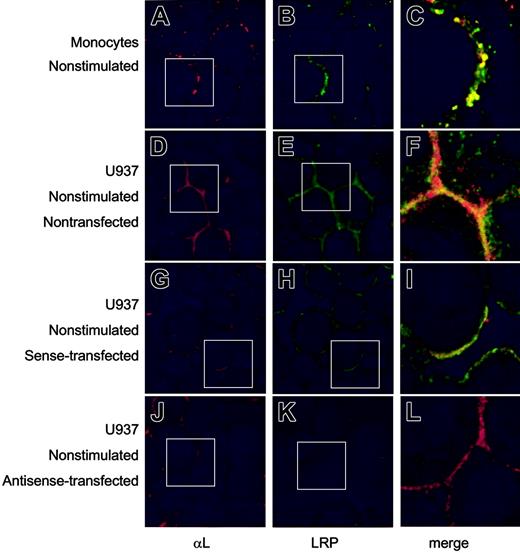
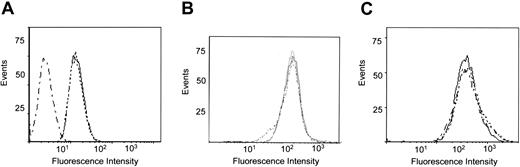
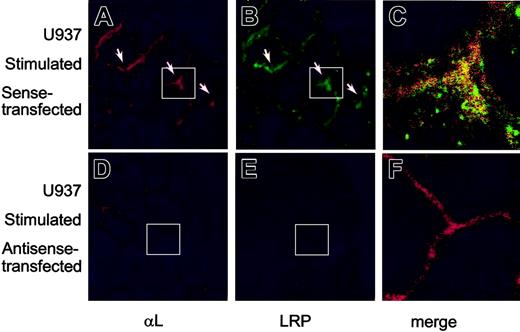
The subcellular localization of endogenously expressed LRP and β2-integrins was further studied by confocal immunofluorescence microscopy. First, the presence of β2-integrins at the cell surface in blood-derived monocytes or the U937 cell line was confirmed using anti-αL antibodies (Figure 4A, D). Similar surface staining was observed for LRP (Figure 4B, E), which overlapped with that of αL to a significant extent (Figure 4C, F). To investigate whether the surface location of αLβ2-integrin was linked to that of LRP, U937 cells were prepared in which LRP expression was down-regulated using LRP-specific phosphorothioate antisense oligodeoxynucleotides. LRP expression appeared to be unaffected when resting cells were treated with sense oligodeoxynucleotides (Figure 4H). In contrast, a strong down-regulation of LRP expression was observed in cells that were transfected with antisense LRP oligodeoxynucleotides (Figure 4K). Down-regulation of LRP-surface expression was examined in a quantitative manner using flow cytometric analysis, which revealed that the amount of surface-exposed LRP was similar in non- and sense-transfected U937 cells but reduced to background levels in antisense-transfected cells (Figure 5A). The expression of the αL subunit at the cell surface remained unaffected in sense- and antisense-transfected cells (Figure 4G, J). Indeed, flow cytometric analysis showed similar mean fluorescence intensities for non-, sense-, and antisense-transfected U937 cells (Figure 5B). In addition, the surface expression of uPAR, which has the potential to associate with LRP and β2-integrins, remained unchanged on transfection of the U937 cells (Figure 5C). These data support the view that the amount of αL expression at the cell surface is independent of the presence of LRP.
Surface expression of LRP and αL-subunit in monocytes and U937 cells. Freshly isolated monocytes (A-C), nontransfected U937 cells (D-F), and U937 cells transfected with sense (G-I) or antisense LRP oligonucleotides (J-L) were collected on object glasses by cytospin centrifugation (105 cells/spin). After fixation, αL-subunit (A, D, G, J) was visualized using monoclonal antibodies and a Texas Red–labeled secondary antibody. LRP (B, E, H, K) was detected using polyclonal antibodies and an FITC-labeled secondary antibody. Original magnification, × 250. (C, F, I, L) Enlarged merge images; original magnification, × 750. White boxes indicate the positions of the enlarged merge images.
Surface expression of LRP and αL-subunit in monocytes and U937 cells. Freshly isolated monocytes (A-C), nontransfected U937 cells (D-F), and U937 cells transfected with sense (G-I) or antisense LRP oligonucleotides (J-L) were collected on object glasses by cytospin centrifugation (105 cells/spin). After fixation, αL-subunit (A, D, G, J) was visualized using monoclonal antibodies and a Texas Red–labeled secondary antibody. LRP (B, E, H, K) was detected using polyclonal antibodies and an FITC-labeled secondary antibody. Original magnification, × 250. (C, F, I, L) Enlarged merge images; original magnification, × 750. White boxes indicate the positions of the enlarged merge images.
Cell surface expression of LRP, αL, and uPAR. Flow cytometric analysis of the cell surface expression of LRP (A), αL (B), and uPAR (C) in non- (—), sense- (....), and antisense- (._._.) transfected U937 cells.
Cell surface expression of LRP, αL, and uPAR. Flow cytometric analysis of the cell surface expression of LRP (A), αL (B), and uPAR (C) in non- (—), sense- (....), and antisense- (._._.) transfected U937 cells.
Reduced PMA-mediated clustering of αL subunit in LRP-deficient cells
Because PMA-mediated stimulation results in the clustering of αLβ2-integrins, we further addressed the effect of PMA stimulation on sense and antisense oligodeoxynucleotide–treated U937 cells. PMA stimulation was indeed associated with an increase in the density of the αL subunit at the cell surface in sense-transfected cells (Figure 6A). A similar increase in density at indicated regions at the cell surface was observed for LRP (Figure 6B-C), suggesting that the translocation of αL and LRP is linked to some extent. With regard to the antisense-transfected cells, no obvious increase in density was observed for the αL subunit (Figure 6D), despite normal expression levels for this subunit. This suggests that clustering of β2-integrins is dependent on the presence of LRP.
Effect of sense and antisense nucleotides on expression of LRP and αL-subunit. U937 cells were transfected with sense (A-C) or antisense oligonucleotides (D-F) and were stimulated with 100 nM PMA for 15 minutes. Cells were collected on object glasses and stained for αL and LRP, as described in the legend to Figure 4. Original magnification, × 250. (C-F) Enlarged merge images; original magnification, × 1375. White boxes indicate the positions of the enlarged merge images.
Effect of sense and antisense nucleotides on expression of LRP and αL-subunit. U937 cells were transfected with sense (A-C) or antisense oligonucleotides (D-F) and were stimulated with 100 nM PMA for 15 minutes. Cells were collected on object glasses and stained for αL and LRP, as described in the legend to Figure 4. Original magnification, × 250. (C-F) Enlarged merge images; original magnification, × 1375. White boxes indicate the positions of the enlarged merge images.
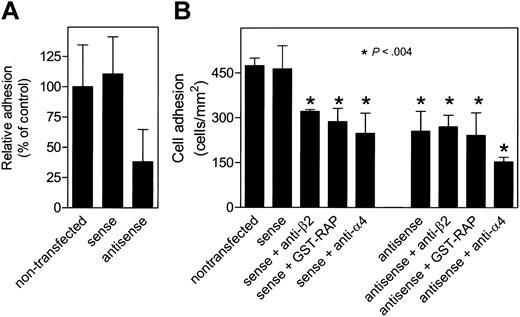
Reduced LRP levels result in impaired β2-dependent adhesion to endothelial cells
β2-Dependent adhesion of leukocytes to the endothelium requires clustering of these integrins. Given that our microscopic analysis suggested that clustering of β2-integrins is LRP dependent, the effect of LRP deficiency on leukocyte adhesion to the endothelial surface was addressed. Therefore, the adhesion of normal U937 cells and U937 cells transfected with sense or antisense LRP oligos to HUVECs was compared. First, in a static adhesion assay using stimulated HUVECs as a surface, sense-transfected U937 cells were similar to untransfected cells (Figure 7A). In contrast, a markedly reduced adhesion was observed for antisense-transfected cells (Figure 7A). This was further addressed in perfusion experiments. As shown in Figure 7B, U937 cells adhered efficiently to the HUVEC layer (474 ± 25 cells/mm2; n = 3). A similar amount of adhesion was detected for sense-transfected cells (463 ± 77 cells/mm2; n = 8; P > .05). Monoclonal antibodies directed against the β2-subunit or the LRP antagonist GST-RAP effectively interfered with the adhesion of sense-transfected cells (321 ± 6 cells/mm2; n = 3; P = .0005 and 287 ± 44 cells/mm2; n = 4; P = .0013, respectively). Thus, the adhesion of U937 cells to endothelial cells is a process that involves β2-integrins and LRP. With regard to antisense-transfected cells, lesser cells were observed to adhere to the endothelial surface compared with non- and sense-transfected cells (255 ± 66 cells/mm2; n = 8; P = .0004 compared with nontransfected cells). Moreover, no further decrease in adhesion was observed in the presence of either anti-β2 antibodies (270 ± 38 cells/mm2; n = 3; P = .0015 compared with nontransfected cells and P > .05 relative to antisense-transfected cells) or the LRP antagonist GST-RAP (241 ± 75 cells/mm2; n = 4; P = .0039 compared with nontransfected cells and P > .05 relative to antisense-transfected cells). In contrast, an additional decrease in adhesion was observed in the presence of an anti-α4 antibody (153 ± 15 cells/mm2; n = 3; P = .003 compared with antisense-transfected cells). In conclusion, these data indicate that the absence of functional LRP is associated with reduced β2-integrin–dependent adhesion to the endothelial surface.
Adhesion of U937 cells to HUVECs under flow conditions. (A) Non-, sense-, and antisense-transfected U937 cells were added to wells coated with HUVECs, which were stimulated with TNF-α (100 U/mL) for 4 hours and fixed afterward. Bound U937 cells were analyzed as described in the legend to Figure 1B. (B) Non-, sense-, and antisense-transfected U937 cells (2 × 106 cells/mL) were perfused for 10 minutes with a wall shear stress of 0.8 dyne/cm2 over a glass coverslip confluently covered with HUVECs, which were stimulated with TNF-α (100 U/mL) for 4 hours before perfusion. Where indicated, cells were preincubated with anti-β2-integrin or anti-α4 antibodies (20 μg/mL) or GST-RAP (50 μg/mL) for 15 minutes. The number of firmly adhered cells per square millimeter was obtained from video-image analysis. Data represent the mean ± SD of 3 to 8 perfusions.
Adhesion of U937 cells to HUVECs under flow conditions. (A) Non-, sense-, and antisense-transfected U937 cells were added to wells coated with HUVECs, which were stimulated with TNF-α (100 U/mL) for 4 hours and fixed afterward. Bound U937 cells were analyzed as described in the legend to Figure 1B. (B) Non-, sense-, and antisense-transfected U937 cells (2 × 106 cells/mL) were perfused for 10 minutes with a wall shear stress of 0.8 dyne/cm2 over a glass coverslip confluently covered with HUVECs, which were stimulated with TNF-α (100 U/mL) for 4 hours before perfusion. Where indicated, cells were preincubated with anti-β2-integrin or anti-α4 antibodies (20 μg/mL) or GST-RAP (50 μg/mL) for 15 minutes. The number of firmly adhered cells per square millimeter was obtained from video-image analysis. Data represent the mean ± SD of 3 to 8 perfusions.
Discussion
Many receptors have evolved to fulfill one specific function, though a subset of receptors is known to display multispecificity and multifunctionality.45 One such example is LRP, a member of the LDL-receptor family that, since its first description in 1988, has been classified as an endocytic receptor.1 However, in the past few years, it has become evident that LRP function encompasses other processes as well. For instance, LRP expressed in brain vascular endothelial cells is involved in the regulation of the vascular tone and permeability of the blood-brain barrier,46 whereas LRP expressed in primary neurons mediates calcium signaling through N-methyl-d-aspartate receptors.12 This suggests that the functionality of LRP is dependent on the cell type in which this receptor is expressed.
LRP is abundantly present in leukocytes,15,16 but its contribution to leukocyte function has remained poorly understood. In the present study, we obtained evidence that LRP is able to bind to leukocyte-specific β2-integrin complexes: (1) adhesion of the monocytic cell line U937 to recombinant fragments of LRP, ie clusters II and IV, could be inhibited by antibodies directed against β2-, αL-, αM-, or αX-subunits (Figure 1C); (2) LRP and β2-integrins coprecipitated in immunoprecipitation experiments (Figure 3); (3) recombinant fragments of the αM- and αL-subunits interacted with full-length LRP (Figure 2B); (4) LRP cluster IV displayed binding to purified αMβ2-integrin (Figure 2A).
The integrin superfamily has been reported to comprise 18 different α-subunits and 8 different β-subunits, which can combine to make up to 24 different heterodimers. To the best of our knowledge, the β2-integrin subfamily is the first to be reported to bind LRP. It should be mentioned that LRP has recently been implicated to promote the maturation and intracellular trafficking of β1-integrins.47 Furthermore, LRP has been reported to mediate endocytosis of complexes between plasminogen activator inhibitor-1 and the αV-subunit.48 However, no evidence of direct interaction between these integrin subunits and LRP could be obtained in these studies. Indeed, we could not detect any inhibition of cell adhesion to LRP fragments in the presence of inhibitors of α4β1or αVβ3 (Figure 1C).
β2-Integrins and LRP are transmembrane proteins containing cytoplasmic and extracellular domains. Our results obtained from cell adhesion and protein-interaction assays demonstrate that at least some of the interactive sites are located in the cluster II and IV regions of LRP and the I-domain of the α-subunits (Figures 1, 2), both of which are part of the respective extracellular regions. Cell adhesion was also inhibited in the presence of the anti-β2 antibody. It seems reasonable to assume that this subunit is involved in complex assembly as well. Alternatively, antibody binding to the β2-subunit may prevent binding to the complementary subunit by sterical hindrance. With regard to the cytoplasmic regions of the receptors, it is unclear whether they are involved in complex formation. This aspect is currently under investigation.
We considered the possibility that LRP is involved in the removal of β2-integrins from the cell surface. As such, the amount of β2-integrins expressed at the cell surface would be increased on the down-regulation of LRP. However, examination of αL (Figure 5B) or αM (data not shown) surface expression using flow cytometric analysis revealed that sense- and antisense-transfected monocytic U937 cells display a fluorescence response similar to that of nontransfected cells. Apparently, the absence of LRP leaves the amount of αL or αM at the cell surface unaffected, suggesting another function for the interaction between LRP and β2-integrins. Data obtained from immunoprecipitation experiments (Figure 3A) and confocal microscopy (Figure 4) point to the possibility that β2-integrins and LRP form a complex at the cell surface. Indeed, we observed not only a cooperative clustering of both receptors (Figure 6) but also an increase of β2-integrin/LRP complex in detergent-insoluble membrane fractions (Figure 3B). The notion that both receptors have the potential to be present in detergent-resistant membrane fractions is in line with previous reports showing the presence of β2-integrins and LRP in lipid rafts of leukocytes and smooth muscle cells, respectively.13,49
Recently, one of us reported that β2-integrins associate with matrix metalloproteases50 and that this interaction is critical for β2-integrin–dependent leukocyte migration. This indicates that β2-integrins have the potential to form supramolecular complexes that are of functional importance. We have addressed this possibility for the LRP/β2-integrin complex by testing β2-dependent adhesion to stimulated HUVECs. Adhesion of U937 cells to HUVECs was reduced by almost 50% in the presence of anti-β2 subunit antibodies and to the same extent in the presence of GST-RAP (Figure 7A). Moreover, a similar reduction in adhesion was observed for LRP-deficient cells, and this reduced adhesion remained unchanged in the presence of anti–β2-integrin antibodies or GST-RAP. It should be noted that the residual adhesion observed could reflect adhesion mediated by selectins and α4β1-integrins. Indeed, the adhesion of LRP-expressing and -deficient cells was significantly decreased in the presence of anti–α4-antibodies (Figure 7B). Nevertheless, our data demonstrate that the absence or inhibition of LRP abrogates β2-dependent cell adhesion. Thus, it seems conceivable that LRP and β2-integrins form a functionally important complex at the leukocyte surface.
Of importance in this regard is the notion that LRP and β2-integrins may form complexes with other receptors as well. For instance, LRP has been shown to be involved in the regulation of uPAR surface expression, whereas urokinase plasminogen activator receptor (uPAR) contributes to β2-integrin function. As such, some of our observations could be explained by a model in which the absence of LRP modulates the surface expression of uPAR, which in turn could affect β2-integrin–dependent adhesion. Thus, LRP and β2-integrins could be part of a larger complex in which uPAR acts as an intermediate between both receptors. However, several observations argue against such a model. First, it has been established that binding of uPAR to LRP requires the presence of the uPA/PAI-1 complex.51 In agreement with this notion, we were unable to detect coprecipitation between LRP and uPAR in immunoprecipitation experiments (data not shown). Second, the surface expression of uPAR was unaffected in senseor antisense-transfected cells (Figure 5C). In view of our experimental data, it seems reasonable to assume that complex formation between LRP and β2-integrins is independent of uPAR.
The β2-integrins are known to recognize a variety of ligands to facilitate leukocyte adhesion to a broad range of surfaces. The main ligands are the ICAM molecules, but also fibrinogen and the platelet glycoprotein Ib/IX/V receptor may provide adhesive surfaces for these integrins. The interaction with these counter-receptors involves the I-domain of αM,34 the same domain that contains a binding site for LRP. Because β2-dependent leukocyte adhesion to these ligands is obviously not hampered by the presence of LRP, the possibility exists that the LRP-binding site is distinct from those of ICAMs, fibrinogen, and glycoprotein Ibα. Alternatively, the relatively low affinity for LRP may allow easy dissociation by ICAMs, fibrinogen, or glycoprotein Ibα, provided that they display higher affinity for αM than LRP. This alternative explanation seems favorable in view of our observation that fibrinogen was found to interfere with the binding of the αM I-domain to LRP (Figure 2B). Thus, it seems conceivable that LRP is required to position β2-integrins appropriately to facilitate the interaction with counterreceptors such as fibrinogen. This “cis” interaction, however, does not exclude the possibility that β2-integrins and LRP may interact in “trans” or, in other words, that β2-integrins exposed on the surface of one cell interact with LRP on the surface of another cell. This possibility is supported by our experiments that show effective leukocyte adhesion to purified LRP fragments (Figure 1).
In conclusion, the present study provides evidence for a previously unrecognized link between LRP and the inflammatory system. Our data point to a model in which LRP is a regulator of β2-integrin function because LRP deficiency abrogates β2-dependent cell adhesion. The possibility that LRP also contributes to other β2-integrin–dependent functions, such as phagocytosis and migration, remains to be investigated.
Prepublished online as Blood First Edition Paper, August 24, 2004; DOI 10.1182/blood-2004-02-0498.
Supported by grants from the Dutch Organization of Scientific Research ZonMW (no. 902-26-236) (P.J.L.), the Finnish Cancer Society and the Sigrid Juselius Foundation (C.G.G.), and the Netherlands Heart Foundation (no. 1999B059) (J.J.Z), and by a travel grant from the Academy of Finland and ZonMW (no. 910-31-410) (C.G.G., P.J.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal