Abstract
Vascular endothelial growth factor (VEGF) not only regulates angiogenesis, vascular permeability, and vasodilation but also promotes vascular inflammation. However, the molecular basis for the proinflammatory effects of VEGF is not understood. We now show that VEGF activates endothelial cell exocytosis of Weibel-Palade bodies, releasing vasoactive substances capable of causing vascular thrombosis and inflammation. VEGF triggers endothelial exocytosis in part through calcium and phospholipase C-γ (PLC-γ) signal transduction. However, VEGF also modulates endothelial cell exocytosis by activating endothelial nitric oxide synthase (eNOS) production of nitric oxide (NO), which nitrosylates N-ethylmaleimide sensitive factor (NSF) and inhibits exocytosis. Thus, VEGF plays a dual role in regulating endothelial exocytosis, triggering pathways that both promote and inhibit endothelial exocytosis. Regulation of endothelial exocytosis may explain part of the proinflammatory effects of VEGF.
Introduction
Vascular endothelial growth factor (VEGF) isoforms regulate a variety of vascular processes, including angiogenesis, vascular permeability, and vasodilation.1-5 VEGF isoforms promote endothelial cell migration, proliferation, and survival by interacting with the VEGF receptors. The VEGF family includes isoforms VEGF-A, VEGF-B, VEGF-C, VEGF-D, placenta growth factor (P1GF), and a virally encoded VEGF-E. Alternative splicing of the human VEGF-A gene generates VEGF-A121, VEGF-A145, VEGF-A165, VEGF-A189, and VEGF-A206; the predominant isoform is VEGF-A165. Receptors for VEGF include VEGFR-1 (Fms-like tyrosine kinase-1 [flt-1]), VEGFR-2 (flk-1, or KDR), and VEGF-3 (flt-4); VEGF isoforms also interact with neuropilin (NRP1 and NRP2) coreceptors. VEGF engagement with its receptors activates at least 3 distinct signal transduction pathways: the phosphatidylinositol 3′ (PI-3) kinase–alpha serine/threonine kinase (Akt) pathway; the mitogen activated protein kinase (MAPK) pathway; and the phospholipase C-γ (PLC-γ) and protein kinase C (PKC) pathway.6,7
VEGF also plays a role in inflammatory disorders, but the molecular basis of this effect is unclear. VEGF and VEGFR levels are increased in psoriasis, dermatitis, and bullous skin diseases.8-12 Furthermore, transgenic expression of VEGF-A in mouse skin leads to psoriasis.13,14 One mechanism by which VEGF causes inflammation is by increasing vascular permeability, but VEGF may have other proinflammatory effects as well. Ectopic VEGF-A expression causes increased leukocyte rolling and adhesion in venules, which can be blocked by antibodies to P-selectin.14 Chronic overexpression of VEGF-A leads to inflammatory infiltrates, associated with endothelial cell expression of E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1).13 These studies suggest that VEGF activates the endothelium, triggering endothelial expression of selectins and adhesion molecules and thus initiating vascular inflammation.
Endothelial activation has 2 stages, the rapid translocation of P-selectin to the endothelial surface, and the slower synthesis and expression of adhesion molecules such as ICAM-1. P-selectin is stored in Weibel-Palade bodies, endothelial storage granules that also contain von Willebrand factor (VWF), interleukin-8 (IL-8), and tissue plasminogen activator.15-24 Rapid exocytosis of Weibel-Palade bodies is activated by vasopressin, hypoxia, histamine, and thrombin.17,25-27 The protein machinery that mediates exocytosis includes N-ethylmaleimide–sensitive factor (NSF) and soluble NSF attachment protein receptors (SNAREs).28-30 Regulation of endothelial cell exocytosis is not well understood, but nitric oxide (NO) can inhibit exocytosis by covalently modifying NSF, thereby inhibiting NSF activity and blocking NSF activation of exocytosis.31 Exocytosis of Weibel-Palade bodies causes rapid translocation of P-selectin from within granules to the endothelial surface, where P-selectin then interacts with P-selectin glycoprotein ligand-1 (PSGL-1) on the surface of leukocytes, triggering leukocyte rolling.32 Thus, exocytosis of Weibel-Palade bodies is a critical early step in vascular inflammation.
We now show that VEGF has 2 opposing effects on Weibel-Palade–body exocytosis. VEGF triggers Weibel-Palade–body exocytosis in part by calcium and PLC-γ–mediated pathways. However, VEGF also modulates Weibel-Palade–body exocytosis by activating the PI-3 kinase/Akt pathway which increases NO synthesis, thereby inhibiting exocytosis.
Materials and methods
Reagents
Recombinant human vascular endothelial growth factor (VEGF-A121), N-nitro-L-arginine methyl ester (L-NAME), U73122, Calphostin C, A23187, and 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-acetoxymethyl ester (BAPTA/AM), were purchased from Sigma (St Louis, MO). 5-nitroso-N-acetyl-D,L-penicillamine (SNAP) was purchased from Cayman Chemical (Ann Arbor, MI). Human α-thrombin was purchased from Enzyme Research Laboratories (South Bend, IN). Soluble Fms-like tyrosine kinase-1 (sFlt-1) was purchased from Merck (Darmstadt, Germany). Fluo-3 acetoxymethyl ester (Fluo-3AM) was purchased from Molecular Probes (Eugene, OR). Rabbit polyclonal antibody to endothelial nitric oxide synthase (eNOS), and mouse monoclonal antibody to phospho-eNOS were purchased from BD Biosciences (San Diego, CA). Rabbit polyclonal antibody to Akt and mouse monoclonal antibody to phospho-Akt were purchased from Upstate Biotechnology (Charlottesville, VA). The antibody to nitrosocysteine was purchased from Calbiochem (San Diego, CA).
Preparation of recombinant adenoviruses
The replication-deficient adenovirus encoding the epitope-tagged dominant-negative Akt cDNA and the adenovirus LacZ were constructed by homologous recombination in 293 cells with use of the adenovirus-based plasmid JM17.33 All viruses were amplified and titered in 293 cells and purified on CsCl gradients.
Cell culture and analysis of VWF release
Human aortic endothelial cells (HAECs) were obtained from Clonetics (Walkersville, MD) and grown in endothelial growth medium 2 (EGM-2) media supplemented with growth factors in a kit (Bullet Kit; Clonetics). To measure the effect of VEGF on VWF release, HAECs were grown in EGM-2 media with growth factors, then washed and placed in EGM-2 media without growth factors or serum, stimulated with various concentration of VEGF, and the amount of VWF released into the media was measured by an enzyme-linked immunosorbent assay (ELISA; American Diagnostica, Greenwich, CT). To clarify the mechanism by which VEGF induces VWF exocytosis, HAECs were pretreated for 30 minutes with U73211, Calphostin C, and sFlt-1 and then stimulated with 50 ng/mL VEGF for 1 hour. The amount of VWF released into the media was measured by an ELISA. HAECs were pretreated with 10 μM BAPTA/AM for 30 minutes in Dulbecco modified Eagle medium (DMEM) or CaCl2-free DMEM. The cells were then stimulated with VEGF. The supernatants were collected, and the concentration of VWF released into the media was measured by an ELISA. To examine the effect of VEGF pretreatment on VEGF-induced VWF exocytosis, we incubated HAECs with various concentration of VEGF or control for 1, 2, 4, and 8 hours. The cells were washed and then stimulated with 50 ng/mL VEGF. To measure the effect of Akt on exocytosis, HAECs were incubated for 48 hours with adenoviral vectors expressing constitutively active Akt [Akt(CA)], dominant-negative Akt [Akt(DN)], or β-galactosidase (LacZ) for 24 hours at a multiplicity of infection (MOI) of 200 before VEGF stimulation.33-35 To measure the effect of NO on VWF release by VEGF, HAECs were pretreated with SNAP for 4 hours. The cells were washed and stimulated with 50 ng/mL VEGF, and the amount of VWF released into the media was measured by an ELISA. To measure the effect of endogenous NO production on exocytosis, HAECs were pretreated for 16 hours with L-NAME before VEGF or thrombin stimulation.
Analysis of P-selectin translocation
HAECs were treated with 100 ng/mL VEGF for 0 and 5 minutes, and the expression of P-selectin on the cell surface was measured by a cell surface ELISA.36 Briefly, HAEC monolayers were fixed with 2% paraformaldehyde in a sodium phosphate buffer (pH 7.4) with 0.1 M L-lysine monohydrochloride and 10 mM sodium m-periodate for 30 minutes at 4°C and then incubated with 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) containing 0.1 M glycine overnight at 4°C. After washing twice with 0.1% BSA in PBS, the fixed cells were probed with a rabbit anti–human p-selection antibody (PharMingen, San Diego, CA) for 1 hour at 37°C. After washing 3 times with 0.1% BSA in PBS, the HAECs were incubated with a peroxidase-conjugated mouse anti–rabbit immunoglobulin G (IgG) antibody (Amersham Biosciences, Piscataway, NJ) for 1 hour at 37°C. After washing 3 times with 0.1% BSA in PBS, 0.2% H2O2 and 3.7 mM O-phenylenediamine (Sigma) was added for 30 minutes and then added 1 M H2SO4 for stopping the reaction. Optical density (OD) 520 nm of the plates was read on a spectrophotometric microplate reader (Molecular Devices, Sunnyvale, CA).
Immunofluorescence of VWF
HAECs were treated with VEGF 50 ng/mL or cyclohexamide (CHX) 10 μM for 2 hours and then stained with anti-VWF mouse monoclonal antibody (BD Biosciences, San Jose, CA) and carbocyanine 3 (Cy3)–conjugated IgG secondary antibody (Jackson Immunoresearch Laboratories, West Grove, PA). The cells were observed with an immunofluorescence microscope (Nikon, Tokyo, Japan).
Determination of [Ca2+]i
[Ca2+]i was determined by using the indicator dye fluo-3. HAECs were plated onto collagen-coated glass coverslips and incubated with fluo-3AM in minimum essential media 2 (MEM-2) for 20 minutes at 37°C. The cells were washed, stimulated with 50 ng/mL VEGF or 1 U/mL thrombin, and then analyzed with epifluorescence microscopy (Nikon).
Western blot analysis of Akt and eNOS phosphorylation
Confluent HAECs were serum-starved in serum-free EGM-2 media for 18 hours. Cell were rinsed and stimulated with various concentrations of VEGF for 30 minutes in PBS. The supernatant was removed, and sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (Bio-Rad, Hercules, CA) was added. The cells were scraped, the lysate were fractionated on a 7.5% SDS-PAGE, and immunoblotted with antibodies to phospho-eNOS and phospho-Akt. To examine the effect of Akt on VEGF-induced eNOS phosphorylation, HAECs were pretreated with adenoviral vectors expressing Akt(CA), Akt(DN), or β-galactosidase at an MOI of 200 for 48 hours before VEGF or thrombin stimulation.33
Measurement of endothelial NO production
Confluent HAECs were serum-starved in serum-free EGM-2 media for 18 hours. Cell were rinsed and stimulated with various concentrations of VEGF for 30 minutes in PBS. NO content in the supernatants was then measured by the Griess reaction.31
Determination of S-nitrosylation of NSF
HAECs were treated with 50 ng/mL VEGF, and cells were harvested at various times after treatment. Measurement of NSF nitrosylated in the cell lysates was performed by immunoprecipitation with antibody to nitrosocysteine, followed by immunoblotting with antibody to NSF (Santa Cruz Biotechnology, Santa Cruz, CA).
Statistical analyses
Data are expressed as the mean ± SD. Statistical comparison was made by analysis of variance followed by a Bonferroni t test for multiple comparison. A P value of less than .05 was considered significant.
Results
VEGF triggers VWF exocytosis
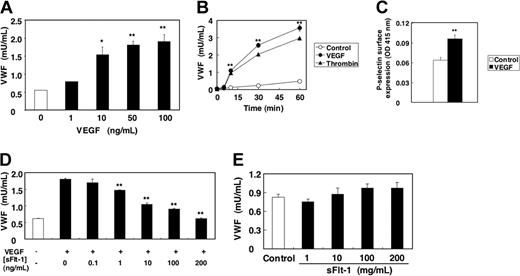
We first examined the effect of VEGF on Weibel-Palade–body exocytosis. HAECs were stimulated with various concentration of VEGF, and the amount of VWF released into the media was measured by an ELISA. VEGF strongly induced VWF exocytosis in a dose-dependent manner, with a maximal effect achieved at 50 ng/mL (Figure 1A). VEGF induced VWF exocytosis within 5 minutes of stimulation, and VWF continues to be released up to 60 minutes after stimulation (Figure 1B). The effect on exocytosis of VEGF was comparable to that of thrombin.
VEGF induces VWF release from human aortic endothelial cells. (A) Dose response. HAECs were treated with VEGF for 1 hour. The amount of VWF released from cells into the media was measured by an ELISA (n = 3 ± SD; *P < .05 versus 0 ng/mL; **P < .01 versus 0 ng/mL). (B) Time course. HAECs were incubated with 50 μg/mL VEGF or 1 U/mL thrombin. The amount of VWF released from cells into the media was measured by an ELISA (n = 3 ± SD; error bars too small to be seen; **P < .01 versus control). (C) VEGF induces P-selectin translocation onto surface of endothelial cells. HAECs were treated with 100 ng/mL VEGF for 5 minutes, and the expression of P-selectin on the cell surface was measured by a cell surface ELISA. (n = 3 ± SD; **P < .01 versus control). (D) Soluble Flt-1 inhibits VEGF-induced VWF release from endothelial cells. HAECs were pretreated with sFlt-1 for 30 minutes, and then incubated with 50 ng/mL VEGF for 1 hour. The amount of VWF released from cells into the media was measured by an ELISA (n = 3 ± SD; **P < .01 versus 0 ng/mL sFlt-1). (E) Soluble Flt-1 alone has no effect on VWF release. HAECs were treated with sFlt-1 for 1 hour. The amount of VWF released from cells into the media was measured by an ELISA (n = 3 ± SD).
VEGF induces VWF release from human aortic endothelial cells. (A) Dose response. HAECs were treated with VEGF for 1 hour. The amount of VWF released from cells into the media was measured by an ELISA (n = 3 ± SD; *P < .05 versus 0 ng/mL; **P < .01 versus 0 ng/mL). (B) Time course. HAECs were incubated with 50 μg/mL VEGF or 1 U/mL thrombin. The amount of VWF released from cells into the media was measured by an ELISA (n = 3 ± SD; error bars too small to be seen; **P < .01 versus control). (C) VEGF induces P-selectin translocation onto surface of endothelial cells. HAECs were treated with 100 ng/mL VEGF for 5 minutes, and the expression of P-selectin on the cell surface was measured by a cell surface ELISA. (n = 3 ± SD; **P < .01 versus control). (D) Soluble Flt-1 inhibits VEGF-induced VWF release from endothelial cells. HAECs were pretreated with sFlt-1 for 30 minutes, and then incubated with 50 ng/mL VEGF for 1 hour. The amount of VWF released from cells into the media was measured by an ELISA (n = 3 ± SD; **P < .01 versus 0 ng/mL sFlt-1). (E) Soluble Flt-1 alone has no effect on VWF release. HAECs were treated with sFlt-1 for 1 hour. The amount of VWF released from cells into the media was measured by an ELISA (n = 3 ± SD).
To confirm that VEGF activates exocytosis, we also determined the effect of VEGF on externalization of P-selectin which is stored in Weibel-Palade bodies along with VWF.15,17 VEGF induced surface expression of P-selectin on HAECs within 5 minutes, as detected by a cell surface ELISA (Figure 1C). To demonstrate the specificity by which VEGF induced Weibel-Palade–body exocytosis, we next examined the effect of recombinant soluble Flt-1 (sFlt-1), 1 of the 3 VEGF receptors, on VWF exocytosis induced by VEGF. Recombinant sFlt-1 inhibited VEGF-induced VWF exocytosis in a dose-dependent manner (Figure 1D). Soluble Flt-1 by itself has no effect on exocytosis (Figure 1E). Furthermore, pretreatment of HAECs with genistein, a nonspecific inhibitor of tyrosine kinase receptor autophosphorylation and activation, strongly blocked VEGF-induced VWF exocytosis (data not shown). These results suggest that VEGF induces Weibel-Palade–body exocytosis through VEGF receptor signaling.
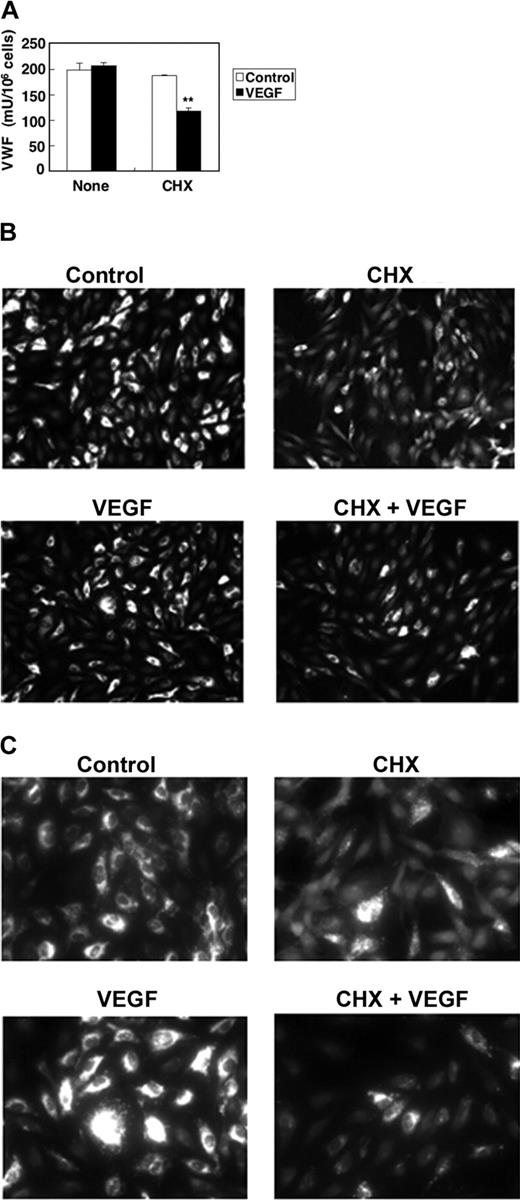
We expected that VEGF treatment would stimulate HAEC exocytosis of all Weibel-Palade bodies, exhausting the intracellular supply of VWF. However, VWF continues to be released by endothelial cells, even 1 hour after treatment. To explore the kinetics of Weibel-Palade–body exocytosis, we measured the steady-state protein levels of VWF inside endothelial cells by ELISA. VEGF does not change the intracellular levels of VWF (Figure 2). However, VEGF treatment does decrease intracellular levels of VWF if the cells are pretreated with the protein synthesis inhibitor CHX (Figure 2). These data suggest that during exposure to VEGF, endothelial cells continually produce Weibel-Palade bodies containing VWF.
Kinetics of VWF expression in VEGF treated cells. (A) Steady-state VEGF protein levels in HAECs. HAECs were treated with 50 ng/mL VEGF or 10 μM CHX 10 μM for 2 hours. The concentration of VWF in the cells was measured by an ELISA. (n = 3 ± SD; *P < .01 compared with VEGF alone). (B) Immunofluorescence of VWF in endothelial cells, low magnification. HAECs were treated with 50 ng/mL VEGF or 10 μM CHX for 2 hours and then stained with anti-VWF antibody and Cy3-conjugated IgG secondary antibody. Magnitude, × 100. (C) Immunofluorescence of VWF in endothelial cells, high magnification. HAECs were treated with 50 ng/mL VEGF or 10 μM CHX for 2 hours and then stained with anti-VWF antibody and Cy3-conjugated IgG secondary antibody. Magnification, × 400. Cells were imaged at 22°C under a Nikon Fluorescent Eclipse E600 microscope (Nikon, Tokyo, Japan) equipped with 10 × 10 and 10 × 40 water immersion objective lenses (Nikon). Images were acquired with a Nikon DXM 1200 digital camera and analyzed with Spot Epi-fl 3.5.2 software (Diagnostic Instruments, Sterling Heights, MI).
Kinetics of VWF expression in VEGF treated cells. (A) Steady-state VEGF protein levels in HAECs. HAECs were treated with 50 ng/mL VEGF or 10 μM CHX 10 μM for 2 hours. The concentration of VWF in the cells was measured by an ELISA. (n = 3 ± SD; *P < .01 compared with VEGF alone). (B) Immunofluorescence of VWF in endothelial cells, low magnification. HAECs were treated with 50 ng/mL VEGF or 10 μM CHX for 2 hours and then stained with anti-VWF antibody and Cy3-conjugated IgG secondary antibody. Magnitude, × 100. (C) Immunofluorescence of VWF in endothelial cells, high magnification. HAECs were treated with 50 ng/mL VEGF or 10 μM CHX for 2 hours and then stained with anti-VWF antibody and Cy3-conjugated IgG secondary antibody. Magnification, × 400. Cells were imaged at 22°C under a Nikon Fluorescent Eclipse E600 microscope (Nikon, Tokyo, Japan) equipped with 10 × 10 and 10 × 40 water immersion objective lenses (Nikon). Images were acquired with a Nikon DXM 1200 digital camera and analyzed with Spot Epi-fl 3.5.2 software (Diagnostic Instruments, Sterling Heights, MI).
Calcium mediates VEGF-activated Weibel-Palade–body exocytosis
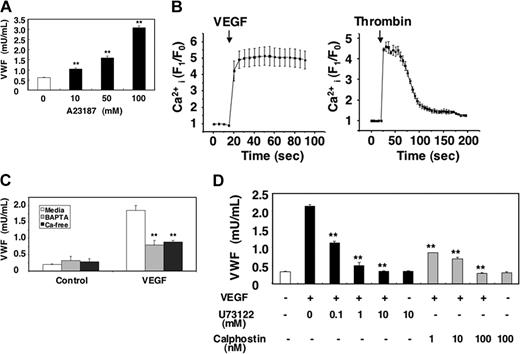
Since calcium can trigger exocytosis, we examined the effects of VEGF on calcium signaling during Weibel-Palade–body exocytosis. Treatment with the Ca2+ ionophore A23187 induced VWF exocytosis from HAECs in a dose-dependent manner, confirming that Ca2+ can activate endothelial exocytosis (Figure 3A). To examine whether or not VEGF itself increases intracellular calcium, we treated HAECs with a calcium-specific fluorescent indicator fluo-3AM and then measured the intracellular calcium levels. VEGF rapidly increased intracellular calcium levels in HAECs (Figure 3B). VEGF elevates intracellular calcium levels for more than 1 minute; in contrast, thrombin elevates intracellular calcium levels transiently (Figure 3B).
Long-term treatment with VEGF inhibits VEGF-induced exocytosis. (A) The calcium ionophore A23187 activates VWF release from endothelial cells. HAECs were incubated with A23187 for 1 hour. The amount of VWF released from cells into the media was measured by an ELISA (n = 3 ± SD; **P < .01 versus 0 μM). (B) VEGF increases intracellular calcium levels. HAECs were incubated with the calcium indicator fluo-3AM for 20 minutes. The integrated fluo-3 fluorescence intensity of each cell was measured in real time after treatment with 50 ng/mL VEGF or 1 U/mL thrombin (n = 3 ± SD). (C) Calcium pools and VWF release from endothelial cells. HAECs were pretreated with 20 μM BAPTA/am for 30 minutes in DMEM or Ca2+-free DMEM and then incubated with 50 ng/mL VEGF for 1 hour. The amount of VWF released from cells into the media was measured by an ELISA (n = 3 ± SD; **P < .01 versus media + VEGF). (D) PLC and PKC inhibitors block VEGF-induced VWF release from endothelial cells. HAECs were pretreated with the PLC-γ inhibitor U73122 for 30 minutes or the PKC inhibitor calphostin C for 30 minutes and then incubated with 50 ng/mL VEGF for 1 hour. The amount of VWF released from cells into the media was measured by an ELISA (n = 3 ± SD; **P < .01 versus no inhibitor).
Long-term treatment with VEGF inhibits VEGF-induced exocytosis. (A) The calcium ionophore A23187 activates VWF release from endothelial cells. HAECs were incubated with A23187 for 1 hour. The amount of VWF released from cells into the media was measured by an ELISA (n = 3 ± SD; **P < .01 versus 0 μM). (B) VEGF increases intracellular calcium levels. HAECs were incubated with the calcium indicator fluo-3AM for 20 minutes. The integrated fluo-3 fluorescence intensity of each cell was measured in real time after treatment with 50 ng/mL VEGF or 1 U/mL thrombin (n = 3 ± SD). (C) Calcium pools and VWF release from endothelial cells. HAECs were pretreated with 20 μM BAPTA/am for 30 minutes in DMEM or Ca2+-free DMEM and then incubated with 50 ng/mL VEGF for 1 hour. The amount of VWF released from cells into the media was measured by an ELISA (n = 3 ± SD; **P < .01 versus media + VEGF). (D) PLC and PKC inhibitors block VEGF-induced VWF release from endothelial cells. HAECs were pretreated with the PLC-γ inhibitor U73122 for 30 minutes or the PKC inhibitor calphostin C for 30 minutes and then incubated with 50 ng/mL VEGF for 1 hour. The amount of VWF released from cells into the media was measured by an ELISA (n = 3 ± SD; **P < .01 versus no inhibitor).
To explore which pools of calcium are mobilized by VEGF and regulate exocytosis, we added thrombin to HAECs pretreated with BAPTA to deplete intracellular calcium stores or to HAECs cultured in Ca2+-free media. Exocytosis in CaCl2-free media is decreased by 68% (Figure 3C). Exocytosis from cells treated with BAPTA also is decreased by 71%. These results suggest that both intracellular and extracellular Ca2+ mediate VEGF-triggered VWF exocytosis. Finally, we used pharmacologic approaches to study mechanism by which VEGF regulates intracellular calcium levels. Inhibition of PLC-γ with U73122 inhibited VEGF-induced exocytosis with an IC50% (inhibitory concentration of 50%) of approximately 90 nM (Figure 3D). Furthermore, inhibition of PKC with calphostin C also inhibited VWF exocytosis with an IC50% of approximately 0.7 nM (Figure 3D). Taken together, these data suggest that VEGF activates Weibel-Palade–body exocytosis in part by increasing intracellular calcium levels.
Pretreatment with VEGF inhibits VEGF-induced Weibel-Palade–body exocytosis
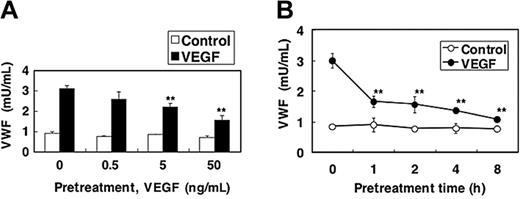
Since biologic stimuli often activate a negative feedback loop, we next examined the effect of VEGF pretreatment on VEGF-induced Weibel-Palade–body exocytosis. HAECs were pretreated with VEGF for 2 hours, washed, and then exposed again to VEGF. VEGF pretreatment of endothelial cells inhibits VEGF activation of Weibel-Palade–body exocytosis in a dose-dependent manner by 62% (Figure 4A). VEGF inhibition of exocytosis occurs within 1 hour of treatment and persists 8 hours after VEGF treatment (Figure 4B).
Akt mediates long-term VEGF inhibition of exocytosis. (A) Dose response. HAECs were pretreated with 0, 0.5, 5, and 50 ng/mL VEGF for 2 hours. Cells were then washed and treated with 50 ng/mL VEGF for 1 hour, and the release of VWF was measured as above (n = 3 ± SD; **P < .01 versus 0 ng/mL VEGF). (B) Time course. HAECs were pretreated with 50 ng/mL VEGF for 0, 1, 2, 4, or 8 hours. Cells were then washed and treated with 50 ng/mL VEGF for 1 hour, and the release of VWF was measured as above (n = 3 ± SD; **P < .01 versus 0 hours).
Akt mediates long-term VEGF inhibition of exocytosis. (A) Dose response. HAECs were pretreated with 0, 0.5, 5, and 50 ng/mL VEGF for 2 hours. Cells were then washed and treated with 50 ng/mL VEGF for 1 hour, and the release of VWF was measured as above (n = 3 ± SD; **P < .01 versus 0 ng/mL VEGF). (B) Time course. HAECs were pretreated with 50 ng/mL VEGF for 0, 1, 2, 4, or 8 hours. Cells were then washed and treated with 50 ng/mL VEGF for 1 hour, and the release of VWF was measured as above (n = 3 ± SD; **P < .01 versus 0 hours).
Akt mediates VEGF inhibition of Weibel-Palade–body exocytosis
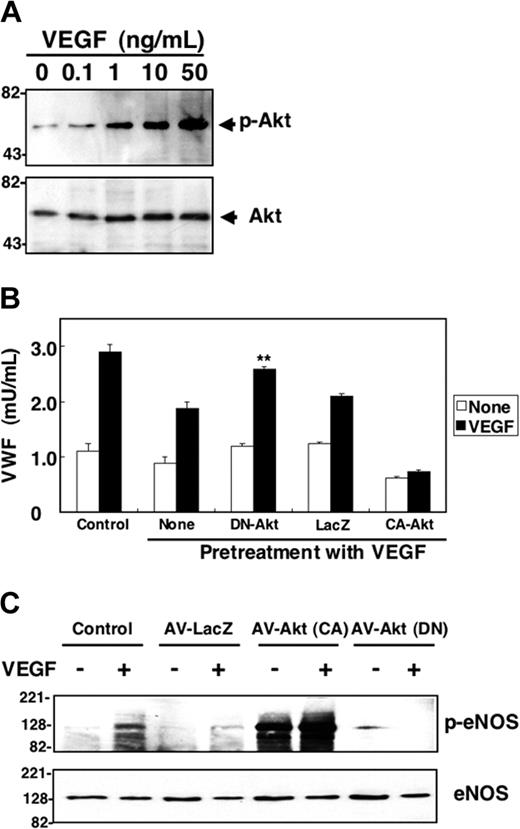
We hypothesized that the Akt pathway mediates VEGF inhibition of exocytosis. To explore the role of Akt in VEGF inhibition of exocytosis, we first examined VEGF-induced Akt phosphorylation. VEGF (50 ng/mL) stimulation of HAECs for 30 minutes elicited an increase in phosphorylation of Akt, a marker of Akt activation (Figure 5A). To explore the role of Akt in VEGF inhibition of exocytosis, we used viral vectors directing the expression of dominant-negative Akt. HAECs were infected with adenovirus-LacZ (AV-LacZ), adenovirus-constitutively active Akt (AV-CA-Akt), or adenovirus–dominant-negative Akt (AV-DN-Akt); HAECs were pretreated with VEGF for 2 hours, and then VEGF was added to trigger exocytosis. Exocytosis is activated in control cells (Figure 5B). VEGF inhibits exocytosis. However, overexpression of dominant-negative Akt blocks the ability of VEGF to inhibit exocytosis (Figure 5B). In contrast, a control adenoviral vector has no effect. Constitutively active Akt increases the ability of VEGF to block exocytosis. These data suggest that Akt mediates VEGF activation of exocytosis of endothelial cell granules.
Long-term treatment with VEGF activates NO synthesis which nitrosylates NSF and inhibits exocytosis. (A) VEGF increases Akt phosphorylation. HAECs were pretreated with 50 ng/mL VEGF for 30 minutes, and total cell lysates were immunoblotted with antibody to phospho-Akt (top row) or total Akt (bottom row). (B) Dominant-negative Akt blocks VEGF inhibition of exocytosis. HAECs were transduced with adenovirus expressing dominant-negative Akt or adenovirus expressing β-galactosidase at an MOI of 200 for 48 hours, then treated with 50 ng/mL VEGF for 2 hours. Cells were then washed and treated with VEGF 50 ng/mL for 1 hour, and exocytosis was measured with an ELISA for VWF as described for Figure 1 (n = 3 ± SD; **P < .01 versus VEGF + LacZ). (C) Dominant-negative Akt inhibits VEGF-activated phosphorylation of eNOS. HAECs were transduced with adenoviral vectors expressing Akt(CA), Akt (DN), or LacZ at an MOI of 200 for 48 hours. Cells were then treated with 50 ng/mL VEGF for 30 minutes, and total cell lysates were immunoblotted with antibody to phospho-eNOS or eNOS.
Long-term treatment with VEGF activates NO synthesis which nitrosylates NSF and inhibits exocytosis. (A) VEGF increases Akt phosphorylation. HAECs were pretreated with 50 ng/mL VEGF for 30 minutes, and total cell lysates were immunoblotted with antibody to phospho-Akt (top row) or total Akt (bottom row). (B) Dominant-negative Akt blocks VEGF inhibition of exocytosis. HAECs were transduced with adenovirus expressing dominant-negative Akt or adenovirus expressing β-galactosidase at an MOI of 200 for 48 hours, then treated with 50 ng/mL VEGF for 2 hours. Cells were then washed and treated with VEGF 50 ng/mL for 1 hour, and exocytosis was measured with an ELISA for VWF as described for Figure 1 (n = 3 ± SD; **P < .01 versus VEGF + LacZ). (C) Dominant-negative Akt inhibits VEGF-activated phosphorylation of eNOS. HAECs were transduced with adenoviral vectors expressing Akt(CA), Akt (DN), or LacZ at an MOI of 200 for 48 hours. Cells were then treated with 50 ng/mL VEGF for 30 minutes, and total cell lysates were immunoblotted with antibody to phospho-eNOS or eNOS.
Akt has many downstream targets. We hypothesized that VEGF may activate Akt phosphorylation of eNOS, as has been shown by others.37-39 VEGF treatment of HAECs activates phosphorylation of eNOS. Expression of a dominant-negative Akt blocks the ability of VEGF to stimulate eNOS phosphorylation (Figure 5C). These results suggest that VEGF pretreatment inhibits exocytosis by triggering Akt to activate eNOS.
VEGF triggers NO synthesis which nitrosylates NSF and inhibits exocytosis
We, therefore, examined the effect of VEGF on eNOS phosphorylation. HAECs were pretreated with 50 ng/mL VEGF for 30 minutes, and total cell lysates were immunoblotted with antibody to phospho-eNOS and eNOS. VEGF induced a dose-dependent increase in eNOS phosphorylation (Figure 6A). VEGF activation of eNOS phosphorylation is dependent on Akt, because overexpression of dominant-negative Akt blocks VEGF-triggered increases in eNOS phosphorylation (Figure 5C). VEGF increases NO production in HAEC cultures in a dose-dependent manner (Figure 6B). Thus, VEGF activates NO production in HAECs.
Long-term treatment with VEGF activates NO synthesis which nitrosylates NSF and inhibits exocytosis. (A) VEGF activates eNOS phosphorylation. HAECs were pretreated with 50 ng/mL VEGF for 30 minutes, and total cell lysates were immunoblotted with antibody to phospho-eNOS or eNOS. (B) VEGF activates NO synthesis. HAECs were treated with 50 ng/mL VEGF for 30 minutes, and the
Long-term treatment with VEGF activates NO synthesis which nitrosylates NSF and inhibits exocytosis. (A) VEGF activates eNOS phosphorylation. HAECs were pretreated with 50 ng/mL VEGF for 30 minutes, and total cell lysates were immunoblotted with antibody to phospho-eNOS or eNOS. (B) VEGF activates NO synthesis. HAECs were treated with 50 ng/mL VEGF for 30 minutes, and the
We then explored the role of endogenous NO in the regulation of VWF exocytosis. We pretreated HAECs with L-NAME for 16 hours to inhibit endogenous NOS, treated the cells with VEGF for 2 hours, and then stimulated the cells with 50 ng/mL VEGF for 1 hour and measured VWF release. VEGF pretreatment blocks exocytosis of VWF (Figure 6C). However, L-NAME blocks VEGF inhibition of exocytosis. These data show that endogenous NO inhibits exocytosis.
To confirm the effect of endogenous NO on VWF exocytosis, we next examined the effect of exogenous NO on VEGF-induced VWF exocytosis. We pretreated HAECs with SNAP for 4 hours, stimulated the cells with VEGF (50 ng/mL) for 1 hour, and then measured the amount of VWF released into the media. VEGF induces a rapid release of VWF from HAECs (Figure 6D). However, exogenous NO blocks VEGF-induced exocytosis. In addition, SNAP did not affect the viability of HAECs at the concentration of which exhibited the inhibitory effect (data not shown).
We have previously shown that NSF regulates endothelial cell exocytosis of Weibel-Palade bodies.31 Nitrosylation of NSF inhibits NSF disassembly activity which is necessary for exocytosis. Accordingly, we next examined the effect of VEGF-induced NO synthesis on nitrosylation of NSF. HAECs were stimulated with VEGF, and the amount of nitrosylated NSF was measured by immunoprecipitation. VEGF increases S-nitrosylation of NSF (Figure 6E). Thus, endogenous NO nitrosylates NSF.
Thrombin does not trigger NO synthesis and does not inhibit exocytosis
We next compared the effects of pretreatment with VEGF to thrombin. In contrast to VEGF, pretreatment with thrombin does not inhibit VEGF-triggered exocytosis (Figure 7A). Two lines of evidence suggest that thrombin does not activate Akt. First, overexpression of dominant-negative Akt has no effect on thrombin-stimulated cells (Figure 7A). Second, thrombin does not trigger phosphorylation of Akt; VEGF does (Figure 7B). Furthermore, thrombin pretreatment does not lead to phosphorylation of eNOS (Figure 7C). Finally, the NOS inhibitor L-NAME blunts the inhibitory effects of VEGF but has no effect on thrombin pretreatment (Figure 7D). Thus, VEGF pretreatment inhibits exocytosis through an Akt and eNOS–dependent pathway; in contrast thrombin, having no effects on Akt or eNOS, does not inhibit exocytosis.
Pretreatment with thrombin does not inhibit VEGF-induced exocytosis. (A) Thrombin pretreatment does not block VEGF activation of exocytosis. HAECs were transduced with adenovirus expressing dominant negative Akt or adenovirus expressing β-galactosidase (LacZ) at an MOI of 200 for 48 hours, then treated with 50 ng/mL VEGF for 2 hours. Cells were then washed and treated with thrombin 1 U/mL for 1 hour, and exocytosis was measured with an ELISA for VWF as above (n = 3 ± SD; **P < .01 versus VEGF + LacZ). (B) VEGF but not thrombin or A23187 increases Akt phosphorylation. HAECs were pretreated with 50 ng/mL VEGF, 1 U/mL thrombin, or 1 μM A23187 for 30 minutes, and total cell lysates were immunoblotted with antibody to phospho-Akt (top row) or total Akt (bottom row). (C) Thrombin does not activate phosphorylation of eNOS. HAECs were transduced with adenoviral vectors expressing constitutively active Akt [Akt (CA)], dominant-negative Akt [Akt (DN)], or β-galactosidase (LacZ) at an MOI of 200 for 48 hours. Cells were then treated with 1 U/mL thrombin for 30 minutes, and total cell lysates were immunoblotted with antibody to phospho-eNOS or eNOS. (D) VEGF but not thrombin pretreatment decreases VEGF-induced exocytosis. HAECs were pretreated with 1 mM L-NAME for 16 hours, washed, and then incubated with 50 ng/mL VEGF or 1 U/mL thrombin for 1 hour. Cells were washed and treated with VEGF 50 ng/mL for 1 hour, and the amount of VWF released from cells into the media was measured by an ELISA (n = 3 ± SD; **P < .01 for VEGF versus VEGF + L-NAME).
Pretreatment with thrombin does not inhibit VEGF-induced exocytosis. (A) Thrombin pretreatment does not block VEGF activation of exocytosis. HAECs were transduced with adenovirus expressing dominant negative Akt or adenovirus expressing β-galactosidase (LacZ) at an MOI of 200 for 48 hours, then treated with 50 ng/mL VEGF for 2 hours. Cells were then washed and treated with thrombin 1 U/mL for 1 hour, and exocytosis was measured with an ELISA for VWF as above (n = 3 ± SD; **P < .01 versus VEGF + LacZ). (B) VEGF but not thrombin or A23187 increases Akt phosphorylation. HAECs were pretreated with 50 ng/mL VEGF, 1 U/mL thrombin, or 1 μM A23187 for 30 minutes, and total cell lysates were immunoblotted with antibody to phospho-Akt (top row) or total Akt (bottom row). (C) Thrombin does not activate phosphorylation of eNOS. HAECs were transduced with adenoviral vectors expressing constitutively active Akt [Akt (CA)], dominant-negative Akt [Akt (DN)], or β-galactosidase (LacZ) at an MOI of 200 for 48 hours. Cells were then treated with 1 U/mL thrombin for 30 minutes, and total cell lysates were immunoblotted with antibody to phospho-eNOS or eNOS. (D) VEGF but not thrombin pretreatment decreases VEGF-induced exocytosis. HAECs were pretreated with 1 mM L-NAME for 16 hours, washed, and then incubated with 50 ng/mL VEGF or 1 U/mL thrombin for 1 hour. Cells were washed and treated with VEGF 50 ng/mL for 1 hour, and the amount of VWF released from cells into the media was measured by an ELISA (n = 3 ± SD; **P < .01 for VEGF versus VEGF + L-NAME).
These results suggest that VEGF has 2 opposing effects on endothelial exocytosis. VEGF not only activates Weibel-Palade–body exocytosis but also triggers NO synthesis which inhibits exocytosis.
Discussion
The major finding of these studies is that VEGF has 2 opposing effects on Weibel-Palade–body exocytosis from endothelial cells. VEGF not only triggers exocytosis, but VEGF also modulates exocytosis by distinct pathways.
VEGF activates exocytosis
Endothelial exocytosis of Weibel-Palade bodies leads to the release of VWF and the translocation of P-selectin.22 Classic activators of endothelial exocytosis include thrombin, histamine, and adenosine triphosphate (ATP). Our data show that the vascular signaling molecule VEGF also activates exocytosis. VEGF activates exocytosis by activating distinct signaling pathways that include elevations in intracellular calcium. Calcium plays a critical role in the final stages of exocytosis leading to membrane fusion in neurons.28-30 The precise identity of the calcium sensors regulating exocytosis in endothelial cells is unknown.
Endothelial exocytosis may play a role in a subset of the physiologic effects of VEGF. For example, VEGF regulates wound repair, and VEGF triggered P-selectin translocation may also play a role in leukocyte recruitment to sites of tissue injury.1-5 However, it is unclear whether or not endothelial exocytosis plays a role in the cellular effects of VEGF, such as endothelial migration, proliferation, differentiation, and survival. Finally, some of the pathophysiologic effects of VEGF are probably not related to exocytosis, such as angiogenesis or permeability. However, our findings provide an explanation for how VEGF causes inflammation: VEGF triggers exocytosis of Weibel-Palade bodies, releasing P-selectin which recruits leukocytes to the vessel wall, and releasing IL-8, which activates them.
VEGF activates NO, which blocks exocytosis
VEGF also modulates exocytosis by activating eNOS through a PI-3 kinase/Akt mechanism previously described.37-40 NO then inhibits Weibel-Palade–body exocytosis triggered by VEGF itself or by other agonists. Previously, we have shown that NO inhibits exocytosis by directly nitrosylating NSF, a key regulator of vesicle trafficking.18,31 The present study shows that not only exogenous but also endogenous NO inhibits exocytosis. These results suggest that other agonists that increase eNOS expression or activity will also inhibit endothelial exocytosis. Furthermore, vascular expression of other NOS isoforms such as the inducible NOS (iNOS) or the neuronal NOS (nNOS) may also lead to regulation of endothelial exocytosis.18
Important studies by Kaufmann and Vischer41 corroborate our work, demonstrating that some agonists activate both exocytosis and NO synthesis. For example, vasopressin and its analog desmopressin (DDAVP) activates endothelial exocytosis of VWF, mediated in part by cyclic adenosine monophosphate (cAMP).25 Furthermore, DDAVP also activates eNOS phosphorylation and NO synthesis from endothelial cells, a process also mediated in part by cAMP.42 However, it is not yet known whether or not the activation of eNOS by DDAVP leads to a decrease in endothelial cell exocytosis. Our data comparing thrombin to VEGF pretreatment of endothelial cells suggest that some but not all agonists of exocytosis activate a negative feedback loop that inhibits exocytosis.
VEGF has opposing effects on exocytosis
Paradoxically, VEGF not only activates endothelial exocytosis through 1 set of pathways, but also inhibits exocytosis through a different set of pathways. Perhaps NO modulates the level of endothelial exocytosis and inflammation following vascular injury. High levels of active eNOS may mitigate vascular inflammation by decreasing exocytosis. However, low levels of eNOS expression or defects in pathways that activate eNOS may permit higher levels of exocytosis, leading to an increase in vascular inflammation. This extrapolation of our results may provide a mechanism by which patients with decreased eNOS activity are predisposed to increased levels of vascular inflammation.43
Prepublished online as Blood First Edition Paper, September 2, 2004; DOI 10.1182/blood-2004-04-1519.
Supported by grants from the National Institutes of Health (NIH) (R01 HL63706-04, R01 HL074061, P01 HL65608, P01 HL56091), the American Heart Association (AHA) (EIG 0140210N), the Ciccarone Center, and the John and Cora H. Davis Foundation (C.J.L.) and by grants from NIH (RR07002 and HL074945) (C.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 6. Long-term treatment with VEGF activates NO synthesis which nitrosylates NSF and inhibits exocytosis. (A) VEGF activates eNOS phosphorylation. HAECs were pretreated with 50 ng/mL VEGF for 30 minutes, and total cell lysates were immunoblotted with antibody to phospho-eNOS or eNOS. (B) VEGF activates NO synthesis. HAECs were treated with 50 ng/mL VEGF for 30 minutes, and the \batchmode \documentclass[fleqn,10pt,legalpaper]{article} \usepackage{amssymb} \usepackage{amsfonts} \usepackage{amsmath} \pagestyle{empty} \begin{document} \(\mathrm{NO}_{2}^{-}\) \end{document} in the supernatant was measured by the Griess reaction (n = 3 ± SD; **P < .01 versus control). (C) Inhibition of endogenous NOS increases VEGF-induced VWF release. HAECs were pretreated with 1 mM L-NAME for 16 hours, washed, and then incubated with 50 ng/mL VEGF for 2 hours. Cells were washed and treated with VEGF 50 ng/mL for 1 hour, and the amount of VWF released from cells into the media was measured by an ELISA (n = 3 ± SD; **P < .01 for VEGF versus VEGF + L-NAME). (D) Exogenous NO decreases VEGF-induced VWF release from human aortic endothelial cells. HAECs were pretreated with SNAP for 4 hours, washed, and then incubated with 50 ng/mL VEGF for 1 hour. The amount of VWF released from cells into the media was measured by an ELISA (n = 3 ± SD; **P < .01 versus 0 μM SNAP). (E) VEGF activates nitrosylation of NSF. HAECs were treated with 50 ng/mL VEGF, and cells were harvested at various times after treatment. Cell lysates were immunoprecipitated with antibody to nitrosocysteine and immunoblotted with antibody to NSF.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/1/10.1182_blood-2004-04-1519/6/m_zh80010571600006.jpeg?Expires=1770559573&Signature=HjIlPWV7KOJNKN5xDqFk1jxGKKPf1etG0uXkd8mWwjRbzTi9rZEWpGGtvxG4ifWnDa80rEFjiFJlCmT8zbMR2BfEjIGAZRwyUWGzm1qwPfY0u7ZpwHvrlk8c8b8hasgkt1FPog~16gaI886ilZhYK179CuICtH5~isdWfl9caBbevIKs~N3zKJdrKfXMDdRJDel4ELMt29u~weCE953~qFcnMRhIsVaI1PfDPMjGFkE7~~Y86nP5EwcR-b6OhpbUewiNv1vnBH0mcx7k31qtE2DzqngYtVieGa9r~I0SnO5mjPNwzimBF2-dGqWQ133sOp7GbRl2Nhj~-qzX3KuRNA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Pretreatment with thrombin does not inhibit VEGF-induced exocytosis. (A) Thrombin pretreatment does not block VEGF activation of exocytosis. HAECs were transduced with adenovirus expressing dominant negative Akt or adenovirus expressing β-galactosidase (LacZ) at an MOI of 200 for 48 hours, then treated with 50 ng/mL VEGF for 2 hours. Cells were then washed and treated with thrombin 1 U/mL for 1 hour, and exocytosis was measured with an ELISA for VWF as above (n = 3 ± SD; **P < .01 versus VEGF + LacZ). (B) VEGF but not thrombin or A23187 increases Akt phosphorylation. HAECs were pretreated with 50 ng/mL VEGF, 1 U/mL thrombin, or 1 μM A23187 for 30 minutes, and total cell lysates were immunoblotted with antibody to phospho-Akt (top row) or total Akt (bottom row). (C) Thrombin does not activate phosphorylation of eNOS. HAECs were transduced with adenoviral vectors expressing constitutively active Akt [Akt (CA)], dominant-negative Akt [Akt (DN)], or β-galactosidase (LacZ) at an MOI of 200 for 48 hours. Cells were then treated with 1 U/mL thrombin for 30 minutes, and total cell lysates were immunoblotted with antibody to phospho-eNOS or eNOS. (D) VEGF but not thrombin pretreatment decreases VEGF-induced exocytosis. HAECs were pretreated with 1 mM L-NAME for 16 hours, washed, and then incubated with 50 ng/mL VEGF or 1 U/mL thrombin for 1 hour. Cells were washed and treated with VEGF 50 ng/mL for 1 hour, and the amount of VWF released from cells into the media was measured by an ELISA (n = 3 ± SD; **P < .01 for VEGF versus VEGF + L-NAME).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/1/10.1182_blood-2004-04-1519/6/m_zh80010571600007.jpeg?Expires=1770559573&Signature=e7JA0HsVokE2bGBinRor5tXlSpB3wHDBY64JZCbxvPsrvEuSf3EFf9OUb0oVAyyzV0sP5cVXQ1q2F7gJC-aZXra-py6ZUx1zVGlbfuwzV~ScBNS15qtiZI4M2EU15Um0SA4y9WRkNgPz27HZA-isBIIiTXAZ6tOblH9HRKaJUM9ZJcprvbZyb2YKs458Awwq0BzzPWJQx88k-DlU3ZtPBdpug6HpfLYpTAK5O4UXWhkfjOrFimm-M0xHInytWvp4ZEXIiK-I3q58BYkk7fb74aSipJaZt9HW4TdgcSl1Lp20WGshv-03BcR2ijWwgBlgt9eNOKXOix~3eB05dGRYhw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal