Abstract
The rapid induction of interferon-γ (IFN-γ) by innate cytokines such as interleukin 12 (IL-12) and IL-18 is critical for immunity against infectious pathogens. We investigated the molecular mechanisms underlying this response. IL-12 and IL-18 rapidly and synergistically induced the secretion of IFN-γ by freshly purified human peripheral blood lymphocytes. At early time points, IFN-γ was expressed almost exclusively by natural killer cells and in both CD56bright and CD56dim subpopulations. Mitogen-activated protein kinase p38 was activated strongly by IL-18 and weakly by IL-12 in natural killer cells but was not activated by either cytokine in T cells. The expression of IFN-γ mRNA and protein was dose-dependently blocked by SB203580, a specific inhibitor of mitogen-activated protein kinase p38, which also caused a dramatic destabilization of IFN-γ mRNA. The 3′ untranslated region (UTR) of IFN-γ mRNA conferred p38 responsiveness to a heterologous reporter mRNA. Therefore, the synergistic induction of IFN-γ by IL-12 and IL-18 in natural killer cells is mediated at least in part by p38-dependent and 3′ UTR-mediated stabilization of IFN-γ mRNA. (Blood. 2005;105:282-288)
Introduction
The production of interferon-γ (IFN-γ) is an early and critical event in the immune response to intracellular pathogens.1-4 As a pleiotropic cytokine, IFN-γ influences both innate and acquired arms of the immune response.5,6 First, it stimulates microbicidal activities of phagocytic cells, for example by activation of the respiratory burst. Second, it favors the development of a proinflammatory, cell-mediated immune response by stimulating antigen presentation and promoting the differentiation of T-helper 1 (Th1) lymphocytes. Mice that lack IFN-γ or its receptor display increased susceptibility to a number of pathogens.5,6 On the other hand, the overexpression or ectopic expression of IFN-γ causes a destructive, even lethal, inflammatory response.7,8 IFN-γ has also been implicated in autoimmune diseases such as insulin-dependent diabetes, multiple sclerosis, and systemic lupus erythematosus.6 Hence, the tight regulation of IFN-γ gene expression is clearly of fundamental importance.
At early time points following infection, the expression of IFN-γ is principally detected in natural killer (NK) cells, later appearing in cytotoxic CD8+ memory T cells, then in Th1 CD4+ lymphocytes as the acquired immune response develops.9-12 The rapid production of IFN-γ by NK cells contributes to host defense prior to the emergence of an effective acquired immune response or even in the absence of such a response.1,13 Although NK cells may respond directly to some pathogens, in many cases the rapid expression of IFN-γ requires the mediation of professional antigen-presenting cells.14 These sentinel cells deploy Toll-like receptors (TLRs) to detect the presence of pathogens and respond by secreting a variety of cytokines, including interleukin-1β (IL-1β), IL-12, IL-15, IL-18, and tumor necrosis factor α (TNFα). Although NK cells constitutively express receptors for both IL-12 and IL-18,15,16 either cytokine alone induces IFN-γ gene expression relatively weakly. In combination, IL-12 and IL-18 synergize to induce IFN-γ expression not only in NK cells but also in B, T, and dendritic cells.17-22
Mitogen-activated protein kinase (MAPK) p38 is required for the induction of IFN-γ gene expression by a variety of agonists in T, NK, and dendritic cells.17,21-27 Mice that lack MAPK-activated protein kinase 2 (MK2), a kinase activated by p38, are defective in expression of IFN-γ and consequently susceptible to the intracellular pathogen Listeria monocytogenes.28,29 MAPK p38 regulates the expression of several proinflammatory genes, including TNFα, IL-6, IL-8, granulocyte/macrophage colony-stimulating factor (GM-CSF), and cyclooxygenase 2 (COX-2) by means of mRNA stabilization.28,29 This posttranscriptional regulation is mediated by MK2 and depends upon adenylate/uridylate-rich elements (AREs), which are located within the 3′ untranslated regions (UTRs) of p38-sensitive transcripts and typically contain several copies of the pentameric motif AUUUA. Human IFN-γ mRNA contains 5 copies of the AUUUA motif within its 3′ UTR, has a short half-life, and is posttranscriptionally regulated by IL-12 and other agonists,30-32 although the mechanism has not been described. We hypothesized that the synergistic induction of IFN-γ protein by IL-12 and IL-18 in human NK cells involves the stabilization of IFN-γ mRNA by the MAPK p38 pathway.
Materials and methods
DNA constructs
Plasmid vectors encoding constitutively active MAPK kinase 6 (MKK6), constitutively active MK2, or dominant-negative MK2 and in vitro transcription vectors for the generation of β-globin and glyceraldehyde phosphate dehydrogenase (GAPDH) riboprobes were previously described.33 The human IFN-γ 3′ UTR was amplified by polymerase chain reaction (PCR) from human genomic DNA using Vent polymerase (New England Biolabs, Hitchin, United Kingdom) and the primers IFN5 (5′-GCGCGGATCCTGGTTGTCCTGCCTGCAATATTTGAATTTTAAATC-3′) and IFN3 (5′-GCGCAGATCTCATATATATTAGATATTGATAATTTTAACAAATG-3′). The PCR product was digested with BamHI and BglII and ligated into BglII-linearized pTetBBB34 (generous gift of Ann-Bin Shyu, University of Texas) to generate pTetBBB-IFN-γ. This construct was verified by sequencing.
Cell isolation, culture, stimulation, and transfection
Human peripheral blood mononuclear cells were prepared by density centrifugation of buffy coats from human venous blood through Ficoll-Hypaque (Nycomed Pharma AS, Oslo, Norway). Lymphocytes were purified by centrifugal elutriation in a Beckman JE6 elutriator (Beckman RIIC, High Wycombe, United Kingdom). Human NK and T cells were enriched by negative selection using cell isolation kits according to manufacturer's instructions (Dynal, Bromborough, United Kingdom). Staining with surface markers routinely revealed greater than 90% purity for NK cells and greater than 95% purity for T cells. Cells were cultured in RPMI 1640 supplemented with 2 mM l-glutamine and 10% heat-inactivated human serum. Freshly purified cells were stimulated with IL-12 and/or IL-18 (R&D systems, Abingdon, United Kingdom; 20 ng/mL unless otherwise stated) and phorbol myristate acetate (PMA; 10 ng/mL) plus ionomycin (1 μg/mL) or sodium arsenite (250 μM). In some experiments, cells were incubated with SB203580 (Sigma Aldrich, Poole, United Kingdom) or with vehicle (0.1% dimethyl sulfoxide [DMSO]) for 5 minutes prior to stimulation. Actinomycin D was used at a final concentration of 5 μg/mL in studies of mRNA stability. Tetracycline-responsive HeLa (HeLa-TO) cells (BD Biosciences, Erembodegem, Belgium) were maintained and transfected as described.33
ELISA
IFN-γ levels in culture supernatants were measured using recombinant human IFN-γ and matched antibody pair sets (BD Biosciences) according to standard enzyme-linked immunosorbent assay (ELISA) protocols. Ninety-six-well ELISA plates were read using a Labsystems Bichromatic plate reader (Lab Systems, Helsinki, Finland), and IFN-γ protein concentrations were calculated using Ascent software (Dynex Labsystems, Ashford, United Kingdom).
Quantification of mRNA
Total RNA was purified from lymphocytes using RNeasy mini kits (Qiagen, Crawley, United Kingdom) and assayed for cytokine mRNAs using the hCK-1 Riboquant Multiprobe RNase Protection Assay system according to manufacturer's instructions (BD Biosciences). For Northern blotting, total RNA samples (10 μg) were subjected to electrophoresis on 1% formaldehyde-agarose gels, 28S rRNA was visualized by staining with SybrGreen (Molecular Probes, Leiden, The Netherlands) and quantified using a Fuji FLA2000 phosphorimager (Raytek Scientific, Sheffield, United Kingdom). Northern blotting was performed as described35 using an IFN-γ cDNA probe labeled using Ready-to-go reagents according to manufacturer's instructions (Amersham Biosciences, Chalfont St Giles, United Kingdom). Measurement of β-globin and GAPDH mRNA in HeLa-TO cells was performed as described.33
Cell staining and flow cytometry
For identification of lymphocyte populations, 106 cells were washed in fluorescence-activated cell sorter (FACS) buffer (phosphate-buffered saline [PBS], 1% fetal calf serum [FCS], 0.1% sodium azide) and stained with fluorochrome-conjugated antibodies directed against cell surface antigens (CD3-peridinin chlorophyll A protein [PerCP] or -fluorescein isothiocyanate [FITC], CD56-phycoerythrin [PE], CD69-PerCP; BD Biosciences) for 20 to 30 minutes on ice. For detection of intracellular IFN-γ, 106 cells were treated with Brefeldin A (10 μg/mL) during stimulation to block cytokine secretion, fixed in 2% paraformaldehyde/PBS for 15 minutes on ice, washed twice with FACS buffer, permeabilized in FACS buffer containing 0.1% saponin on ice for 10 minutes, and incubated with IFN-γ-FITC antibody (BD Biosciences) for 30 minutes on ice and in the dark. For detection of activated p38, 106 cells were fixed immediately in 2% paraformaldehyde/Tris-buffered saline (TBS) for 10 minutes at 37°C, washed twice with modified FACS buffer (TBS, 1% FCS, 0.1% sodium azide), and permeabilized in 90% methanol on ice for 30 minutes. After washing in modified FACS buffer, cells were stained with PE-conjugated phospho-p38 antibody (BD Biosciences) for 1 hour at room temperature. To confirm the specificity of the phospho-p38 antibody, some flow cytometry experiments were performed in the presence of p38 phospho T180/Y182 or non-phospho-peptide competitors (Abcam, Cambridge, United Kingdom). FACS analyses were performed using a FACSvantage scanner and CellQuest software (BD Biosciences).
Western blotting
Western blotting was performed as described36 using a rabbit polyclonal serum against p38 (gift of J. Saklatvala, Imperial College London, United Kingdom) or a phospho-p38-specific serum (Cell Signaling Technology, Hitchin, United Kingdom).
Results
IL-12 and IL-18 rapidly and synergistically induce IFN-γ mRNA and protein expression in a p38-dependent manner
Preliminary experiments investigated the time course of expression of IFN-γ mRNA and protein in response to IL-12 and IL-18 (data not shown). On this basis, 7 hours was selected as a convenient time point at which both mRNA and protein could consistently be detected. Peripheral blood lymphocytes (PBLs) were treated for 7 hours with IL-12 and IL-18, alone or in combination, in the absence or presence of the p38 inhibitor SB203580 (Figure 1A). IL-12 alone consistently induced low levels of IFN-γ mRNA and protein (0.35-3.25 ng/mL, n = 4), which were both reduced to background levels by 1 μM SB203580. Induction of IFN-γ gene expression by IL-18 alone was too weak for accurate measurement at this early time point. In combination, IL-12 and IL-18 strongly induced expression of IFN-γ mRNA and protein, which were both inhibited by 1 μM SB203580. Although there was considerable donor variation in protein expression (3.5-14.2 ng/mL, n = 12), the induction of protein and mRNA was consistently inhibited to a similar extent by 1 μM SB203580 (Figure 1B). The induction of IFN-γ mRNA by IL-12 and -18 was similarly inhibited by 1 μM SB203580 at earlier time points, whereas the induction of IFN-γ protein was similarly inhibited by 1 μM SB203580 if measured after 24 hours (data not shown). IFN-γ expression was significantly induced by stimulation with as little as 1 ng/mL IL-12, and IL-18 and was similarly inhibited by 1 μM SB203580 at all cytokine concentrations tested (data not shown). SB203580 inhibits the kinase activity of p38 with a 50% inhibitory concentration (IC50) of approximately 0.6 μM but can inhibit other kinases at higher concentrations. SB203580 dose dependence was tested to confirm the involvement of p38 in IFN-γ gene expression induced by IL-12 plus IL-18 (Figure 1C). The induction of both IFN-γ mRNA and protein was inhibited significantly at 0.1 μM, greater than 50% at 1 μM, and almost completely at 10 μM SB203580, consistent with the drug's IC50 for inhibition of p38.
Synergistic and p38-dependent induction of IFN-γ mRNA and protein by IL-12 and IL-18. (A) Freshly isolated human PBLs (4 × 106/mL) were treated with 1 μM SB203580 (SB; ▪) or vehicle (0.1% DMSO; □), IL-12 (20 ng/mL) and IL-18 (20 ng/mL) as indicated for 7 hours. Supernatants were collected and IFN-γ protein content was measured by ELISA (top). Error bars indicate SD of triplicate measurements. Total RNA was purified and subjected to ribonuclease protection assay to quantitate IFN-γ mRNA, plus L32 and GAPDH mRNAs as loading controls (bottom). (B) Freshly isolated human PBLs were treated with 1 μM SB203580 (SB; ▪) or vehicle (0.1% DMSO; □) and then stimulated with IL-12 and IL-18 (20 ng/mL each) for 7 hours. IFN-γ protein and mRNA were quantified as described for panel A, and calculated as percentage of expression in the absence of SB203580. Mean values from 7 different donors are plotted. Error bars indicate SEM. (C) Freshly isolated human PBLs (7 × 106/mL) were treated with the indicated doses of SB203580 or vehicle (0.1% DMSO) and stimulated with IL-12 and IL-18 (20 ng/mL each) for 7 hours. IFN-γ protein content of supernatants was measured by ELISA (top). Error bars indicate SD of triplicate measurements. Total RNA was purified; IFN-γ mRNA was quantified by Northern blotting and 28S rRNA was quantified as a loading control (bottom). This experiment was performed twice with very similar results.
Synergistic and p38-dependent induction of IFN-γ mRNA and protein by IL-12 and IL-18. (A) Freshly isolated human PBLs (4 × 106/mL) were treated with 1 μM SB203580 (SB; ▪) or vehicle (0.1% DMSO; □), IL-12 (20 ng/mL) and IL-18 (20 ng/mL) as indicated for 7 hours. Supernatants were collected and IFN-γ protein content was measured by ELISA (top). Error bars indicate SD of triplicate measurements. Total RNA was purified and subjected to ribonuclease protection assay to quantitate IFN-γ mRNA, plus L32 and GAPDH mRNAs as loading controls (bottom). (B) Freshly isolated human PBLs were treated with 1 μM SB203580 (SB; ▪) or vehicle (0.1% DMSO; □) and then stimulated with IL-12 and IL-18 (20 ng/mL each) for 7 hours. IFN-γ protein and mRNA were quantified as described for panel A, and calculated as percentage of expression in the absence of SB203580. Mean values from 7 different donors are plotted. Error bars indicate SEM. (C) Freshly isolated human PBLs (7 × 106/mL) were treated with the indicated doses of SB203580 or vehicle (0.1% DMSO) and stimulated with IL-12 and IL-18 (20 ng/mL each) for 7 hours. IFN-γ protein content of supernatants was measured by ELISA (top). Error bars indicate SD of triplicate measurements. Total RNA was purified; IFN-γ mRNA was quantified by Northern blotting and 28S rRNA was quantified as a loading control (bottom). This experiment was performed twice with very similar results.
NK cells are activated and synthesize IFN-γ in response to IL-12 plus IL-18
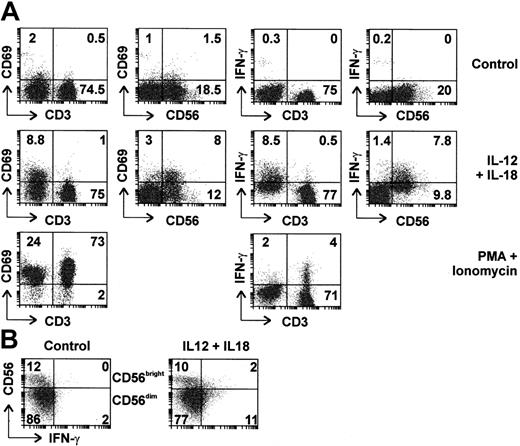
To determine which cells were involved in the response to IL-12 plus IL-18, PBLs were purified by centrifugal elutriation and analyzed by flow cytometry (Figure 2A). Six percent to 22% of PBLs were CD56+ CD3- (NK cells), whereas 2.5% to 10% were CD56+ CD3+ (NKT cells). As expected, CD56+ cells did not express CD4, however, 30% to 50% expressed the CD8 marker as previously reported37 (data not shown). After treatment with IL-12 and IL-18 for 4 hours, 45% of CD56+ cells expressed IFN-γ, whereas a similar proportion (40%) showed up-regulation of CD69, a cell surface marker of activated lymphocytes. Induction of IFN-γ was detected in both CD56+/CD8+ and CD56+/CD8- cells (data not shown). NK cells expressing high levels of CD56 (so-called CD56bright cells) have been characterized as a subclass specialized in the production of cytokines such as IFN-γ, whereas CD56dim cells are specialized in cytotoxic functions.38 In our initial experiments, intracellular IFN-γ was clearly detected in CD56dim cells, however, CD56bright cells were too few for meaningful assessment. Therefore, NK cells were purified by negative selection, treated for 4 hours with IL-12 and IL-18, then assessed by flow cytometry. Under these conditions, both CD56dim and CD56bright cells expressed IFN-γ (for example see Figure 2B).
Both CD56bright and CD56dim NK cells are activated and express IFN-γ in response to IL-12 and IL-18. (A) Freshly isolated human PBLs were left untreated, stimulated with IL-12 and IL-18 (20 ng/mL each), or with PMA and ionomycin for 4 hours in the presence of Brefeldin A. Expression of intracellular IFN-γ and cell surface markers CD3, CD56, and CD69 was assessed by 2-color flow cytometry. Percentages of cells in top left, top right, and bottom right quadrants are indicated. (B) NK cells were purified by negative selection and left untreated or stimulated with IL-12 and IL-18 (20 ng/mL each) for 4 hours. Cell surface CD56 and intracellular IFN-γ expression were assessed by flow cytometry. Percentages of cells present in each quadrant are indicated.
Both CD56bright and CD56dim NK cells are activated and express IFN-γ in response to IL-12 and IL-18. (A) Freshly isolated human PBLs were left untreated, stimulated with IL-12 and IL-18 (20 ng/mL each), or with PMA and ionomycin for 4 hours in the presence of Brefeldin A. Expression of intracellular IFN-γ and cell surface markers CD3, CD56, and CD69 was assessed by 2-color flow cytometry. Percentages of cells in top left, top right, and bottom right quadrants are indicated. (B) NK cells were purified by negative selection and left untreated or stimulated with IL-12 and IL-18 (20 ng/mL each) for 4 hours. Cell surface CD56 and intracellular IFN-γ expression were assessed by flow cytometry. Percentages of cells present in each quadrant are indicated.
At the 4-hours time point, CD3+ cells showed marginal up-regulation of cell surface CD69 and intracellular IFN-γ in response to IL-12 and IL-18. T lymphocytes were capable of IFN-γ expression, since they stained positive for this cytokine following treatment with PMA and ionomycin. In samples that contained higher numbers of NKT cells, significant IFN-γ expression could be detected in CD3+ cells following treatment with IL-12 and IL-18, however, little expression was seen in CD56- cells (data not shown). Therefore, T lymphocytes responded weakly to IL-12 and IL-18, whereas these cytokines induced IFN-γ expression by NK or NKT cells. These experiments did not suggest major differences in the responsiveness of CD56bright and CD56dim or CD8+ and CD8- subpopulations of NK cells.
MAPK p38 is activated by IL-12 and IL-18 in NK cells
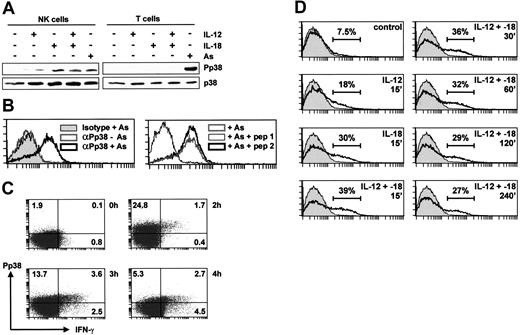
NK and T cells were purified by negative selection from peripheral blood and stimulated for 15 minutes with IL-12, IL-18, or arsenite, a reagent that activates p38 in most cell types. The activation of p38 was monitored by Western blotting with an antibody specific for the phosphorylated (activated) kinase (Figure 3A). Arsenite activated p38 in purified T cells but neither cytokine did so, presumably reflecting the absence of functional receptors for these cytokines in naive T cells. In contrast, p38 was activated strongly by IL-18 and more weakly by IL-12 in purified NK cells.
MAPK p38 is activated by IL-12 and IL-18 only in NK cells. (A) NK or T cells (3 × 106), purified by negative selection from PBLs, were stimulated for 15 minutes with sodium arsenite (As), IL-12, and/or IL-18 (20 ng/mL). Lysates were prepared and Western blotted for phospho-p38 (top) or total p38 (bottom). (B) NK cells were purified by negative selection from PBLs, left untreated or stimulated with sodium arsenite (As) for 15 minutes, then stained with PE-conjugated anti-phospho-p38 antibody or its isotype control (left). In the right-hand panel, cells were treated with sodium arsenite and stained with PE-conjugated anti-phospho-p38 antibody in the presence of 0.1 mg/mL of a phosphorylated p38 peptide corresponding to the antigen (pep 1) or the corresponding nonphosphorylated peptide (pep 2). (C) NK cells were purified by negative selection from PBLs and left untreated or stimulated with IL-12 and IL-18 (20 ng/mL each) for 2, 3, or 4 hours in the presence of Brefeldin A. Intracellular IFN-γ and phospho-p38 content were assessed by 2-color flow cytometry. Note that methanol fixation is required for detection of phospho-p38 but is not optimal for detection of IFN-γ; therefore, expression of the cytokine is underestimated. (D) NK cells were purified by negative selection from PBLs, left untreated or treated with IL-12 and/or IL-18 (20 ng/mL) for the indicated times, and stained with PE-conjugated anti-phospho-p38 antibody. The isotype control is shown in gray in each panel, and estimated percentages of phospho-p38-positive cells are indicated.
MAPK p38 is activated by IL-12 and IL-18 only in NK cells. (A) NK or T cells (3 × 106), purified by negative selection from PBLs, were stimulated for 15 minutes with sodium arsenite (As), IL-12, and/or IL-18 (20 ng/mL). Lysates were prepared and Western blotted for phospho-p38 (top) or total p38 (bottom). (B) NK cells were purified by negative selection from PBLs, left untreated or stimulated with sodium arsenite (As) for 15 minutes, then stained with PE-conjugated anti-phospho-p38 antibody or its isotype control (left). In the right-hand panel, cells were treated with sodium arsenite and stained with PE-conjugated anti-phospho-p38 antibody in the presence of 0.1 mg/mL of a phosphorylated p38 peptide corresponding to the antigen (pep 1) or the corresponding nonphosphorylated peptide (pep 2). (C) NK cells were purified by negative selection from PBLs and left untreated or stimulated with IL-12 and IL-18 (20 ng/mL each) for 2, 3, or 4 hours in the presence of Brefeldin A. Intracellular IFN-γ and phospho-p38 content were assessed by 2-color flow cytometry. Note that methanol fixation is required for detection of phospho-p38 but is not optimal for detection of IFN-γ; therefore, expression of the cytokine is underestimated. (D) NK cells were purified by negative selection from PBLs, left untreated or treated with IL-12 and/or IL-18 (20 ng/mL) for the indicated times, and stained with PE-conjugated anti-phospho-p38 antibody. The isotype control is shown in gray in each panel, and estimated percentages of phospho-p38-positive cells are indicated.
To confirm this finding, flow cytometry was employed to monitor p38 phosphorylation in purified NK cells, using a PE-conjugated antibody against phosphorylated p38. This antibody showed increased staining of arsenite-stimulated NK cells, whereas an isotype-matched control antibody showed no equivalent increase (Figure 3B). Furthermore, staining of arsenite-stimulated NK cells was blocked by a p38 phosphopeptide, but not by the equivalent unphosphorylated peptide, demonstrating the specificity of the phospho-p38 antibody (Figure 3B). Flow cytometry confirmed that p38 was activated strongly by IL-18 and weakly by IL-12, with a slight additive effect in the presence of both cytokines (Figure 3D). The latter effect was consistently detected by flow cytometry but was too subtle to be observed by Western blotting. The activation of p38 by IL-12 and -18 was prolonged, remaining detectable up to 4 hours after the stimulus (Figure 3D). No activation of p38 by IL-12 or IL-18 was observed by flow cytometric analysis of purified T lymphocytes (data not shown).
Flow cytometry was used to investigate the relationship between p38 activation and IFN-γ gene expression (Figure 3C). Intracellular IFN-γ could be detected 2 hours after treatment of cells with IL-12 and -18 and first appeared in a subset of cells staining positive for activated p38. Because p38 activation preceded and was necessary for IFN-γ expression, we suggest that stimulated cells first became phospho-p38 positive (Figure 3C top left quadrant), then positive for both phospho-p38 and IFN-γ (Figure 3C top right quadrant), and then IFN-γ positive but phospho-p38 negative (Figure 3C bottom right quadrant) as the activity of the kinase declined.
IFN-γ mRNA is stabilized by MAPK p38
The involvement of MAPK p38 in the regulation of IFN-γ mRNA stability was investigated by actinomycin D chase experiments. In PBLs stimulated with IL-12 and IL-18, IFN-γ mRNA was potently destabilized by 1 μM SB203580, its half-life decreasing from 83 to 32 minutes (Figure 4A). A similar destabilization of IFN-γ mRNA by 1 μM SB203580 occurred in purified IL-12 + IL-18-treated NK cells (Figure 4B).
MAPK p38 regulates the stability of IFN-γ mRNA. (A) Freshly isolated human PBLs were stimulated with IL-12 and IL-18 (20 ng/mL each) for 4 hours, and then actinomycin D was added in the presence of 1 μM SB203580 or vehicle (0.1% DMSO). Total RNA was purified at the intervals shown and Northern blotted for IFN-γ mRNA. 28S rRNA was quantified as a loading control. (B) The experiment described in panel A was performed 4 times and IFN-γ mRNA was normalized against 28S rRNA and plotted against time on a semilogarithmic graph. Error bars indicate SEM. (C) NK cells were purified by negative selection and stimulated with IL-12 and IL-18 for 4 hours, then actinomycin D was added in the presence of 1 μM SB203580 or vehicle (0.1% DMSO). Total RNA was purified at the intervals shown and IFN-γ mRNA was quantified by ribonuclease protection assay. This experiment was performed twice with identical results.
MAPK p38 regulates the stability of IFN-γ mRNA. (A) Freshly isolated human PBLs were stimulated with IL-12 and IL-18 (20 ng/mL each) for 4 hours, and then actinomycin D was added in the presence of 1 μM SB203580 or vehicle (0.1% DMSO). Total RNA was purified at the intervals shown and Northern blotted for IFN-γ mRNA. 28S rRNA was quantified as a loading control. (B) The experiment described in panel A was performed 4 times and IFN-γ mRNA was normalized against 28S rRNA and plotted against time on a semilogarithmic graph. Error bars indicate SEM. (C) NK cells were purified by negative selection and stimulated with IL-12 and IL-18 for 4 hours, then actinomycin D was added in the presence of 1 μM SB203580 or vehicle (0.1% DMSO). Total RNA was purified at the intervals shown and IFN-γ mRNA was quantified by ribonuclease protection assay. This experiment was performed twice with identical results.
A tetracycline-regulated posttranscriptional reporter system34 was used to determine whether the IFN-γ 3′ UTR mediates mRNA stabilization by the MAPK p38 pathway. Briefly, a rabbit β-globin reporter mRNA is transcribed under the control of a tetracycline-regulated promoter in HeLa-TO cells, which stably express a tetracycline-repressible transcription factor. The reporter mRNA is allowed to accumulate for 24 hours, then its transcription is halted by the addition of tetracycline and its subsequent decay is quantified by ribonuclease protection assay. The β-globin mRNA is stable and unresponsive to p38 activation or inhibition; however, p38-responsive reporter mRNA decay is conferred by the insertion of 3′ UTR elements derived from p38-regulated transcripts. Although IFN-γ gene expression is restricted to certain hematopoietic cells, the components of p38-regulated mRNA decay appear to be well conserved between cells. For example, the p38-mediated stabilization of TNFα, GM-CSF, and tristetrapolin (TTP) mRNAs can be recapitulated in HeLa-TO cells that do not normally express these transcripts.35,39,40 The HeLa-TO system can therefore be used as a convenient assay system to investigate cis-acting sequences and regulatory pathways involved in the control of IFN-γ mRNA stability.
A cDNA fragment corresponding to the full-length human IFN-γ 3′ UTR was cloned downstream of the β-globin coding region in the tetracycline-responsive reporter construct pTetBBB to generate the construct pTetBBB-IFN-γ. The chimeric β-globin-IFN-γ mRNA expressed from this construct decayed with a half-life of approximately 1 hour (Figure 5A) in contrast to a β-globin reporter mRNA, which had a half-life in excess of 4 hours (data not shown). An active mutant of MKK6 was coexpressed with the reporter mRNA to activate the MAPK p38 pathway in transfected cells. This resulted in significant stabilization of the β-globin-IFN-γ reporter mRNA, which could be reversed by addition of 1 μM SB203580. The mechanism of regulation of β-globin-IFN-γ mRNA stability was investigated further by coexpression of constitutively active or dominant-negative forms of MK2, the downstream kinase that mediates mRNA stabilization by p38. A constitutively active mutant of MK2 stabilized the reporter mRNA (Figure 5B), whereas a dominant-negative mutant inhibited the stabilization of reporter mRNA by MKK6 (Figure 5C). Together, these data demonstrate that the IFN-γ 3′ UTR mediates mRNA stabilization by the MKK6-p38-MK2 pathway.
The IFN-γ 3′ UTR mediates regulation of mRNA stability by the p38 pathway. (A) HeLa-TO cells were transfected with 100 ng of pTetBBB-IFN-γ and 100 ng of pCDNA3 or pCDNA3-MKK6E (which expresses a constitutively active mutant of MKK6). Total DNA was made up to 1 μg by addition of carrier (pBluescript; Stratagene, La Jolla, CA). After 24 hours, cells were treated with 1 μM SB203580 (SB) or vehicle (0.1% DMSO) for 5 minutes prior to addition of tetracycline (final concentration 100 ng/mL). Cells were harvested at the intervals shown and ribonuclease protection assays performed to quantify β-globin-IFN-γ (reporter) and GAPDH (loading control) mRNAs. This experiment was performed 3 times, reporter mRNA levels were normalized against GAPDH, and mean outcomes plotted on a semilogarithmic graph (bottom). Error bars indicate SEM. (B) HeLa-TO cells were transfected with 100 ng of pTetBBB-IFN-γ and 800 ng of pEF or pEF-MK2ca (which expresses a constitutively active mutant of MK2). Total DNA was made up to 1 μg by addition of carrier (pBluescript). After 24 hours, tetracycline was added and cells were processed as described above. Mean outcomes of 3 independent experiments were plotted as described for panel A. (C) HeLa-TO cells were transfected with 100 ng of pTetBBB-IFN-γ, 100 ng of pCDNA3 or pCDNA-MKK6E, and 800 ng of pEF or pEF-MK2dn (which expresses a dominant-negative form of MK2). After 24 hours, tetracycline was added, cells were processed, and mean outcomes of 3 independent experiments were plotted as described for panel A.
The IFN-γ 3′ UTR mediates regulation of mRNA stability by the p38 pathway. (A) HeLa-TO cells were transfected with 100 ng of pTetBBB-IFN-γ and 100 ng of pCDNA3 or pCDNA3-MKK6E (which expresses a constitutively active mutant of MKK6). Total DNA was made up to 1 μg by addition of carrier (pBluescript; Stratagene, La Jolla, CA). After 24 hours, cells were treated with 1 μM SB203580 (SB) or vehicle (0.1% DMSO) for 5 minutes prior to addition of tetracycline (final concentration 100 ng/mL). Cells were harvested at the intervals shown and ribonuclease protection assays performed to quantify β-globin-IFN-γ (reporter) and GAPDH (loading control) mRNAs. This experiment was performed 3 times, reporter mRNA levels were normalized against GAPDH, and mean outcomes plotted on a semilogarithmic graph (bottom). Error bars indicate SEM. (B) HeLa-TO cells were transfected with 100 ng of pTetBBB-IFN-γ and 800 ng of pEF or pEF-MK2ca (which expresses a constitutively active mutant of MK2). Total DNA was made up to 1 μg by addition of carrier (pBluescript). After 24 hours, tetracycline was added and cells were processed as described above. Mean outcomes of 3 independent experiments were plotted as described for panel A. (C) HeLa-TO cells were transfected with 100 ng of pTetBBB-IFN-γ, 100 ng of pCDNA3 or pCDNA-MKK6E, and 800 ng of pEF or pEF-MK2dn (which expresses a dominant-negative form of MK2). After 24 hours, tetracycline was added, cells were processed, and mean outcomes of 3 independent experiments were plotted as described for panel A.
Discussion
We set out to investigate the mechanisms of regulation of IFN-γ gene expression by the innate cytokines IL-12 and IL-18. We demonstrate, for the first time to our knowledge, that these cytokines induce rapid and p38-dependent expression of IFN-γ by NK cells. Furthermore, this induction involves the stabilization of IFN-γ mRNA by the MAPK p38 signaling pathway.
Many inflammatory mediators and other immunomodulators are encoded by mRNAs that contain AREs and, as a consequence, are rapidly deadenylated and degraded in the absence of cell stimulation.41 The MAPK p38 signaling pathway is strongly activated by proinflammatory stimuli and controls the inflammatory response in part by stabilizing such transcripts,42,43 possibly by preventing their deadenylation.44 In several respects, IFN-γ behaved like other well-characterized posttranscriptional targets of the p38 pathway. In actinomycin D chase experiments, the p38 inhibitor SB203580 reduced the half-life of IFN-γ mRNA from 83 to 32 minutes, similar to its effect upon COX-2 mRNA stability in lipopolysaccharide (LPS)-stimulated human monocytes,45 TTP mRNA stability in LPS-stimulated mouse macrophages,39 or IL-6 mRNA stability in human fibroblast-like synoviocytes treated with IL-1β.46 Destabilization of IFN-γ mRNA by SB203580 was accompanied by a decrease in its apparent molecular weight, consistent with rapid shortening of the poly(A) tail preceding decay of the mRNA body.44 The human IFN-γ 3′ UTR, like those of TNFα, IL-6, IL-8, COX-2, TTP, and GM-CSF,33,35,39,40 destabilized a heterologous reporter mRNA and conferred stabilization by the MKK6-p38-MK2 pathway in HeLa-TO cells. It is possible that mRNA stabilization by MAPK p38 is involved in the induction of IFN-γ by other agonists. Indeed, our preliminary results indicate p38-mediated stabilization of IFN-γ mRNA following stimulation of PBLs with anti-CD3 and anti-CD28 antibodies (data not shown).
IL-18 shares some, though not all, signaling pathways with IL-1 and is capable of activating p38.47,48 We show that IL-18 activates p38 strongly in NK cells but not detectably in T lymphocytes. NK cells constitutively express the IL-18 receptor whereas naive T cells do not.15,16 IL-12 up-regulates the IL-18 receptor in T cells, providing a mechanism for synergy between the 2 cytokines.20,49 This mechanism is presumably too slow to drive significant T-lymphocyte IFN-γ expression at the early time points investigated here. In NK cells, MAPK p38 was also activated weakly by IL-12, consistent with the weak but p38-dependent induction of IFN-γ by this cytokine.50,51 In contrast to the strong, rapid, and transient activation of p38 by IL-18, IL-12 induced a gradual increase in activity over an extended period (data not shown). This suggests that the activation of p38 by IL-12 may be indirect, for example mediated by up-regulation of GADD45-β or -γ, which can activate the p38 pathway.21,52,53 This is currently under investigation.
Neither IL-18 nor IL-12 alone strongly induced IFN-γ gene expression, therefore, the activation of p38 and stabilization of mRNA are insufficient and transcriptional activation is likely to play a significant role in the synergistic induction of IFN-γ by the 2 cytokines. IL-12 induces transcription by means of tyrosine phosphorylation and activation of signal transducer and activator of transcription 4 (STAT4), a member of the signal-transducer-and-activator-of-transcription family,54-57 whereas IL-18 activates members of the nuclear factor κB (NFκB) and activator protein-1 (AP-1) families.47,54,58 These different families of transcription factors can cooperate to induce IFN-γ expression.54,55 MAPK p38 may participate in transcriptional activation of IFN-γ either through activatory serine phosphorylation of STAT450,59 or via activating transcription factor 2 (ATF2), a member of the AP-1 family.51 These transcriptional mechanisms have been well characterized elsewhere and were not the focus of the studies undertaken here. However, it should be pointed out that transcriptional and posttranscriptional regulation of gene expression by MAPK p38 are not mutually exclusive. For example, p38 was shown to regulate the expression of COX-2 at both of these levels in LPS-stimulated human monocytes.45
In NK cells, the IFN-γ promoter is primed for transcriptional activation, as reflected by constitutive demethylation of the locus, acetylation of histone H3, and generation of transcripts.60,61 The short half-life of these transcripts prevents synthesis of protein in the absence of appropriate stimuli.62 These conditions make the cell exquisitely poised for rapid and efficient induction of IFN-γ biosynthesis in response to signals that activate transcription on one hand and stabilize the mRNA on the other. Our data suggest that the stabilization of IFN-γ mRNA by the p38 pathway is an important component of this response.
Flow cytometry is potentially a powerful technique for the study of MAPK signaling in cell populations but has been described in relatively few publications.63 We used this method to confirm the strong activation of MAPK p38 by IL-18 and the weak activation by IL-12 in NK but not T cells. Kinase activation was observed in only a proportion of NK cells (approximately 40% at the 15-minutes time point). As expected, expression of IFN-γ was first detected in cells that contained phosphorylated (activated) p38. It is difficult to detect cell surface markers and phosphorylated kinases simultaneously by flow cytometric methods because of incompatibilities of fixation and permeabilization methods.63 For this reason we have been unable to characterize in more detail the subsets of NK cells in which p38 is activated. However, the expression of IFN-γ (and by inference, the activation of p38) was clearly not restricted to CD56bright cells. These cells preferentially expressed IFN-γ after prolonged stimulation with IL-12 and IL-18,64-66 yet the more numerous CD56dim cells appeared to constitute a significant source of IFN-γ at the early time points examined here. For example, 4 hours after stimulation with IL-12 and IL-18, 80% to 85% of IFN-γ-expressing NK cells were CD56dim. Although we found expression of IFN-γ in CD8+ cells, these were apparently NK cells rather than T lymphocytes. Four hours stimulation with IL-12 and IL-18 was presumably insufficient for strong induction of IFN-γ in CD8+ memory T cells, as described elsewhere.9,10,67
Inflammatory, autoimmune, and immunodeficient pathologies are associated with overexpression, ectopic expression, or underexpression of IFN-γ. For example, splenocytes from MK2 knock-out mice are deficient in the induction of IFN-γ gene expression by Listeria monocytogenes, and the mice display increased susceptibility to this intracellular pathogen.29 These observations suggest that an MKK6/p38/MK2-dependent mechanism for the stabilization of IFN-γ mRNA may play an important role in host defenses against intracellular pathogens, although it is necessary to investigate the stability of IFN-γ mRNA in the MK2-/- mice to establish this with certainty. Defects in the posttranscriptional regulation of gene expression may contribute to a wide range of pathologies68 ; therefore, in diseases characterized by dysregulation of IFN-γ, it would be interesting to investigate the function of the p38-mediated regulatory mechanism described in this manuscript and the potential impact of previously defined polymorphisms of the human IFN-γ 3′ UTR.69,70
Prepublished online as Blood First Edition Paper, September 2, 2004; DOI 10.1182/blood-2004-07-2782.
Supported by the Arthritis Research Campaign, Chesterfield, United Kingdom.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal