Comment on Koschmieder et al, page 324
A novel binary transgenic mouse model utilizing the SCL enhancer to conditionally express BCR-ABL in hematopoietic stem cells is a promising new tool to dissect the molecular pathogenesis of CML.
Since the late 1980s, modeling human leukemia in laboratory mice through expression of leukemia oncogenes in bone marrow has been a major goal. Two main experimental approaches have been employed: generation of transgenic mice, and ex vivo retroviral transduction of bone marrow followed by transplantation into recipient mice. For modeling chronic myeloid leukemia (CML), the retroviral method has thus far proven more fruitful. Induction of CML-like myeloproliferative disease in recipients of marrow transduced with BCR-ABL retrovirus implicated the Bcr-Abl tyrosine kinase as the direct cause of CML and validated it as a drug target.1 Subsequent studies have identified leukemogenic signaling pathways and tested novel therapies for CML.2
In contrast, BCR-ABL transgenic mice have been more problematic (see the review in Van Etten3 ). Expression of BCR-ABL under an immunoglobulin enhancer caused sporadic lymphomas but BCR-ABL was not expressed in pre-B cells in young transgenic mice, suggesting the oncogene was silenced during development. Embryonic toxicity was confirmed when BCR-ABL was expressed from the murine bcr promoter. Subsequent models using the metallothionein and tec promoters to direct BCR-ABL expression yielded B-lymphoid and myeloid leukemias, but concerns about toxicity and silencing of BCR-ABL in transgenic mice have persisted.FIG1
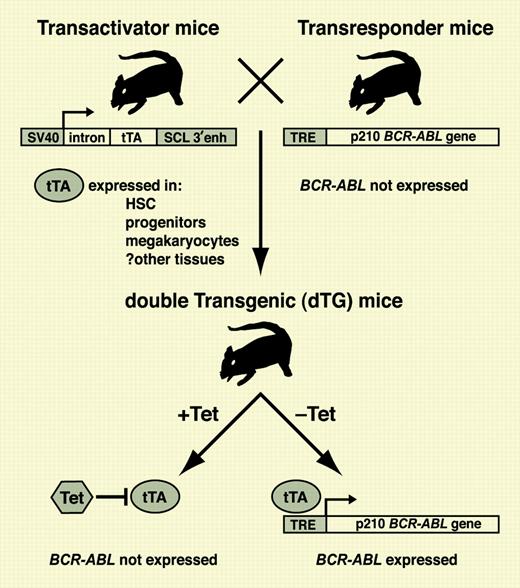
In transactivator mice, tTA is expressed in HSCs and progenitors under control of the SCL enhancer, whereas transresponder mice do not express the BCR-ABL transgene regulated by the tetracycline response element (TRE). In double-transgenic progeny, tTA is repressed in the presence of tetracycline (Tet) but can transactivate the BCR-ABL gene in HSCs upon withdrawal of antibiotic. For details, see the article by Koschmieder et al beginning on page 324.
In transactivator mice, tTA is expressed in HSCs and progenitors under control of the SCL enhancer, whereas transresponder mice do not express the BCR-ABL transgene regulated by the tetracycline response element (TRE). In double-transgenic progeny, tTA is repressed in the presence of tetracycline (Tet) but can transactivate the BCR-ABL gene in HSCs upon withdrawal of antibiotic. For details, see the article by Koschmieder et al beginning on page 324.
A potential solution is transgenic mice where the expression of the BCR-ABL gene can be controlled in vivo and activated postnatally. One approach, pioneered by the laboratories of Dan Tenen at Harvard and Claudia Huettner at the Blood Center of Southeastern Wisconsin, is a binary transgene system employing tTA, a prokaryotic transcriptional activator protein whose function can be negatively regulated in mice by tetracycline antibiotic in the drinking water (see figure). A “transactivator” strain of mice expressing tTA in a tissue-specific manner is mated with a “transresponder” strain carrying the BCR-ABL gene under the control of a tetracycline response element. In progeny inheriting both transgenes, removal of doxycycline from the water leads to specific induction of BCR-ABL in those cells expressing tTA. The key element of this high-tech system is the promoter/enhancer driving tTA. For CML, expression of tTA should ideally be restricted to the hematopoietic stem cell and perhaps some downstream progenitors. Previous binary BCR-ABL models using the mouse mammary tumor virus enhancer or human CD34 promoter to express tTA yielded B-lymphoid leukemia and essential thrombocythemia-like disease, but not bona fide CML.4 In the current issue of Blood, Koschmieder and colleagues utilize the 3′ enhancer of the murine stem cell leukemia (SCL) gene, known to direct transgene expression in murine long-term repopulating cells, to express tTA. Upon withdrawal of doxycycline, double-transgenic progeny developed fatal CML-like illness characterized by neutrophilia and splenomegaly, with some animals succumbing to superimposed B-lymphoid leukemia suggestive of blast crisis. Myeloid cells expressed BCR-ABL transcripts and protein, and the syndrome was reversible upon readministration of antibiotic.
Thus, the long-awaited transgenic model of CML may be in hand. One cautionary note is that because some SCL transactivator lines expressed tTA in lung and intestines, it will be important to demonstrate that CML-like disease can be reproduced in nontransgenic recipients by transplantation of double-transgenic bone marrow. In the present study, attempts to transfer the disease to immunodeficient recipients were unsuccessful, possibly due to poor engraftment. This transgenic model could be superior to retroviral models for investigating many aspects of CML pathophysiology because of the ease of generation of a uniform cohort of diseased mice and the ability to switch BCR-ABL expression on and off. For example, a recent study suggested that the size of the hematopoietic stem cell (HSC) compartment is normal in patients with chronicphase CML,5 but Koschmieder and colleagues found the corresponding population to be increased 7-fold in leukemic double-transgenic mice. The opportunity to carry out functional transplantation studies of these putative BCR-ABL–expressing HSCs will be invaluable for resolving this paradox.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal