Abstract
After invading human red blood cells (RBCs) the malaria parasite Plasmodium falciparum remodels the host cell by trafficking proteins to the RBC compartment. The virulence protein P falciparum erythrocyte membrane protein 1 (PfEMP1) is responsible for cytoadherence of infected cells to host endothelial receptors. This protein is exported across the parasite plasma membrane and parasitophorous vacuole membrane and inserted into the RBC membrane. We have used green fluorescent protein chimeras and fluorescence photobleaching experiments to follow PfEMP1 export through the infected RBC. Our data show that a knob-associated histidine-rich protein (KAHRP) N-terminal protein export element appended to the PfEMP1 transmembrane and C-terminal domains was sufficient for efficient trafficking of protein domains to the outside of the P falciparum–infected RBC. The physical state of the exported proteins suggests trafficking as a complex rather than in vesicles and supports the hypothesis that endogenous PfEMP1 is trafficked in a similar manner. This study identifies the sequences required for expression of proteins to the outside of the P falciparum–infected RBC membrane.
Introduction
Plasmodium falciparum, the causative agent of the most severe form of malaria in humans, is responsible for over 2 million deaths per year. The parasite invades red blood cells (RBCs) and initiates remodeling resulting in dramatic morphologic changes that play a role in the pathogenesis of disease. An important property of parasite-infected RBCs is their ability to adhere to receptors on the host microvasculature.1 The key protein involved in cytoadherence is the antigenic variant P falciparum erythrocyte membrane protein 1 (PfEMP1).2-4 PfEMP1 binds to different host receptors such as CD36, intercellular adhesion molecule-1 (ICAM-1), and chondroitin sulfate and is important for immune evasion.
Electron micrographs of parasite-infected RBCs reveal electron dense cup-shaped structures underlying the RBC membrane known as knobs.5 Knobs are composed of the knob-associated histidinerich protein (KAHRP). Knob proteins interact with the host cytoskeleton6,7 and KAHRP binds to the cytoplasmic tail of PfEMP1, which is known as the acidic terminal segment (ATS). The putative transmembrane (Tm) region of PfEMP1 is thought to be integrated into the RBC membrane thereby presenting the large extracellular domain at the external surface (see Kyes et al1 for review). The ectodomain comprises an N-terminal segment followed by modular Duffy-binding–like (DBL) domains and cysteinerich interdomain regions (CIDRs) of variable number that provide adhesive properties.
P falciparum invades the RBC by invagination of the host membrane whereby the parasite becomes surrounded by a parasitophorous vacuolar membrane (PVM). As a consequence exported proteins traverse the parasite plasma membrane and PVM to reach the host cell cytosol. In the case of PfEMP1, additional events insert the protein into the RBC membrane. Exported proteins have a hydrophobic signal near the N-terminus that directs translocation into the endoplasmic reticulum (ER) and default trafficking to the parasitophorous vacuole.8,9 PfEMP1 lacks an N-terminal hydrophobic signal and it has been assumed that the Tm acts as a “start transfer” signal for insertion into the ER membrane.10 Recent work has identified a protein export element (PEXEL) in the N-terminus of exported proteins required for transport across the PVM.11,12 KAHRP and other exported proteins possess a conserved PEXEL motif whereas a related translocation motif appears to be important for trafficking PfEMP1 across the PVM.11,12 It is not clear what additional information is required for trafficking of PfEMP1 across the RBC cytosol and its insertion into the RBC membrane.
The RBC lacks the trafficking machinery present in other cells and the parasite transposes structures outside into the host cell.13 Current evidence suggests structures known as Maurer clefts, which are elaborated in the host cytosol, are important in protein trafficking and sorting within the RBC.8,14 For example, both KAHRP and PfEMP1 transiently associate with Maurer clefts before relocating to the host membrane.8 However both the signals and the molecular machinery responsible for trafficking of PfEMP1 across the host cell remain undefined.
To understand trafficking of PfEMP1 to the surface of P falciparum–infected RBCs we have generated parasites expressing PfEMP1–green fluorescent protein (GFP) chimeras and identified sequences required for this process. In this study, we provide the first example of trafficking of a transgenic protein to the external surface of the P falciparum–infected RBC.
Materials and methods
Plasmid constructs and transfection, Western blotting, and antibodies
We prepared transfection constructs in the plasmid vector pARL and generated transfectants as described15 (Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Infected RBCs were purified by gelatin16 or magnetic sorting (Miltenyi Biotec, Auburn, CA). Protein samples were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and probed with mouse αGFP (Roche, Indianapolis, IN; 1:1000), mouse αKAHRP-His (1:1000), rabbit αKAHRP-3′ repeats (1:2000), or rabbit αheat-shock protein 70 (αhsp70) antiserum (1:6000) and visualized using enhanced chemiluminescence (ECL; Amersham, Uppsala, Sweden). Antibodies were raised in mice to amino acids 38 to 126 of KAHRP (histidine-rich domain).
Brefeldin A and microscopy
Brefeldin A (Sigma, St Louis, MO) was added to a final concentration of 5 μg/mL for 16 hours. GFP-expressing parasites were imaged using a Leica TCS SP2 confocal microscope (Heidelberg, Germany) or a Carl Zeiss Axioskop 2 (Thornwood, NY) with a PCO SensiCam (Motion Engineering Company, Indianapolis, IN) and Axiovision 3 software (Carl Zeiss). Immunoelectron microscopy was performed as described.17 Sections were incubated with a rabbit αGFP antibody (1:500). Sections were viewed on a Philips CM120 transmission electron microscope. For indirect immunofluorescence, parasites were fixed with 4% formaldehyde/phosphate-buffered saline (PBS) and permeabilized in 0.05% Triton X-100/PBS. Slides were incubated with antisera (rabbit αGFP [1:1000], mouse αP falciparum skeleton-binding protein 1 [αPfSBP1; 1:400], mouse α exported protein-1 [αEXP1; 1:2000], rat αPfBiP [1:1000], mouse PfEMP1 αATS [1:100], rabbit αKAHRP 3′repeats [1:1000], and mouse αKAHRP-His [1:200]) and detected with appropriate secondary antibodies. ER was stained with 500 nM ER Tracker Blue White DPX (Molecular Probes, Eugene, OR). Live parasitized RBCs were labeled with antibody by incubating with either rabbit αGFP (1:1000) or mouse αKAHRP-His (1:200) antibody in 3% (wt/vol) bovine serum albumin (BSA)/PBS. As control, cells were permeabilized with streptolysin O (Sigma) as described.14 A Zeiss Axioskop 2 microscope equipped with a 100×/1.4 Plan-Apochrome oil objective lens and acquisition software (Carl Zeiss, Mannheim, Germany) was used to collect the images in Figures 2, 3, 4, 5. A TCS SP2 confocal microscope was used to collect the images in Figures 4B and 6 as described previously.18
Fluorescence recovery after photobleaching
Results
PfEMP1-GFP chimeras in P falciparum–infected RBCs
Marti et al11 appended the N-terminal region of PfEMP1 (including its PEXEL motif) to the Tm and ATS of PfEMP1 and showed that this chimera was able to direct a yellow fluorescent protein (YFP) tag to the RBC cytosol. However, this construct was expressed at low levels, making further analysis difficult. Moreover, it was not possible to determine if PfEMP1 molecules were delivered to the external surface of the RBC as the N-terminal region lacked a tag that could be recognized. To analyze elements required for targeting PfEMP1 to the RBC membrane and determine if the PEXEL in KAHRP was sufficient for trafficking to the erythrocyte surface, we generated a series of GFP-fusion constructs in the vector pARL11 (Figure 1). The first construct fused the N-terminal segment (NTS)/DBL1α domain of PfEMP1 (Figure 1A) to GFP (NTS/DBL1α-GFP; Figure 1B). This region lacks an ER entry sequence but includes the PEXEL. The second construct comprised 20 amino acids of the CIDR2β domain followed by the PfEMP1 Tm and the first 113 amino acids of ATS (TmATSs-GFP). The third construct consisted of 20 amino acids of the CIDR2β domain followed by the PfEMP1 Tm with the full-length ATS (TmATS-GFP). The fourth construct placed the acyl carrier protein (ACP) N-terminal signal sequence21 upstream of the CIDR2β fragment followed by the Tm and ATS of PfEMP1 (ACPTmATSs). To determine the effect of adding an N-terminal hydrophobic signal sequence and a PEXEL to the Tm of PfEMP1 we designed additional constructs. The fifth construct had the first 60 amino acids of KAHRP followed by PfEMP1 CIDR2β fragment, the Tm, and a partial ATS fused to GFP (K60TmATSs-GFP). The sixth construct had the first 60 amino acids of KAHRP followed by PfEMP1 CIDR2β fragment and the Tm but included the full-length ATS (K60TmATS-GFP). The seventh construct contained 120 amino acids of KAHRP including the histidine-rich domain (from amino acids 60-119) together with the CIDR2β fragment, the Tm, and full-length ATS (K60TmATS-GFP). The KAHRP histidinerich domain could be recognized using an αKAHRP-His anti-serum. Finally, we sought to determine if the CIDR2β-PfEMP1 Tm region could be replaced with the Tm region of the apical membrane antigen-1 (AMA1), a type 1 integral membrane protein. This eighth construct contained the N-terminal 60 amino acids of KAHRP followed by 41 amino acids of AMA1 (20–amino acid N-terminal to the Tm plus the Tm region) followed by truncated ATS of PfEMP1 fused to GFP (K60Tm(ama1)ATSs).22,23 The resultant GFP chimeras were expressed in transfected 3D7 (KAHRP+) or D10 (KAHRP-) P falciparum.
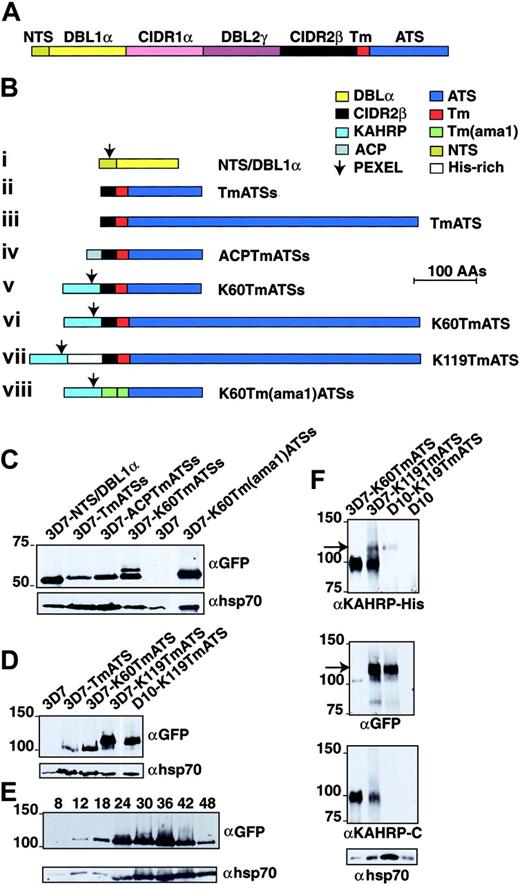
PfEMP1 structure and chimeric proteins expressed in P falciparum. (A) PfEMP1 has a putative Tm region (Tm; red segment) followed by a conserved acidic terminal segment (ATS; dark blue). The extracellular domain is variable in sequence and size but assembled from 3 main building blocks: NTS (N-terminal segment; dark yellow), DBL (Duffy-binding–like) domain (yellow and purple segments), and CIDR (cysteine-rich interdomain region; pink and black segments). Sizes of each domain are not to scale. (B) Structures of the proteins expressed from plasmid constructs. The color of each domain is the same as in panel A. All are chimeric proteins with GFP at the C-terminus. (i) NTS and DBL1α. (ii) Twenty amino acids (AAs) of CIDR2β, PfEMP1 Tm, and 113 amino acids of ATS (ATSs). (iii) The CIDR2β fragment, PfEMP1 Tm, and 447 amino acids of ATS. (iv) The signal peptide of ACP (amino acids 1-20; gray segment), the CIDR2β fragment, PfEMP1 Tm, and ATSs. (v) The first 60 amino acids of KAHRP (light blue), the CIDR2β fragment, PfEMP1 Tm, and ATSs. (vi) KAHRP1-60, CIDR2β fragment, PfEMP1 Tm, and full-length ATS. (vii) KAHRP1-119 including the His-rich domain, CIDR2β domain, PfEMP1 Tm, and full-length ATS. (viii) KAHRP1-60, 20 amino acids of the AMA1 exodomain (green), AMA1 Tm, and ATSs. The arrow shows the position of the PEXEL in each construct. (C) Transgene expression of GFP chimeric proteins in P falciparum transfected with the PfEMP1 constructs. The 3D7-NTS/DBL1α, 3D7-TmATSs, 3D7-ACPTmATSs, 3D7-K60TmATSs, 3D7, and 3D7-K60Tm(ama1)ATSs were subjected to Western analysis and probed with αGFP antibody. The top band in 3D7-K60TmATSs may represent full-length chimeric protein before cleavage of the KAHRP signal peptide. Shown below is the same blot probed with αhsp70 antibodies. (D) The 3D7, 3D7-TmATS, 3D7-K60TmATS, and 3D7-K119TmATS were probed with αGFP antibody. Shown below is the same blot probed with αhsp70 antibodies. (E) The 3D7-K119TmATS parasites were synchronized and samples were taken at various intervals after invasion. Shown below is the same blot probed with αhsp70 antibodies. (F) The 3D7-K60TmATS, 3D7-K119TmATS, D10-K119TmATS, and D10 parasitized RBCs were probed with αKAHRP-His, αGFP, αKAHRP-C (3′ repeat region of C-terminus), and αhsp70 antibodies. The arrow indicates the 120-kDa K119-TmATS-GFP protein.
PfEMP1 structure and chimeric proteins expressed in P falciparum. (A) PfEMP1 has a putative Tm region (Tm; red segment) followed by a conserved acidic terminal segment (ATS; dark blue). The extracellular domain is variable in sequence and size but assembled from 3 main building blocks: NTS (N-terminal segment; dark yellow), DBL (Duffy-binding–like) domain (yellow and purple segments), and CIDR (cysteine-rich interdomain region; pink and black segments). Sizes of each domain are not to scale. (B) Structures of the proteins expressed from plasmid constructs. The color of each domain is the same as in panel A. All are chimeric proteins with GFP at the C-terminus. (i) NTS and DBL1α. (ii) Twenty amino acids (AAs) of CIDR2β, PfEMP1 Tm, and 113 amino acids of ATS (ATSs). (iii) The CIDR2β fragment, PfEMP1 Tm, and 447 amino acids of ATS. (iv) The signal peptide of ACP (amino acids 1-20; gray segment), the CIDR2β fragment, PfEMP1 Tm, and ATSs. (v) The first 60 amino acids of KAHRP (light blue), the CIDR2β fragment, PfEMP1 Tm, and ATSs. (vi) KAHRP1-60, CIDR2β fragment, PfEMP1 Tm, and full-length ATS. (vii) KAHRP1-119 including the His-rich domain, CIDR2β domain, PfEMP1 Tm, and full-length ATS. (viii) KAHRP1-60, 20 amino acids of the AMA1 exodomain (green), AMA1 Tm, and ATSs. The arrow shows the position of the PEXEL in each construct. (C) Transgene expression of GFP chimeric proteins in P falciparum transfected with the PfEMP1 constructs. The 3D7-NTS/DBL1α, 3D7-TmATSs, 3D7-ACPTmATSs, 3D7-K60TmATSs, 3D7, and 3D7-K60Tm(ama1)ATSs were subjected to Western analysis and probed with αGFP antibody. The top band in 3D7-K60TmATSs may represent full-length chimeric protein before cleavage of the KAHRP signal peptide. Shown below is the same blot probed with αhsp70 antibodies. (D) The 3D7, 3D7-TmATS, 3D7-K60TmATS, and 3D7-K119TmATS were probed with αGFP antibody. Shown below is the same blot probed with αhsp70 antibodies. (E) The 3D7-K119TmATS parasites were synchronized and samples were taken at various intervals after invasion. Shown below is the same blot probed with αhsp70 antibodies. (F) The 3D7-K60TmATS, 3D7-K119TmATS, D10-K119TmATS, and D10 parasitized RBCs were probed with αKAHRP-His, αGFP, αKAHRP-C (3′ repeat region of C-terminus), and αhsp70 antibodies. The arrow indicates the 120-kDa K119-TmATS-GFP protein.
To confirm expression of PfEMP1-GFP proteins we used Western blots probed with αGFP. No reactivity was observed in 3D7 (Figure 1C) or D10 (Figure 1F) parasites, whereas all transfected parasites expressed GFP chimeras. The 3D7-NTS/DBL1α parasites expressed a protein of approximately 52 kDa, whereas 3D7-TmATSs, 3D7-ACPTmATSs, 3D7-K60TmATSs, and 3D7-K60Tm(ama1)ATSs showed GFP chimera between 54 and 62 kDa. Parasites transfected with full-length ATS (ie, 3D7-TmATS, 3D7-K60TmATS, 3D7-K119TmATS, and D10-K119TmATS) expressed proteins of approximately 100 to 120 kDa (Figure 1D). The K119TmATS-chimera was first detected at 12 hours with increasing amounts in later stages (Figure 1E). Transcription of var commences early with maximal levels at 12 hours after invasion24 and PfEMP1 is first detected on the surface 16 hours after invasion.16,25 Thus, the chimeric PfEMP1-GFP proteins, expressed from the Pfcrt promoter in the vector pARL, are expressed at a similar stage to endogenous PfEMP1.
Antibodies against the N-terminal domain (38-126 amino acids) of KAHRP were used to detect K119TmATS-GFP chimeras but did not detect fusion proteins with only the N-terminal 60 amino acids of KAHRP (Figure 1F). Endogenous KAHRP (98 kDa) was detected in knob+ 3D7 but not in D10 parasites, which lack this protein due to deletion of the gene26 (Figure 1F). Endogenous KAHRP was the only protein detected with αKAHRP-His antibodies in parasites expressing 3D7-K60TmATS, which lacks the KAHRP-His region. In contrast, 3D7-K119TmATS and D10-K119TmATS express a protein of approximately 120 kDa detected with αKAHRP-His and αGFP (Figure 1F arrow). Antibodies recognizing the C-terminal region of KAHRP (αKAHRP-C) detected endogenous KAHRP in 3D7-K60TmATS and 3D7-K119TmATS but not in D10-K119TmATS and D10 as expected (Figure 1F third panel). These results demonstrated that transgenic parasites expressed chimeric PfEMP1-GFP proteins.
Trafficking of GFP chimeras
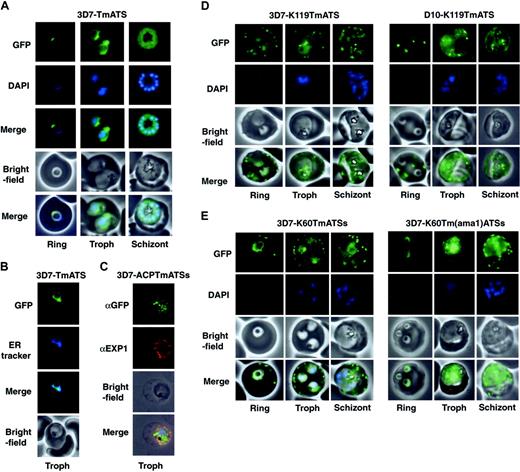
Expression of GFP chimeras in parasites resulted in green fluorescence in defined regions. The NTS/DBL1α-GFP protein showed a diffuse pattern in the parasite cytoplasm indicating this region of PfEMP1 was insufficient for entry into the secretory system (Figure S1). In contrast, 3D7 parasites expressing TmATS-GFP (Figure 2A) or TmATSs-GFP (data not shown) displayed a perinuclear pattern that matched ER tracker (Figure 2B). Whilst this is consistent with the Tm of PfEMP1 being sufficient for entry into the ER it is possible that the protein has associated with the cytosolic face of the ER.
The N-terminal signal sequence of ACP is sufficient for protein transport to the parasitophorous vacuole.21 Therefore, we attached the ACP signal to the Tm and ATS of PfEMP1 (3D7-ACPTmATSs; Figure 1B). The resultant transgenic parasites express low levels of chimera, however detection of the protein by immunofluorescence using αGFP revealed punctate structures within the parasite (Figure 2C). These structures partly overlapped the parasitophorous vacuole as defined by labeling with EXP1 (Figure 2C). Thus, a classical hydrophobic signal sequence appears to direct a part of the population of PfEMP1 chimeras to the parasitophorous vacuole but not beyond.
We also generated transfected 3D7 (Figure 2D; Video S1) and D10 (Figure 2D) parasites expressing the first 60 or 119 amino acids of KAHRP followed by the PfEMP1 Tm and ATS (K119TmATS) or ATSs (K60TmATSs). In each case and in all life cycle stages the chimera was transported into the host cell, which associated with punctate structures resembling Maurer clefts. A rim fluorescence pattern was also observed consistent with host cell membrane association. The data indicate that the KAHRP hydrophobic signal peptide and PEXEL motif are sufficient to transport PfEMP1 domains into the host cell. Transit across the PVM and Maurer-cleft association both appear to be independent of the presence (3D7) or absence (D10) of endogenous KAHRP and PfEMP3. A chimera comprising the first 60 amino acids of KAHRP plus a CIDR2β fragment fused to GFP was trafficked to the RBC cytosol but did not associate with punctate structures in the host cell (Figure S2), indicating that the Tm was needed for Maurer cleft association.
The PfEMP1 Tm and the 20–amino acid CIDR2β region were substituted with the Tm of AMA122 to determine if the PfEMP1 Tm was required for correct trafficking (3D7-K60Tm(ama1)ATSs; Figure 2E). The chimeric protein was transported into the RBC cytoplasm and associated with Maurer clefts although export efficiency was low. It appears that the AMA1 Tm region can at least partially complement the function of the equivalent region in PfEMP1.
Trafficking of PfEMP1-GFP to Maurer clefts
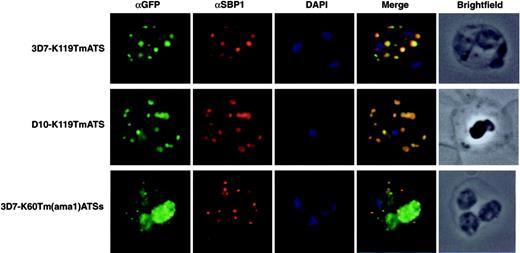
To determine whether the GFP fluorescence detected in foci within the RBC of K119TmATS and K60TmATSs transfectants was correspondent with Maurer clefts association we tested colocalization with PfSBP1, a known marker of these structures.27 Immuno-fluorescence confirmed that both K119TmATS-GFP (Figure 3) and K60TmATSs-GFP (data not shown) colocalized with PfSBP1 in Maurer clefts. The same result was observed for the knob- D10 parasites expressing K119TmATS. Similarly, the K60Tm(ama1)ATSs-GFP protein was exported to the RBC cytoplasm and colocalized with PfSBP1.
Expression of PfEMP1-GFP chimeras inP falciparum–infected RBCs. (A) Fluorescence images of 3D7-TmATS at different stages. The first row represents the GFP fluorescence; second row, the DAPI (4,6 diamidino-2-phenylindole)–stained nuclei; third row, an overlay of the GFP and DAPI images; fourth row, the bright-field images; last row, overlays of all 3 images. (B) ER localization of GFP fluorescence in 3D7-TmATS transfectants. First panel, GFP fluorescence; second panel, ER tracker fluorescence; third panel, overlay of GFP and ER tracker fluorescence; fourth panel, bright-field images. (C) Immunofluorescence imaging of fixed 3D7-ACPTmATSs transfectants. First row, αGFP; second row, αEXP1; third row, bright-field image; fourth row, overlay of all images. (D-E) Expression of K119TmATS-GFP, K60TmATSs-GFP, and K60Tm(ama1)ATSs-GFP proteins. All images represent live parasites of the 3D7 or D10 strains collected at ring, trophozoite (Troph), and schizont stages during the 48-hour asexual life cycle and were stained with DAPI. First row, GFP fluorescence; second row, DAPI; third row, bright-field images; fourth row, overlay of GFP fluorescence and bright-field image. DNA in early ring stages did not stain with DAPI; only nuclei of parasites more than 12 hours past invasion were visible after DAPI staining.
Expression of PfEMP1-GFP chimeras inP falciparum–infected RBCs. (A) Fluorescence images of 3D7-TmATS at different stages. The first row represents the GFP fluorescence; second row, the DAPI (4,6 diamidino-2-phenylindole)–stained nuclei; third row, an overlay of the GFP and DAPI images; fourth row, the bright-field images; last row, overlays of all 3 images. (B) ER localization of GFP fluorescence in 3D7-TmATS transfectants. First panel, GFP fluorescence; second panel, ER tracker fluorescence; third panel, overlay of GFP and ER tracker fluorescence; fourth panel, bright-field images. (C) Immunofluorescence imaging of fixed 3D7-ACPTmATSs transfectants. First row, αGFP; second row, αEXP1; third row, bright-field image; fourth row, overlay of all images. (D-E) Expression of K119TmATS-GFP, K60TmATSs-GFP, and K60Tm(ama1)ATSs-GFP proteins. All images represent live parasites of the 3D7 or D10 strains collected at ring, trophozoite (Troph), and schizont stages during the 48-hour asexual life cycle and were stained with DAPI. First row, GFP fluorescence; second row, DAPI; third row, bright-field images; fourth row, overlay of GFP fluorescence and bright-field image. DNA in early ring stages did not stain with DAPI; only nuclei of parasites more than 12 hours past invasion were visible after DAPI staining.
Trafficking of a protein domain to the outside of P falciparum–infected RBCs
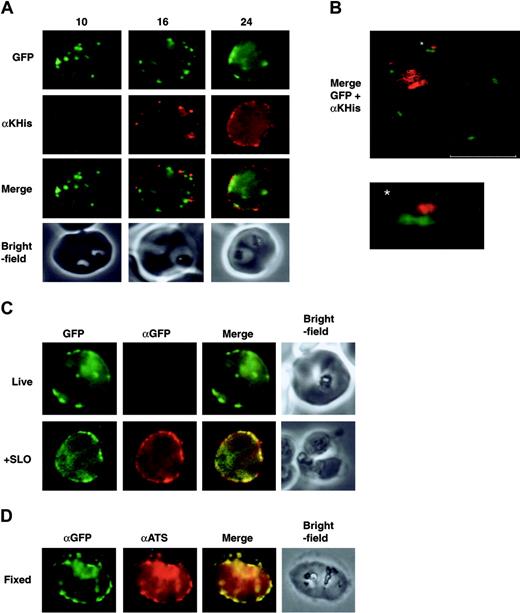
The N-terminal domain of endogenous PfEMP1 is localized on the surface of parasitized RBCs. If K119TmATS-GFP was trafficked correctly to the RBC surface, we expected the antibody-reactive His-rich region to be exposed at the external surface (Figure 4A). We immunolabeled intact cells and showed that the KAHRP-His domain was detectable on the RBC surface of 3D7 transfectants at approximately 16 hours after invasion. At 24 hours the level of protein had increased and an even distribution over the membrane surface was observed. Dual-color confocal images of intact parasitized RBCs labeled with αKAHRP-His antibody illustrate the presence of a subpopulation of surface-exposed chimera as well as a population of GFP-PfEMP1 chimeras associated with punctate structures underlying the RBC membrane (Figure 4B).
To confirm that the orientation of K119TmATS-GFP fusion protein on the RBC surface was identical to endogenous PfEMP1 we examined if the GFP domain in all transfected parasites was facing the RBC cytoplasm. We incubated intact or permeabilized cells with αGFP antibody. No reactivity to the GFP domain was observed in live cells (Figure 4C) but labeling was detected in permeabilized cells (Figure 4C +SLO). The data suggest that the GFP-ATS domains of both the Maurer cleft–associated and surface-exposed populations of the PfEMP1 chimera face the RBC cytoplasm the same as endogenous PfEMP1.14 The GFP domain was also accessible to specific antibodies in fixed K119TmATS transfectants; in these samples the αGFP and αATS signals appeared to be colocalized (Figure 4D). Additional controls show that neither αGFP nor the αKAHRP-His antibody react with the surface of intact RBCs infected with nontransfected 3D7 P falciparum, whereas αKAHRP-His detected KAHRP in fixed parasitized RBCs (Figure S3). Taken together these results are consistent with the presence of the KAHRP-His domain on the outside of RBCs infected with 3D7-K119TmATS transfectants. The transit of the PfEMP1 chimera via the Maurer clefts and appearance on the surface at about 16 hours after invasion suggest that they are trafficked by the same route and mechanism as endogenous PfEMP1.
The K119TmATS-GFP and K60Tm(ama1)ATSs chimeric proteins associate with Maurer clefts. Immunofluorescence microscopy of trophozoite stage parasites from 3 different transgenic cell lines with αGFP and αSBP1 as a Maurer cleft resident marker. First image in each row represents the chimera detected with a rabbit αGFP antibody; the second displays the Maurer cleft protein, SBP1; the third DAPI staining of the nuclei; the fourth image is an overlay of all 3; and the last image represents a brightfield image of the infected RBC. First row, 3D7-K119TmATS parasites; second row, D10-K119TmATS parasites; third row, 3D7-K60Tm(ama1)ATSs parasites.
The K119TmATS-GFP and K60Tm(ama1)ATSs chimeric proteins associate with Maurer clefts. Immunofluorescence microscopy of trophozoite stage parasites from 3 different transgenic cell lines with αGFP and αSBP1 as a Maurer cleft resident marker. First image in each row represents the chimera detected with a rabbit αGFP antibody; the second displays the Maurer cleft protein, SBP1; the third DAPI staining of the nuclei; the fourth image is an overlay of all 3; and the last image represents a brightfield image of the infected RBC. First row, 3D7-K119TmATS parasites; second row, D10-K119TmATS parasites; third row, 3D7-K60Tm(ama1)ATSs parasites.
Expression of a protein domain on the surface ofP falciparum–infected RBCs. All panels display parasites expressing the 3D7-K119TmATS chimeric protein. The images are from synchronized cultures of transfectants at 10, 16, and 24 hours after invasion. (A) First row, GFP fluorescence; second row, live cells incubated with αKAHRP-His (αKHis) antibodies showing appearance of the KAHRP domain on the surface 16 hours after synchronization; third row, merge of rows 1 and 2; fourth row, bright-field images. (B) Confocal images of live 3D7-K119TmATS parasites 24 hours after invasion incubated with αKAHRP-His antibodies. The image shows an overlay of the GFP fluorescence (green) and αKAHRP-His labeling (red) for a single z section (0.3 μm). The bar equals 4 μm. The asterisk indicates the adjacent locations of GFP on the inside and KAHRP-His on the outside of the RBC plasma membrane, with an enlarged image below. The internal hemozoin-associated fluorescence represents partial reflection of the excitation beam and not labeling with the antibodies. (C) Control experiments examining the specificity of antibody labeling of intact cells. First column, GFP fluorescence; second column, αGFP; third column, overlay of fluorescence images; fourth column, brightfield image. The top row shows intact parasitized RBCs with no access of the αGFP to the GFP domain inside the cell. The bottom row represents a SLO-permeabilized cell where the antibody gains access to the domain on the inside of the RBC. (D) Formaldehyde-fixed parasites show accessibility to αATS and αGFP antibodies.
Expression of a protein domain on the surface ofP falciparum–infected RBCs. All panels display parasites expressing the 3D7-K119TmATS chimeric protein. The images are from synchronized cultures of transfectants at 10, 16, and 24 hours after invasion. (A) First row, GFP fluorescence; second row, live cells incubated with αKAHRP-His (αKHis) antibodies showing appearance of the KAHRP domain on the surface 16 hours after synchronization; third row, merge of rows 1 and 2; fourth row, bright-field images. (B) Confocal images of live 3D7-K119TmATS parasites 24 hours after invasion incubated with αKAHRP-His antibodies. The image shows an overlay of the GFP fluorescence (green) and αKAHRP-His labeling (red) for a single z section (0.3 μm). The bar equals 4 μm. The asterisk indicates the adjacent locations of GFP on the inside and KAHRP-His on the outside of the RBC plasma membrane, with an enlarged image below. The internal hemozoin-associated fluorescence represents partial reflection of the excitation beam and not labeling with the antibodies. (C) Control experiments examining the specificity of antibody labeling of intact cells. First column, GFP fluorescence; second column, αGFP; third column, overlay of fluorescence images; fourth column, brightfield image. The top row shows intact parasitized RBCs with no access of the αGFP to the GFP domain inside the cell. The bottom row represents a SLO-permeabilized cell where the antibody gains access to the domain on the inside of the RBC. (D) Formaldehyde-fixed parasites show accessibility to αATS and αGFP antibodies.
Interestingly, we could not detect the KAHRP-His domain on the surface of the D10 K119TmATS transfectants (Figure S4). This suggests that either KAHRP and/or PfEMP3 are involved in the last step of trafficking from Maurer clefts to the RBC plasma membrane or that D10 parasites lack another component required for the process. Labeling of fixed D10 transfectants with αKAHRP-His showed that these cells lack endogenous KAHRP but express the K119TmATS-GFP fusion protein (Figure S4). The N-terminal region of KAHRP has been shown to be sufficient for trafficking to Maurer clefts and assembly underneath the RBC surface.8 Our results show that this sequence was able to complement PfEMP1 trafficking to Maurer clefts and together with the Tm allow insertion into the RBC membrane with the N-terminal domain on the outside of the cell.
Trafficking of the PfEMP1-GFP chimeras is brefeldin-A sensitive
In higher eukaryotes, brefeldin A (BFA) blocks secretion and causes redistribution of Golgi markers to the ER.28 PfEMP1 trafficking is BFA sensitive as it is trafficked through the classical secretory system of the parasite.8 To determine the effect of BFA on trafficking of K119TmATS-GFP and TmATS-GFP in 3D7 transfectants, the inhibitor was added after invasion and live parasites were examined at 16 hours (Figure 5). Trafficking of K119TmATS-GFP to the parasite membrane and beyond was inhibited by BFA (Figure 5A). In BFA-treated cells a focus of fluorescence was observed near the periphery of the parasite with a diffuse pattern surrounding it. Removal of BFA allowed normal growth and trafficking of K119TmATS-GFP. We examined whether K119TmATS-GFP colocalized with the ER luminal protein PfBiP29 in BFA-treated cells (Figure 5Ax-xiii). Immunofluorescence of K119TmATS-GFP showed a similar pattern of fluorescence to that observed using αPfBiP, providing evidence that this protein was localized in the ER after BFA treatment. However, the localization of TmATS-GFP was not affected by BFA as expected for an ER-localized protein (Figure 5B). Therefore both K119TmATS-GFP and endogenous PfEMP1 are trafficked to the parasite plasma membrane via the classical secretory pathway.8
Trafficking of the K119TmATS-GFP protein is brefeldin A (BFA) sensitive. (Ai-ix) Live cells. GFP fluorescence (i,iv,vii); bright-field image (ii,v,viii); overlay (iii,vi,ix). Normal trafficking of the K119TmATS-GFP chimera was observed in the absence of BFA (-) or after washing, whereas BFA (+) treatment inhibited trafficking. (Ax-xiii) Immunofluorescence microscopy of fixed BFA-treated cells. (x) αGFP; (xi) αBiP; (xii) DAPI; (xiii) merge of subpanels x-xii. The chimera colocalizes with the ER marker BiP after incubation with BFA. (B) Brefeldin A treatment of 3D7-TmATS transfectants. (Bi-vi) Live cells in the presence (+) and absence (-) of BFA. GFP fluorescence (i,iv); phase-contrast images (ii,v); overlays (iii,vi). (Bvii-x) Immunofluorescence microscopy of fixed BFA-treated cells. (vii) αGFP antibody; (viii) αBiP antibody; (ix) DAPI; (x) merge of subpanels vii-ix.
Trafficking of the K119TmATS-GFP protein is brefeldin A (BFA) sensitive. (Ai-ix) Live cells. GFP fluorescence (i,iv,vii); bright-field image (ii,v,viii); overlay (iii,vi,ix). Normal trafficking of the K119TmATS-GFP chimera was observed in the absence of BFA (-) or after washing, whereas BFA (+) treatment inhibited trafficking. (Ax-xiii) Immunofluorescence microscopy of fixed BFA-treated cells. (x) αGFP; (xi) αBiP; (xii) DAPI; (xiii) merge of subpanels x-xii. The chimera colocalizes with the ER marker BiP after incubation with BFA. (B) Brefeldin A treatment of 3D7-TmATS transfectants. (Bi-vi) Live cells in the presence (+) and absence (-) of BFA. GFP fluorescence (i,iv); phase-contrast images (ii,v); overlays (iii,vi). (Bvii-x) Immunofluorescence microscopy of fixed BFA-treated cells. (vii) αGFP antibody; (viii) αBiP antibody; (ix) DAPI; (x) merge of subpanels vii-ix.
Exported PfEMP1-GFP chimeras become tightly associated with Maurer clefts
We performed photobleaching studies18 of Maurer cleft–associated K119TmATS-GFP in 3D7 and D10 parasites to determine if this population was tightly associated with these structures or rapidly exchanging with a cytosolic pool (Figure 6). Selective bleaching of the chimera associated with individual clefts was achieved with a 0.1- to 0.2-second exposure to a laser pulse (Figure 6A-B white arrows). There was no recovery of the fluorescence onto the Maurer clefts within the time scale examined (2-3 minute), indicating that K119TmATS-GFP is tightly bound. The data are consistent with K119TmATS-GFP being embedded in the Maurer cleft membrane and indicate that the Maurer clefts are not connected to each other.
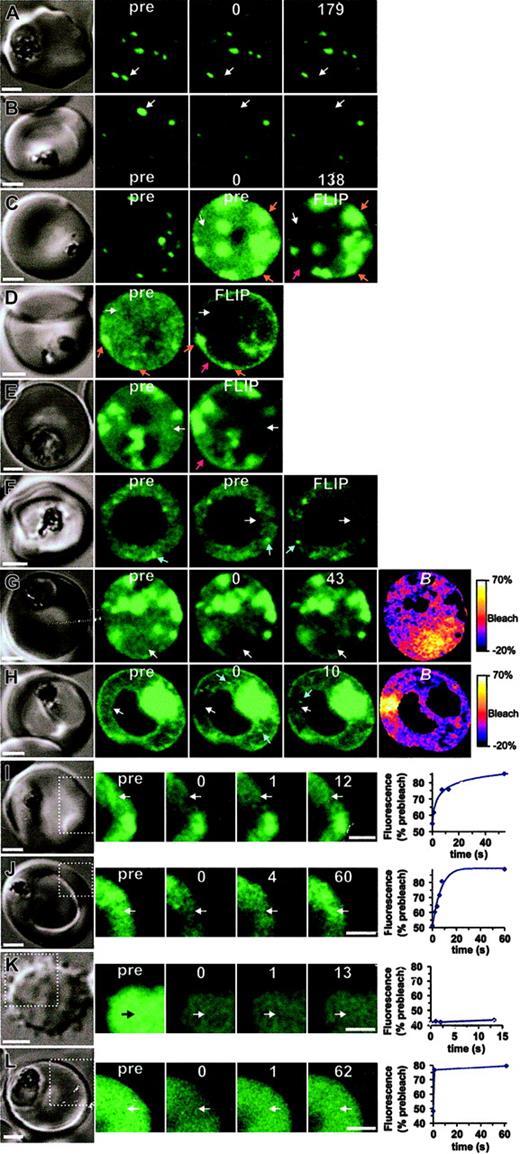
The molecular organization of GFP chimeras. The dynamics of K119TmATS-GFP (A-J) expressed in 3D7 (left panels) or D10 (right panels) was compared with that of KAHRP1-119-GFP in 3D7 transfectants (L) and fluorescein-BSA in resealed RBCs (K). In each case, the first panel shows the differential interference contrast image. The fluorescence images (green) comprise prebleach (pre) and postbleach images at the times (s) indicated following the bleach pulse. (The first postbleach image is defined as zero time.) The position of the bleach pulse is indicated by white arrows. In some images of D10 transfectants, bright “particles” that move position between successive images are marked with blue arrows. Scale bars = 2 μm. Panels A and B show bleaching of Maurer cleft–associated K119TmATS-GFP with 100- and 200-ms bleach pulses, respectively. Panels C-F show FLIP measurements of K119TmATS-GFP where the region indicated by the arrow was subjected to 5 or ten 1- to 2-second bleach pulses separated by 10 to 20 seconds. The 2 prebleach images shown for the cell in panel C were taken at different photomultiplier gains to illustrate the faint nature of the fluorescence associated with the RBC cytosol. Note that the FLIP images have had their brightness and contrast enhanced relative to the prebleach images to permit direct comparisons. Panels G and H illustrate the localized nature of bleaching of RBC cytosol obtained with 0.5- and 1.0-second bleach pulses, respectively, and subsequent recovery. The B image was calculated from the prebleach and postbleach images. Panels I and J show photobleaching measurements of K119TmATS-GFP, illustrating recovery on a time scale of 5 to 10 seconds. Panel K shows photobleaching of a resealed ghost containing fluorescein-BSA with a bleach pulse of 25 ms, indicating very rapid diffusion. Panel L shows photobleaching of a 3D7-KAHRP1-119-GFP with a bleach pulse of 1 second and recovery on a time scale of a few hundred milliseconds. In the case of panels I-L, only the regions indicated by the dotted lines in the differential interference contrast (DIC) image were imaged in the photobleaching measurements. The graphs shown in panels I-L show the temporal dependence of fluorescence intensity in the bleached region following the bleach event, relative to prebleach intensity.
The molecular organization of GFP chimeras. The dynamics of K119TmATS-GFP (A-J) expressed in 3D7 (left panels) or D10 (right panels) was compared with that of KAHRP1-119-GFP in 3D7 transfectants (L) and fluorescein-BSA in resealed RBCs (K). In each case, the first panel shows the differential interference contrast image. The fluorescence images (green) comprise prebleach (pre) and postbleach images at the times (s) indicated following the bleach pulse. (The first postbleach image is defined as zero time.) The position of the bleach pulse is indicated by white arrows. In some images of D10 transfectants, bright “particles” that move position between successive images are marked with blue arrows. Scale bars = 2 μm. Panels A and B show bleaching of Maurer cleft–associated K119TmATS-GFP with 100- and 200-ms bleach pulses, respectively. Panels C-F show FLIP measurements of K119TmATS-GFP where the region indicated by the arrow was subjected to 5 or ten 1- to 2-second bleach pulses separated by 10 to 20 seconds. The 2 prebleach images shown for the cell in panel C were taken at different photomultiplier gains to illustrate the faint nature of the fluorescence associated with the RBC cytosol. Note that the FLIP images have had their brightness and contrast enhanced relative to the prebleach images to permit direct comparisons. Panels G and H illustrate the localized nature of bleaching of RBC cytosol obtained with 0.5- and 1.0-second bleach pulses, respectively, and subsequent recovery. The B image was calculated from the prebleach and postbleach images. Panels I and J show photobleaching measurements of K119TmATS-GFP, illustrating recovery on a time scale of 5 to 10 seconds. Panel K shows photobleaching of a resealed ghost containing fluorescein-BSA with a bleach pulse of 25 ms, indicating very rapid diffusion. Panel L shows photobleaching of a 3D7-KAHRP1-119-GFP with a bleach pulse of 1 second and recovery on a time scale of a few hundred milliseconds. In the case of panels I-L, only the regions indicated by the dotted lines in the differential interference contrast (DIC) image were imaged in the photobleaching measurements. The graphs shown in panels I-L show the temporal dependence of fluorescence intensity in the bleached region following the bleach event, relative to prebleach intensity.
PfEMP1 chimeric proteins are trafficked as complexes
The K119TmATS-GFP chimera was concentrated at Maurer clefts and these structures appear as bright puncta (Figure 6A-B). However, images collected at increased photomultiplier gains showed there is an additional population of the chimera in the RBC cytosol (Figure 6C-J). This population may represent molecules en route to Maurer clefts and the RBC surface. The fluorescence profile of this population appears too smooth to represent small vesicles and seems more likely to arise from individual proteins or protein complexes. As this was an unexpected finding for a membrane protein such as PfEMP1, we further analyzed the physical organization of this population of K119TmATS-GFP. The diffuse staining in the host cytoplasm of 3D7-K119TmATS-GFP (Figure 6C,E) was subjected to a series of intense 2-second laser pulses (Figure 6C,E; white arrow). Fluorescence loss in photobleaching (FLIP) was examined in regions distal to the bleach spot. We observed selective loss of fluorescence from the entire RBC cytosol indicating diffusion within a single continuous compartment. In contrast, there was less loss of fluorescence arising from overexposed Maurer clefts (Figure 6C, orange arrows), confirming that the Maurer cleft and cytosolic populations of chimera are not in rapid equilibrium. The extensive bleaching of the cytosol compartment revealed a small population of chimera associated with the RBC rim (Figure 6C,E red arrow). This presumably represents K119TmATS-GFP inserted into the RBC membrane.
We also examined the mobility of the K119TmATS-GFP chimera in knob- D10-parasitized RBCs. Again, application of high-intensity laser pulses (Figure 6D white arrow in pre image) produced selective loss of cytosolic fluorescence, leaving behind fluorescence associated with Maurer clefts and the RBC rim (Figure 6D orange and red arrows, respectively). However given that the His-rich domain is not exposed at the external surface (Figure S3), this rim labeling may represent Maurer clefts closely docked to the RBC membrane. In D10 transfectants, the cytosollocated population of GFP appeared more granular and in some cells small mobile puncta of fluorescence, possibly representing aggregates of protein, or nascent Maurer clefts were observed in the RBC cytosol (Figure 6F blue arrows). Unlike Maurer clefts, which remain stationary during photobleaching measurements (Figure 6A-B), these particles were mobile (Figures 6F,H, and Video S2, blue arrows). Bleaching of the cytosolic fluorescence (Figure 6F white arrow) did not ablate these puncta (Video S2).
We used photobleaching recovery to monitor the rate of diffusion of the RBC cytosol-located population of K119TmATS-GFP in 3D7 transfectants (Figure 6G-J). Application of a short laser pulse resulted in localized bleaching in the RBC cytosol (Figure 6G 0 time panel). This was visualized as a dark region in the RBC cytosol. Similar results were observed for D10 transfectants (Figure 6H). This was particularly evident in the B images constructed from prebleach and postbleach images (Figure 6G-H right-hand panels). These color-coded images show bleaching largely restricted to the illuminated region. Following the localized bleaching events, we observed signal recovery in subsequent images (Figure 6G and H, 43-s and 10-s time panels, respectively). Using a decreased pixel resolution and bidirectional line scanning, it was possible to semiquantitate the recovery for cytosolic K119TmATS-GFP in 3D7 and D10, which indicated a half-time of a few seconds (Figure 6I-J).
The fact that we were able to monitor recovery of fluorescence for the RBC cytosol population of the PfEMP1-GFP chimera suggests it is not present as a monomeric species. Diffusion of soluble proteins usually occurs on low millisecond time scales that are too rapid to be measured using confocal microscope–based photobleaching.18 To examine freely diffusing monomeric protein we resealed fluorescein-BSA inside RBCs (Figure 6K). A short bleach pulse (25 milloseconds) and a short image acquisition time (down to 250 milliseconds) were applied to capture the bleach process. We observed an even bleaching of the entire cytosol and no recovery of signal (Figure 6K). Thus fluorescein-BSA equilibrates throughout the RBC cytoplasm with a half-time of significantly less than the 250 milliseconds.
We have previously reported a KAHRP-GFP chimera consisting of the first 119 amino acids (KAHRP1-119-GFP) that displayed rapid diffusion measured using fluorescence recovery after photobleaching (FRAP)8 ; however, in these experiments the time resolution achievable was much less than now possible with new equipment. In order to compare the diffusion of KAHRP1-119-GFP with that of K119TmATS-GFP, we re-examined this transfectant.8 Using a short illumination pulse (1 second) and fast image acquisition, we detected recovery of the signal between the first and second postbleach images (Figure 6L). A semiquantitative analysis indicates recovery with a half-time of hundreds of milliseconds. This is an order of magnitude slower than expected for a monomeric protein of the size of KAHRP1-119-GFP but faster than the diffusion of the PfEMP1 chimera, indicating that KAHRP-GFP is likely to be in a different complex.
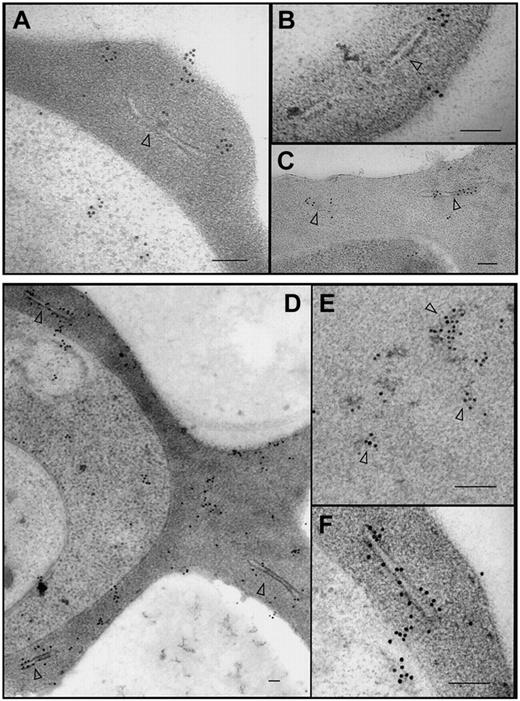
Subcellular localization and organization of PfEMP1-GFP. The 3D7-K60TmATSs-GFP (A-B), 3D7-K60Tm(ama1)ATSs-GFP (C), and 3D7-K119TmATS-GFP (D-F) transfectants were labeled with αGFP antibody. In each case the chimeric protein was observed within the parasite, in aggregates at and near Maurer clefts (arrowheads), and near the surface of the RBC. Gold particles were also often observed associated with electron dense aggregates in the red blood cell cytosol (D-E, arrowheads). Bars represent 100 nm.
Subcellular localization and organization of PfEMP1-GFP. The 3D7-K60TmATSs-GFP (A-B), 3D7-K60Tm(ama1)ATSs-GFP (C), and 3D7-K119TmATS-GFP (D-F) transfectants were labeled with αGFP antibody. In each case the chimeric protein was observed within the parasite, in aggregates at and near Maurer clefts (arrowheads), and near the surface of the RBC. Gold particles were also often observed associated with electron dense aggregates in the red blood cell cytosol (D-E, arrowheads). Bars represent 100 nm.
Ultrastructural analysis of PfEMP1 trafficking
In an effort to determine whether the population of K119TmATS-GFP in the RBC cytosol was present in large protein complexes or in membrane vesicles we have analyzed 3D7-K60TmATSs-GFP (Figure 7A-B), K60Tm(ama1)ATSs-GFP (Figure 7C), and K119TmATS-GFP (Figure 7D-F) transfectants by immunoelectron microscopy using αGFP antibody. In each case, we observed gold particles in the parasite cytoplasm, within the RBC cytosol, and at the host cell membrane. In the RBC cytoplasm numerous gold particles were located at or in close proximity to Maurer clefts (Figure 7A-D,F). In some cells we observed gold particle–studded protrusions from Maurer clefts (Figure 7A-B), which might represent PfEMP1-GFP–containing structures en route to the RBC surface. These extensions are reminiscent of the electron-lucent extensions on the surface of Maurer clefts observed with αPfEMP1 antibodies introduced into streptolysin O (SLO)–permeabilized infected RBCs14 and suggest PfEMP1-GFP chimeras form structures equivalent to those formed by endogenous PfEMP1. We also detected patches of gold particles associated with electron dense material in the RBC cytoplasm (Figure 7E, arrowheads). This material may correspond to the slowly diffusing cytosolic species observed in photobleaching measurements. Despite examining many sections we saw no evidence of membrane-bound vesicles in the host cell cytosol. Taken together, the electron microscopy and FRAP data suggest that chimeric fusion proteins in the cytosol are trafficked as membrane-free complexes and suggest that endogenous PfEMP1 is trafficked via the same process.
Discussion
The virulence protein PfEMP1 is displayed on the surface of P falciparum–infected RBCs and is responsible for adherence and antigenic variation.1 Recently, it was shown that a pentameric export element (PEXEL) is required for transport of proteins across the PVM.11,12 Indeed, a PEXEL motif is found in exported proteins in all Plasmodium species examined. In most exported proteins, the PEXEL motif is located downstream of the hydrophobic N-terminal signal sequence. In PfEMP1 (which lacks a signal sequence) it is located 20 to 25 amino acids from the first methionine and we have previously shown that the N-terminal segment of PfEMP1 followed by its Tm and ATS region can direct a YFP chimera to the RBC cytosol.11 In our previous study it was not possible to determine whether the PfEMP1 chimera was exported to the RBC surface. However, in the current work, we used the KAHRP-PEXEL to replace the PfEMP1 motif and to introduce an epitope that can be detected at the external surface.
Fusion of the first 60 or 120 amino acids of KAHRP to the PfEMP1 Tm region allowed translocation across the PVM, whereas the Tm region alone allowed entry into the ER but was not sufficient for trafficking into the RBC. Fusion proteins containing the KAHRP N-terminal region followed by the PfEMP1 Tm and ATS regions were trafficked to the Maurer clefts and became associated with these structures. At least part of the K119TmATS-GFP population of molecules becomes exposed at the RBC surface. Our data indicate that the KAHRP N-terminal signal information (including its PEXEL motif), the PfEMP1 Tm, and the ATS region provide sufficient information to direct domains to the external surface. Interestingly, the His-rich domain was not exposed at the external surface of D10 transfectants, indicating that KAHRP, PfEMP3, or other proteins not expressed in D10 parasites are needed for the final step in trafficking of PfEMP1 to the RBC surface.
A sequence between amino acids 60 and 120 of KAHRP is required for association with Maurer clefts9 and we have shown that an equivalent sequence is contained within PfEMP3 (E.K., M.R., N.K., L.T., and A.F.C., manuscript in preparation). Indeed we have shown previously that KAHRP1-119-GFP associates reversibly with Maurer clefts.8 Although this motif might contribute to the binding of K119TmATS-GFP to Maurer clefts, the interaction between the PfEMP1-GFP fusion proteins and Maurer clefts is much tighter, suggesting that the Tm region of the PfEMP1-GFP fusions is embedded in the Maurer cleft membrane. This is supported by the tight interaction of K60TmATSs-GFP fusions with Maurer clefts that lack the histidine-rich region of KAHRP involved in Maurer cleft association and only contain a truncated ATS.
A recent study used fluorescence microscopy of cells labeled with lipid probes and electron microscopy of serial sections to examine the structures in the P falciparum–infected RBC cytosol.30 These authors postulated that Maurer clefts and the tubovesicular network form part of a continuous meshwork. However, our results indicate a physical barrier that prevents the diffusion of GFP chimeras between adjacent Maurer clefts. Similarly, we find no evidence for continuity of these structures with the PVM.
Exchange of the putative Tm region of PfEMP1 with the equivalent region of another single transmembrane domain protein, AMA1, which is trafficked to the micronemes,23 still allowed the chimeric protein to be trafficked into the RBC cytosol and to associate with Maurer clefts. However, the transport efficiency was low suggesting that in addition to the translocation motif, the PfEMP1 Tm domain plays a role in promoting export across the PVM. Once out in the RBC cytoplasm this fusion protein associates with Maurer clefts, showing that Tm domains other than PfEMP1 can embed into these structures. K60TmATSs-GFP and K60Tm(ama1)ATSs-GFP fusion proteins are both successfully targeted to Maurer clefts, suggesting that the first 113 amino acids of ATS are sufficient for directing trafficking to these structures.
It is interesting to note that the PEXEL motif appears to be used not only by proteins such as KAHRP and PfEMP3 that do not have Tm regions but also by PfEMP1, rifins, and stevor proteins that have one or more predicted Tm regions.11,12 This suggests that these proteins are trafficked across the PVM via the same putative translocase. This finding appears to be inconsistent with vesicular trafficking suggested previously for PfEMP1 and other Tm segment–containing proteins31 and leads to the unusual suggestion that PfEMP1 may be trafficked as a chaperoned complex and inserted into a membrane only after it reaches the RBC cytoplasm. Our FRAP analysis of the GFP-PfEMP1 chimeras is consistent with the presence of complexes of the chimera in the RBC cytosol. There are precedents for the posttranslational insertion of membrane proteins. For example, the Toxoplasma gondii protein, dense granule protein 5 (GRA5), is inserted into the PVM only after release from secretory organelles known as the dense granules.32 Indeed our immunoelectron microscopy studies revealed gold-labeled material that may represent protein complexes but did not identify any vesicles containing these proteins. Previous studies31,33 detected PfEMP1 associated with small vesicles in the host cell cytosol of aluminum fluoride–treated parasites; however, we found no evidence for vesicle-mediated trafficking of the chimera under the conditions of our experiments. Indeed, our data support the suggestion that PfEMP1 transgenic proteins are present within the host cell cytoplasm as large multiprotein complexes. Recent work analyzing the solubility properties of PfEMP1 predicted it was not trafficked as a membrane-embedded protein in classical transport vesicles but rather as a soluble protein prior to insertion into the Maurer clefts or RBC membrane.34 This is consistent with our suggestion that trafficking of PfEMP1 to Maurer clefts occurs via a protein complex.
The ability to traffic transgenic proteins to the P falciparum–infected RBC surface provides an important tool for expression of domains for functional analysis. Many P falciparum proteins are difficult to express in correctly folded forms and the ability to traffic domains to the RBC surface of infected cells would provide engineered domains that could be cleaved from the surface for purification. Additionally, because of the conserved nature of the PEXEL in Plasmodium it will be possible to express protein domains on the outside surface of RBCs infected with transgenic parasites, such as the mouse malaria species P berghei. This will provide a useful system for probing protein function and analysis of immune responses in an infection model in whole animals.
In conclusion, we have shown that a translocation motif, the Tm, and C-terminal regions of PfEMP1 are sufficient for export to the outside of the RBC surface. Our data suggest that PfEMP1 traffics as a complex rather than in vesicles and that Maurer clefts represent an important intermediate compartment. It is likely that helper proteins are required for each step in this complex process. The availability of short transgenic proteins that transit to the RBC surface provides tools to assist in identifying these proteins, which represent potential drug targets for novel classes of antimalarial drugs.
Prepublished online as Blood First Edition Paper, February 3, 2005; DOI 10.1182/blood-2004-12-4666.
E.K. was supported by an International Travelling Research Fellowship from the Wellcome Trust. A.F.C. was supported by an International Research Fellowship from Howard Hughes Medical Institute (HHMI). This work was supported by the National Institutes of Health (NIH; RO1 AI44008), the National Health and Medical Research Council (NHMRC) of Australia, and the Australia Research Council.
An Inside Blood analysis of this article appears in the front of this issue.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Braun-Breton and Ryan, as well as the Malaria Research Reference Reagent Resource Center, for antibodies. We thank the Red Cross Blood Service (Melbourne, Australia) for red cells.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal