Abstract
Angiopoietin 1 (Ang1), a ligand for the receptor tyrosine kinase Tie2, regulates the formation and stabilization of the blood vessel network during embryogenesis. In adults, Ang1 is associated with blood vessel stabilization and recruitment of perivascular cells, whereas Ang2 acts to counter these actions. Recent results from gene-targeted mice have shown that Ang2 is also essential for the proper patterning of lymphatic vessels and that Ang1 can be substituted for this function. In order to characterize the effects of the angiopoietins on lymphatic vessels, we employed viral vectors for overexpression of Ang1 in adult mouse tissues. We found that Ang1 activated lymphatic vessel endothelial proliferation, vessel enlargement, and generation of long endothelial cell filopodia that eventually fused, leading to new sprouts and vessel development. Cutaneous lymphatic hyperplasia was also detected in transgenic mice expressing Ang1 in the basal epidermal cells. Tie2 was expressed in the lymphatic endothelial cells and Ang1 stimulation of these cells resulted in up-regulation of vascular endothelial growth factor receptor 3 (VEGFR-3). Furthermore, a soluble form of VEGFR-3 inhibited the observed lymphatic sprouting. Our results reinforce the concept that Ang1 therapy may be useful in settings of tissue edema. (Blood. 2005;105:4642-4648)
Introduction
Members of the vascular endothelial growth factor (VEGF) and angiopoietin families regulate both angiogenesis and lymphangiogenesis by acting on vascular endothelial cells.1-4 Angiopoietins (Ang1 and Ang2) bind to the Tie2 receptor tyrosine kinase, expressed almost exclusively on the surface of endothelial cells, and regulate interactions between endothelial and periendothelial cells. Ang1 is an obligate agonist of Tie2, whereas Ang2 can act as either an agonist or an antagonist, depending on the cell type and the surrounding microenvironment.5-7 Ang1 expression in the mouse embryo occurs first in the myocardium and later in a more widespread manner around the developing vessels.5 Ang2 is expressed in the embryonic dorsal aorta and the major aortic branches and in adults in tissues that are undergoing vascular remodeling.6,8
Gene-targeting experiments have indicated that Ang1 is necessary for maintaining maximal interactions between endothelial cells, periendothelial cells, and the extracellular matrix.9 In adult tissues, exogenous Ang1 prevents leakage of plasma components into the interstitium caused by powerful vascular permeability agents, such as VEGF.10 Furthermore, abundant evidence suggests that members of the angiopoietin and VEGF families collaborate during different stages of angiogenesis. Ang2 is expressed at sites of pericyte detachment and blood vessel remodeling in conjunction with VEGF, whereas in the absence of VEGF, Ang2 activity leads to endothelial cell apoptosis.6,11,12 In addition, factors that induce angiogenesis in vivo, such as hypoxia and VEGF, have been shown to up-regulate Ang2 in endothelial cells.13
The role of angiopoietins in lymphangiogenesis has remained unclear although Tie2 mRNA and protein have been detected at least in cultured lymphatic endothelial cells.14,15 Ang2 knockout mice have lymphatic defects, suggesting that Ang2 is needed for normal lymph vessel formation and stabilization.8 Replacement of the Ang2 gene with a cDNA encoding Ang1 was sufficient to rescue the lymphatic phenotype but not the blood vascular phenotype.8 Thus, it is possible that both Ang2 and Ang1 act as agonists of Tie2 in lymphangiogenesis, whereas Ang2 is a context-dependent antagonist of Tie2 in angiogenesis. However, it is not known if the angiopoietins collaborate with the key regulators of lymphangiogenesis, VEGF-C and VEGF-D, that induce lymphatic vessel growth via their receptor, VEGFR-3, expressed predominantly in the lymphatic endothelium of adult tissues.16
We chose to address the effects of angiopoietin-1 on lymphatic vasculature via a viral gene delivery system. We show that angiopoietin-1 overexpression induces lymphatic vessel enlargement and sprouting, proliferation, and eventually even formation of new branches. These effects are associated with VEGFR-3 up-regulation in lymphatic vessels and blocked by systemic inhibition of VEGFR-3 signaling.
Materials and methods
Generation and in vitro analysis of viral vectors
The adenovirus encoding Ang1 was produced by cloning the full-length human Ang1 into the BglII site of the previously used adenovirus vector.17 For the adeno-associated virus (AAV) constructs, the Ang1 cDNA was cloned as a blunt-end fragment into the MluI site of psub-CMV-WPRE plasmid and the recombinant AAVs (rAAVs; serotype 2) were produced as previously described.18 For the analysis of protein expression, HeLa cells were transduced by AAVs in medium containing 2% fetal calf serum. After 40 hours, the cells were metabolically radiolabeled (100 μCi/mL [3.7 MBq/mL] ProMix; Amersham, Arlington Heights, IL) for 8 hours and the lysed cells and media were subjected to precipitation with antibodies against Ang1 (SC-6319 and SC-6320; Santa Cruz Biotechnology, Santa Cruz, CA) and a soluble fusion protein composed of the extracellular domain of Tie2 and the Fc domain of human immunoglobulin G1 (IgG1; 313-TI-100; R&D Systems, Minneapolis, MN). An AAV-encoding enhanced green fluorescent protein (EGFP) was used as a negative control. The bound proteins were precipitated with protein G Sepharose, separated by 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and analyzed by autoradiography.
In vivo use and analysis of the viral vectors
The studies were approved by the Committee for Animal Experiments of the University of Helsinki. Recombinant AAV particles (5 × 109-5 × 1010) were injected intradermally into the ears of FVB/n mice. The mice were killed 3 to 8 weeks after gene transduction. At least 3 mice were used in each study group for each analytical technique and time point. Total RNA was extracted from the ears (RNAeasy Kit; Qiagen, Valencia, CA) 5 weeks after AAV infection. Ten micrograms of RNA was subjected to Northern blotting and hybridization with [γ-32P] deoxycytosine triphosphate (Amersham) labeled cDNAs specific for Ang1 (GenBank accession no. NM001146; base pairs 469-1962). A glyceraldehyde-3-phosphate dehydrogenase probe was used as an internal control for equal loading. EGFP expression in AAV-EGFP-transduced ears was visualized under a fluorescent microscope at 3 to 8 weeks after infection. For the adenoviral experiments, 2.5 × 108 to 5 × 108 plaque-forming units (pfu's) of recombinant replication-deficient adenoviruses were injected intravenously via the tail vein or intradermally into the ears of NMRI nu/nu mice. Vectors encoding the growth factors were injected into the right ear, whereas the control virus was injected into the left ear of the same animal.
Analysis of lymphatic and blood vessels
For immunohistochemical analysis, mice were anesthetized and then perfused with 1% paraformaldehyde (PFA) for 2 to 3 minutes. The ears were collected, immersed in 4% PFA for 2 hours, and dissected for whole-mount staining. The tissues were blocked with 5% normal goat or donkey serum in 0.3% Triton-X100 (Fluka Biochemika, Steinheim, Switzerland) in phosphate-buffered saline (PBS) overnight. For staining of lymphatic vessels, tissues were incubated overnight with rabbit antiserum against the lymphatic vessel marker LYVE-119 and/or goat polyclonal anti-mouse VEGFR-3 antibodies (AF743; R&D Systems). Blood vessels in the ear were visualized by using hamster monoclonal anti-mouse platelet endothelial cell adhesion molecule 1 (PECAM-1; CD31) antibodies (clone 2H8, MAB-13982Z; Chemicon, Temecula, CA). Human Ang1 was stained from whole-mount preparations by simultaneous incubation with goat monoclonal antibodies to the N- and C-termini of Ang1 (SC-6319 and SC-6320, respectively; Santa Cruz Biotechnology). Goat IgG was used as a negative control. VEGF-C staining was carried out with rabbit polyclonal antibody no. 6, with preimmune serum from the same rabbit serving as a negative control.20 Incubation with the primary antibody was followed by overnight incubation with appropriate fluorochrome-conjugated secondary antibodies (Alexa 488 or Alexa594; Molecular Probes, Eugene, OR; or fluorescein isothiocyanate; Jackson ImmunoResearch, Bar Harbor, ME). For detection of Tie2 on lymphatic vessels of the ear, frozen sections were fixed with -20°C acetone and incubated with rabbit antiserum for LYVE-1 and rat monoclonal antibodies against Tie2.21 Biotinylated anti-rat secondary antibodies (VectorLabs, Burlingame, CA) were then applied, followed by signal enhancement with the tyramide signal amplification kit (NEN Life Sciences, Boston, MA) and cyanine-conjugated streptavidin (Jackson ImmunoResearch). All fluorescently labeled samples were mounted with Vectashield (VectorLabs) and analyzed with a compound fluorescent microscope (Zeiss 2; Carl Zeiss, Göttingen, Germany; objective × 10 with numeric aperture [NA] 0.30) equipped with a digital camera (AxioCam HR, Carl Zeiss; used to capture Figures 1E-J and 7A) or a confocal microscope (Zeiss LSM 510, objectives × 40 with NA 1.3 and × 63 with NA 1.4; used to capture Figures 2A-L, 3A-D, 4A-B, 5C-D, 6A-C, and 7C-E) by using multichannel scanning in frame mode. Three-dimensional projections were digitally constructed from confocal z-stacks. Alternatively, VEGFR-3 was stained in whole-mount preparations by using the avidin-biotin complex-diaminobenzidine (ABC-DAB) peroxidase method and visualized with a light microscope (Olympus AX70; Olympus, Tokyo, Japan) equipped with a digital camera (Olympus DP50; used to capture Figures 3E and 5A-B).19 For quantitative analysis of the lymphatics in the K14-Ang1 mice,22 the ears were removed from K14-Ang1 transgenic mice and their wild-type littermates, sectioned, and stained with anti-mouse LYVE-1 antibodies. Horseradish peroxidase-conjugated secondary antibodies were added and samples were incubated with PBS containing 0.3 mg/mL DAB (Dojin Chem, Kumamoto, Japan) in the presence of 0.05% NiCl2 and 0.01% hydrogen peroxide at room temperature. The circumference of lymphatic vessels in LYVE-1-stained ears was quantified with the Kurabo angiogenesis image analyzer (Kurabo, Osaka, Japan; n = 3 animals per group).
Analysis of lymphatic vascular endothelial cell proliferation in vivo
Whole-mount preparations of mouse ears were double stained with goat anti-mouse VEGFR-3 and rabbit anti-mouse phosphohistone H3 (pHH3) (US06-570; Upstate Biotechnology, Lake Placid, NY) antibodies.20,23 For double staining of LYVE-1 and proliferating cell nuclear antigen (PCNA), 7-μm deparaffinized sections of ear skin were subjected to trypsin digestion, as previously described.24 The endogenous peroxidase activity was blocked with 3% H2O2 in methanol for 20 minutes, followed by staining for PCNA (93-1143; Zymed, Basel, Switzerland) according to the manufacturer's instructions. Samples were then incubated with affinity-purified rabbit polyclonal antibodies against LYVE-1 at +4°C overnight. Biotinylated antirabbit antibodies (VectorLabs) were then applied, and staining was carried out using 3-amino-9-ethyl carbazole (Sigma-Aldrich, St Louis, MO) as the substrate. Hematoxylin was used for counterstaining. Samples were analyzed under a light microscope, and the percentages of LYVE-1- and PCNA-positive cells were normalized to the number of LYVE-1-positive, PCNA-negative cells in each section.
Analysis of VEGFR-3 by immunofluorescence microscopy
For grading of the VEGFR-3 staining intensity on lymphatic vessels, whole-mount preparations of ears were double stained with antibodies against LYVE-1 and VEGFR-3 and analyzed by fluorescent microscopy. Images were acquired by setting a constant exposure time to the camera (AxioCam). The color images were converted to 8-bit grayscale using Adobe PhotoShop software (San Jose, CA). The images were then exported to ImageJ software25 for quantification of staining intensities.26 Areas formed by VEGFR-3-positive pixels were calculated in the intensity range of 25 to 50 and then normalized to the total area of LYVE-1-positive pixels, in the range of 50 to 100, of the same sample. These intensity values were selected in order to exclude background fluorescence and bright nonspecific staining that was not colocalized with LYVE-1-positive vessels. Staining intensities were also color graded using ImageJ25 in conjunction with a custom look-up table (generously provided by Dr Donald McDonald, University of California, San Francisco [UCSF], San Francisco, CA).
Cell culture and isolation of lymphatic endothelial cells
Human dermal microvascular endothelial cells (HDMECs; PromoCell, Heidelberg, Germany) were cultured in growth medium provided by the supplier. Podoplanin antibodies (a kind gift from Dr Dontscho Kerjaschki, Vienna, Austria27 ), magnetic-activated cell separation (MACS) colloidal superparamagnetic MicroBeads conjugated to goat anti-rabbit IgG antibodies, type LD depletion columns, type MS separation columns, and Midi/MiniMACS separators (Miltenyi Biotech, Bergisch Gladbach, Germany) were used for cell sorting according to the manufacturer's instructions.28 Blood vascular endothelial cells (BECs; PECAM-1+/podoplanin-) were isolated from HDMECs using LD-negative selection columns and a pure population of lymphatic endothelial cells (LECs) was subsequently obtained using MS-positive selection columns. Both populations were maintained on dishes coated with 1 μg/mL human fibronectin (Sigma-Aldrich), and LECs were propagated in the presence of 100 ng/mL VEGF-C (R&D Systems) added to the growth medium.
Adenovirus infection and Northern blot analysis
Confluent plates of endothelial cells were infected for 1.5 hours with adenoviral LacZ (AdLacZ) or AdAng1 (100 pfu/cell) in serum-free medium (PromoCell). After infection, the cells were grown in complete growth medium for 48 hours, and total RNA was extracted with the RNeasy kit (Qiagen), electrophoresed, transferred to nylon filters, and hybridized with 32P-labeled cDNA probes for human VEGFR-3 (bp 20-1005; NM002020), human VEGF-C (bp 80-2076; NM005429.2), or human VEGF-D (bp 509-1572; NM004469.2).
Results
Viral vectors encoding Ang1 induce lymphatic vessel dilation and formation of lymphatic endothelial filopodia
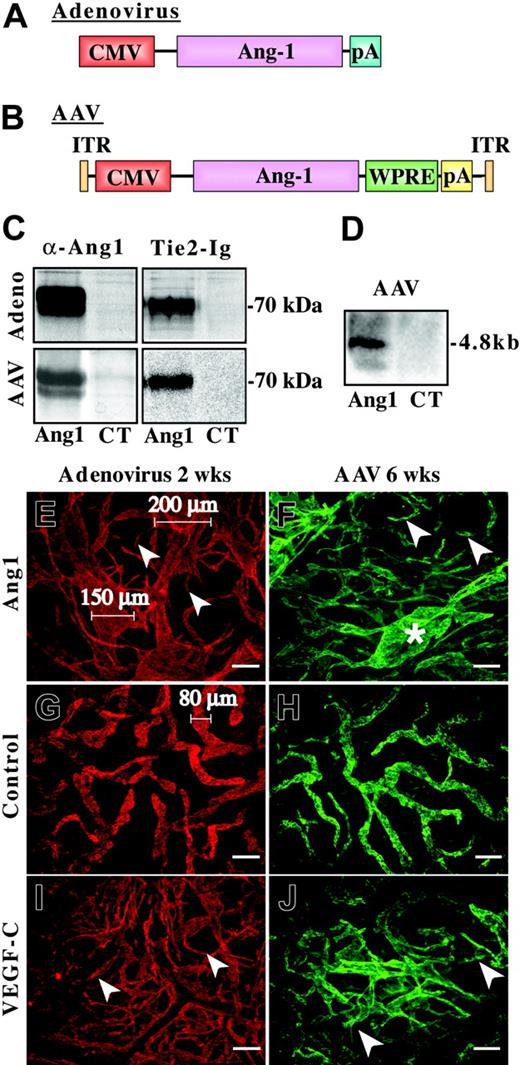
In order to analyze the effect of Ang1 on the lymphatic vessels, adenoviral and AAV vectors encoding the full-length human Ang1 were constructed (Figure 1A-B). HeLa cells were transduced with the viruses, and medium from metabolically labeled cells was precipitated with Ang1 antibodies or soluble Tie2-Ig chimeric protein. As shown in Figure 1C, Ang1 polypeptides of approximately 70 kDa were precipitated from the conditioned medium, indicating that cells transduced by either virus produced a functional growth factor.
Our previous results using adenovirus vectors encoding various VEGF family members and Ang1 have shown that this virus gives a robust expression in mouse skin.17 In order to verify AAV-mediated Ang1 expression in vivo, we extracted total RNA from the ears infected with AAV-Ang1 or AAV-EGFP. Northern blot analysis of the transduced tissues revealed strong expression of the transduced gene (Figure 1D).
For the analysis of Ang1 effects on the lymphatic vessels, AdAng1 virus was injected into the ears of nude mice. Similar doses of AdLacZ or AdVEGF-C were used as negative and positive controls, respectively. The ears were analyzed 2, 4, or 14 days after injection by whole-mount immunofluorescent staining for the lymphatic endothelial marker LYVE-130 and for the pan-endothelial marker PECAM-1. Numerous lymphatic capillary segments in the AdAng1-transduced tissue were grossly dilated, reaching diameters of up to 200 μm (Figure 1E), whereas the diameters of lymphatic capillaries in untreated skin or skin treated with the AdLacZ did not exceed 80 μm (Figure 1G-H). Interestingly, AdAng1 also induced lymphatic sprouting, as did AdVEGF-C (Figure 1E,I arrowheads). Similar findings were observed when AAVs encoding Ang1 or VEGF-C were used: AAV-Ang1 also caused dilation of lymphatic vessels, and both growth factors induced sprouting (Figure 1F,H,J). However, the effects induced by AAVs were more localized and not as prominent as those of the adenoviral vectors. In our experiments AdAng1 and AAV-Ang1 did not significantly affect blood vessels (data not shown).
Viral Ang1 vectors and their effects on lymphatic vessels. (A-B) Schematic structures of the viral Ang1 constructs used in the study. (A) The adenoviral Ang1 vector.29 (B) The AAV vector containing the full-length human Ang1 cDNA. CMV indicates cytomegalovirus promoter and early enhancer; WPRE, Woodchuck hepatitis virus posttranscriptional enhancer element; pA, polyadenylation signal (from Simian virus 40); and ITR, inverted terminal repeats. (C) Comparison of AdAng1 and AAV-Ang1 recombinant protein production in vitro by precipitation using Ang1 antibodies or soluble Tie2-Fc protein. Media from AdLacZ- and AAV-EGFP-infected cells were used as negative controls (CT). (D) Ang1 RNA expression in a Northern blot containing total RNA from mouse ears 6 weeks after infection with AAV-Ang1 or AAV-EGFP. (E,G,I) Lymphatic vessels visualized with fluorescent LYVE-1 (red) whole-mount staining 2 weeks after infection with the adenoviruses. Lymphatic sprouts are indicated with arrowheads in panels E and I. (G) Lymphatics after AdLacZ infection. (F,H,J) LYVE-1-stained lymphatic vessels (green) in ears 6 weeks after AAV transduction. Ang1-induced lymphatic enlargement is shown with an asterisk in panel F. Scale bars: 100 μm.
Viral Ang1 vectors and their effects on lymphatic vessels. (A-B) Schematic structures of the viral Ang1 constructs used in the study. (A) The adenoviral Ang1 vector.29 (B) The AAV vector containing the full-length human Ang1 cDNA. CMV indicates cytomegalovirus promoter and early enhancer; WPRE, Woodchuck hepatitis virus posttranscriptional enhancer element; pA, polyadenylation signal (from Simian virus 40); and ITR, inverted terminal repeats. (C) Comparison of AdAng1 and AAV-Ang1 recombinant protein production in vitro by precipitation using Ang1 antibodies or soluble Tie2-Fc protein. Media from AdLacZ- and AAV-EGFP-infected cells were used as negative controls (CT). (D) Ang1 RNA expression in a Northern blot containing total RNA from mouse ears 6 weeks after infection with AAV-Ang1 or AAV-EGFP. (E,G,I) Lymphatic vessels visualized with fluorescent LYVE-1 (red) whole-mount staining 2 weeks after infection with the adenoviruses. Lymphatic sprouts are indicated with arrowheads in panels E and I. (G) Lymphatics after AdLacZ infection. (F,H,J) LYVE-1-stained lymphatic vessels (green) in ears 6 weeks after AAV transduction. Ang1-induced lymphatic enlargement is shown with an asterisk in panel F. Scale bars: 100 μm.
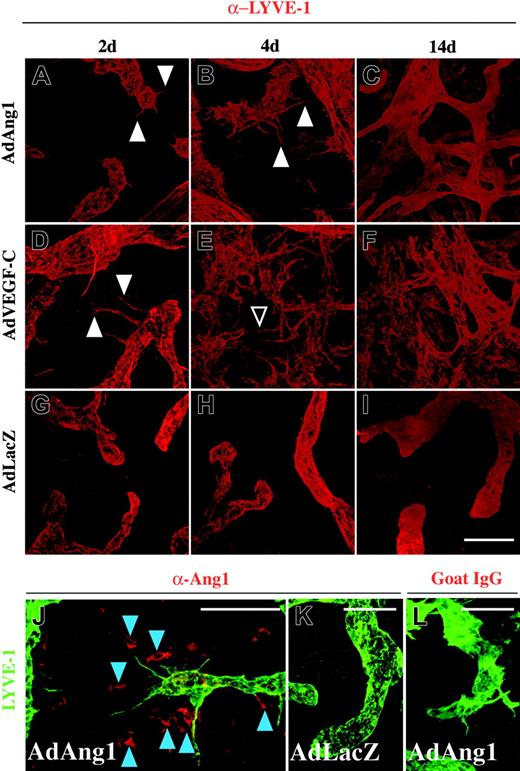
More detailed analysis of lymphatic vessels by high-power confocal microscopy revealed pronounced activation of the endothelium of the initial lymphatic capillaries in response to AdAng1 or AdVEGF-C treatment. Formation of filopodia and endothelial sprouts was observed already 2 days after adenoviral delivery of Ang1 and VEGF-C (Figure 2A,D arrowheads), although the sprouts induced by Ang1 were somewhat less numerous and shorter than those induced by VEGF-C at this time point. At 4 days, the sprouting and endothelial activation increased in the Ang1-treated ears (Figure 2B), whereas VEGF-C-treated tissues displayed excessive sprouting and also fusion of individual sprouts in some instances (Figure 2E arrowhead). After 14 days, both Ang1- and VEGF-C-treated samples showed vessel network formation and stabilization of the continuity of the lymphatic endothelium (Figure 2C,F). However, filopodia were still seen in the remaining initial lymphatics of the AdAng1- and AdVEGF-C-treated ears even 2 weeks after gene transduction (Figure 5C). In contrast, treatment with the control AdLacZ virus did not affect the lymphatics of the ear skin at any time point studied (Figure 2G-I). Lymphatic sprouts were formed in close proximity to cells expressing human Ang1 in the ears transduced with the AdAng1 vector (Figure 2J), whereas neither sprouting nor Ang1 expression could be detected in the control ears (Figure 2K).
Changes in cutaneous lymphatic vessels after adenoviral transduction. (A-I) The vessels were stained for LYVE-1 (red). Sprouts formed by adenoviral Ang1 or VEGF-C are indicated with arrowheads in panels A, B, and D. Fusion of individual sprouts 4 days after AdVEGF-C administration is shown with an open arrowhead in panel E. At the 14-day time point, a dense network of lymphatic vessels can be seen in both Ang1- and VEGF-C-treated ears (C,F). (G-I) Lymphatic vessels after AdLacZ transduction. (J-K) Staining of Ang1 (red) and LYVE-1 (green). Localization of Ang1 positive cells (blue arrowheads) and lymphatic sprouts in the ear transduced with AdAng1 (J). AdLacZ-transduced (K) and AdAng1-transduced goat IgG-stained controls (L) are shown for comparison. Scale bars: 100 μm.
Changes in cutaneous lymphatic vessels after adenoviral transduction. (A-I) The vessels were stained for LYVE-1 (red). Sprouts formed by adenoviral Ang1 or VEGF-C are indicated with arrowheads in panels A, B, and D. Fusion of individual sprouts 4 days after AdVEGF-C administration is shown with an open arrowhead in panel E. At the 14-day time point, a dense network of lymphatic vessels can be seen in both Ang1- and VEGF-C-treated ears (C,F). (G-I) Lymphatic vessels after AdLacZ transduction. (J-K) Staining of Ang1 (red) and LYVE-1 (green). Localization of Ang1 positive cells (blue arrowheads) and lymphatic sprouts in the ear transduced with AdAng1 (J). AdLacZ-transduced (K) and AdAng1-transduced goat IgG-stained controls (L) are shown for comparison. Scale bars: 100 μm.
Lymphatic hyperplasia in the dermis of K14-Ang1 transgenic mice
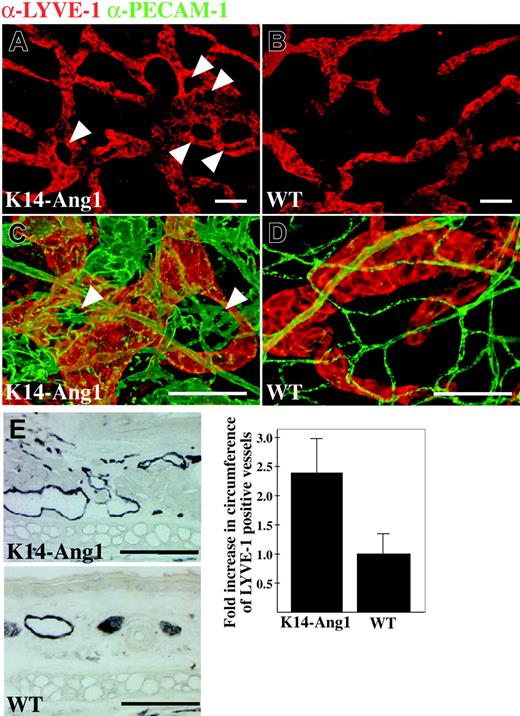
The K14-Ang1 transgenic mice that express Ang1 in basal epidermal cells have been shown to exhibit an increase in the surface area of cutaneous blood vessels.22 We found that these mice have dysmorphic lymphatic vessels concentrated around hair follicles that are known to express the transgene (Figure 3A,C arrowheads). Shown for comparison are normal lymphatic vessels from littermate control mice (Figure 3B,D). Venules of the K14-Ang1 mice were grossly enlarged in the vicinity of dysmorphic lymphatic vessels (Figure 3C-D green staining). LYVE-1 staining of the ear skin and image analysis showed that the lymphatic vessels of the Ang1 overexpressing mice are enlarged (Figure 3E).
Lymphatic vascular effects of Ang1 are inhibited by soluble VEGFR-3-Ig fusion protein
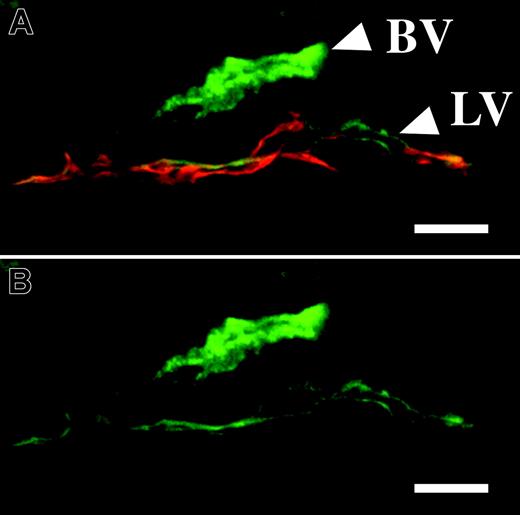
Immunohistochemistry of mouse ear sections revealed the presence of the Ang1 receptor Tie2 in the lymphatic endothelium, although the staining was weaker when compared with the blood vascular endothelium (Figure 4A-B). In order to determine if the effects of Ang1 on the lymphatic endothelium involved the VEGFR-3 ligand-receptor system, we injected mice intravenously with adenoviruses coding for a soluble form of VEGFR-3 (AdVEGFR-3-Ig) to block VEGFR-3 signaling in these mice. Adenoviruses administered via the intravenous route are known to home to the liver where transduced hepatocytes secrete the protein into the circulation (for an example, see Thurston et al10 ). As shown in Figure 5A and B, the lymphatic sprouting induced by AdAng1 in the ear skin was inhibited by systemic VEGFR-3-Ig delivery. Confocal microscopy revealed that the lymphatic capillaries in ears treated with AdAng1 extend endothelial cell filopodia and display an irregular endothelial cell surface (Figure 5C arrows). In contrast, ear samples from mice treated with AdAng1 plus systemic AdVEGFR-3-Ig displayed quiescent and stable lymphatic capillaries similar to the ears treated with the AdLacZ control virus only (Figure 5C inset and D).
Lymphatic capillary hyperplasia in K14-Ang1 transgenic mice. (A-B) Lymphatic vessels in the ear of a K14-Ang1 transgenic mouse (A) and littermate control (B) whole-mount-stained with antibodies to LYVE-1. Note lymphatic vessels around hair follicles (arrowheads). (C-D) Double staining of lymphatic (red) and blood vessels (green). (E) Quantitative analysis of the circumference of LYVE-1-stained lymphatic vessels in ear sections (see micrographs to the left of the graph) of K14-Ang1 transgenic mice relative to controls. Bars represent mean values ± SD (n = 3) and P < .01 compared with wild-type (WT) controls. Scale bars: 100 μm.
Lymphatic capillary hyperplasia in K14-Ang1 transgenic mice. (A-B) Lymphatic vessels in the ear of a K14-Ang1 transgenic mouse (A) and littermate control (B) whole-mount-stained with antibodies to LYVE-1. Note lymphatic vessels around hair follicles (arrowheads). (C-D) Double staining of lymphatic (red) and blood vessels (green). (E) Quantitative analysis of the circumference of LYVE-1-stained lymphatic vessels in ear sections (see micrographs to the left of the graph) of K14-Ang1 transgenic mice relative to controls. Bars represent mean values ± SD (n = 3) and P < .01 compared with wild-type (WT) controls. Scale bars: 100 μm.
Adenoviral Ang1 stimulates proliferation of lymphatic endothelial cells in vivo
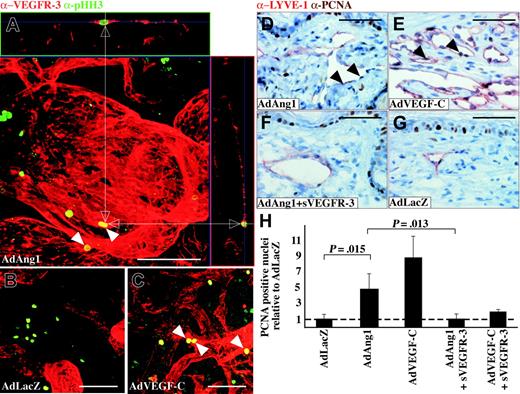
Because AdAng1 could induce formation of lymphatic endothelial filopodia, we were interested in assessing the rate of LEC proliferation in Ang1-treated tissues. Double staining for VEGFR-3 and the proliferation marker phosphohistone H3 revealed proliferating lymphatic endothelial cells in the AdAng1- and AdVEGF-C-treated ears (Figure 6A,C) but not in AdLacZ-treated ears (Figure 6B). For quantitative analysis of the LEC proliferation rates, ear sections were stained with LYVE-1 and PCNA. Ang1 stimulated LEC proliferation, although the proliferative response was more pronounced in AdVEGF-C-treated ears (Figure 6D-E arrowheads). Systemic administration of AdVEGFR-3-Ig inhibited the AdAng1-induced proliferation (Figure 6F-G). Figure 6H shows the quantification of lymphatic endothelial cell proliferation as a proportion of PCNA-positive cells relative to LYVE-1-positive cells with AdLacZ representing baseline levels. It is evident from these results that both Ang1 and VEGF-C significantly increased the lymphatic endothelial cell proliferation rate when compared with the control. However, a combination of AdAng1 or AdVEGF-C plus systemic AdVEGFR-3-Ig treatment reduced the proliferation rate back to baseline levels.
The Tie2 receptor is expressed in the lymphatic vessels. (A-B) Immunofluorescence for Tie2 (green) in blood vessels (BV) and lymphatic vessels (LV). (A) Double staining for LYVE-1 (red) and Tie2. Scale bars: 10 μm.
The Tie2 receptor is expressed in the lymphatic vessels. (A-B) Immunofluorescence for Tie2 (green) in blood vessels (BV) and lymphatic vessels (LV). (A) Double staining for LYVE-1 (red) and Tie2. Scale bars: 10 μm.
Angiopoietin-1 up-regulates VEGFR-3 expression in vivo and in vitro
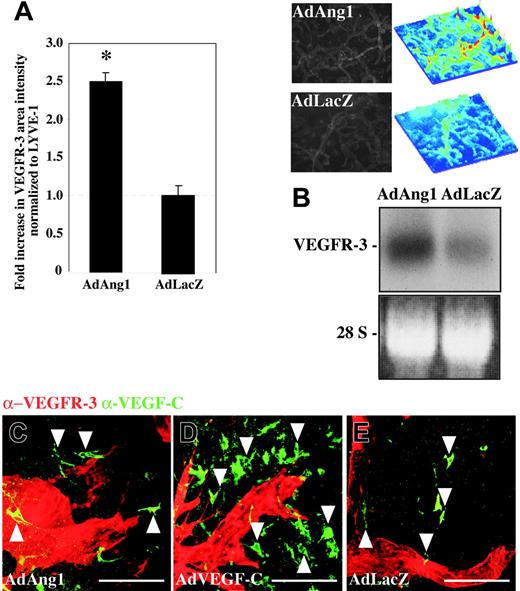
The experiments employing soluble VEGFR-3 suggested that the effects of Ang1 are dependent on VEGFR-3 signaling in lymphatic endothelial cells. In order to assess if Ang1 affected the levels of VEGFR-3 in lymphatic vessels, ears from AdAng1- and AdLacZ-treated mice were double stained with fluorescent antibodies for VEGFR-3 and LYVE-1. A measurement of VEGFR-3 staining intensities, normalized to LYVE-1 intensities, indicated a 2.5-fold increase of lymphatic endothelialVEGFR-3 levels in samples 4 days after treatment with AdAng1 when compared to the AdLacZ controls (Figure 7A). Accordingly, AdAng1 transduction of cultured lymphatic endothelial cells increased VEGFR-3 mRNA levels (Figure 7B), whereas AdAng1 transduction of BECs did not induce VEGFR-3 (data not shown), consistent with the fact that VEGFR-3 was not detected in blood vascular endothelial cells in vivo. As the lymphatic vascular effects of Ang1 were associated with VEGFR-3 up-regulation, the endogenous baseline expression of VEGF-C could be important for conveying the sprouting signals to LECs. VEGF-C was found to be expressed by cells with either a dendritic or a fibroblastic morphology in the AdAng1- and AdLacZ-treated ears (Figure 7C,E arrowheads). Abundant VEGF-C-expressing cells were found in AdVEGF-C-transduced ears (Figure 7D arrowheads).
Discussion
Our data show that viral overexpression of Ang1 induces lymphangiogenesis in adult skin. After Ang1 induced lymphatic vessel enlargement and extension of endothelial filopodia, sprouts were formed, which finally could lead even to the formation of additional lymphatic branches. Lymphangiogenesis induced by adenoviral VEGF-C has been shown to be maximal around the 2-week time point in immunocompromised mice.17 At this point, most of the vessel network was stabilized, and sprouting and filopodia were only seen in the remaining initial lymphatics of the AdAng1- or AdVEGF-C-treated ears.
Adenoviral transgene expression is transient, whereas transgene expression from AAV vectors is prolonged even in immunocompetent mice. AAV was therefore used as the vector for longer-term experiments. AAV-Ang1 transduction resulted in dilation of lymphatics in most of the treated samples. The response was less prominent than with the adenoviral vector, probably reflecting lower expression of the transgene compared with that of the adenoviral vectors. In particular, the ear offers relatively little target tissue for the AAV-2 vector, which is known to transduce primarily muscle and neural tissue.31 In addition, the multimeric and matrix binding properties of native Ang1 may decrease the availability of Ang1 for endothelial cells in the skin.5,32
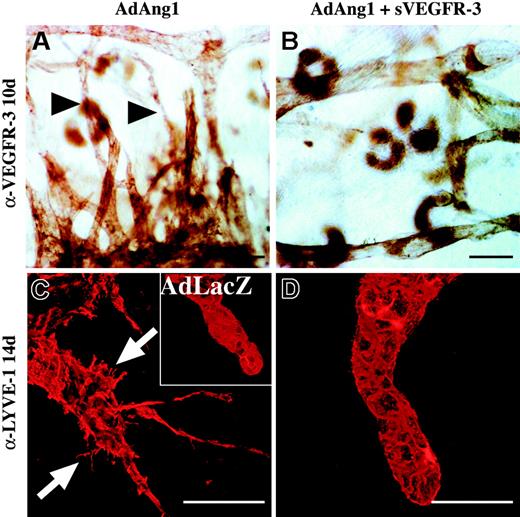
Lymphatic vascular effects of Ang1 are inhibited by systemic delivery of adenoviruses encoding the VEGFR-3-Ig fusion protein. (A-B) Anti-VEGFR-3-stained lymphatic vessel sprouts 10 days after transduction of AdAng1 (arrowheads in A) or AdAng1 plus systemic (s) AdVEGFR-3-Ig (B). (C-D) Confocal imaging of LYVE-1-stained lymphatic capillary ends 14 days after AdAng1 administration. Filopodia formed in response to AdAng1 treatment are indicated with arrows in panel C, while no filopodia can be seen in the AdAng1 plus AdVEGFR-3-Ig-treated ears (D). (C inset) A lymphatic vessel after AdLacZ transduction. Scale bars: 100 μm.
Lymphatic vascular effects of Ang1 are inhibited by systemic delivery of adenoviruses encoding the VEGFR-3-Ig fusion protein. (A-B) Anti-VEGFR-3-stained lymphatic vessel sprouts 10 days after transduction of AdAng1 (arrowheads in A) or AdAng1 plus systemic (s) AdVEGFR-3-Ig (B). (C-D) Confocal imaging of LYVE-1-stained lymphatic capillary ends 14 days after AdAng1 administration. Filopodia formed in response to AdAng1 treatment are indicated with arrows in panel C, while no filopodia can be seen in the AdAng1 plus AdVEGFR-3-Ig-treated ears (D). (C inset) A lymphatic vessel after AdLacZ transduction. Scale bars: 100 μm.
AdAng1 was able to induce proliferation of lymphatic endothelial cells in vivo and we could show that its Tie2 receptor is present in the lymphatic capillaries. Ang1 may exert direct effects promoting LEC proliferation and sprouting, as it has been shown to stimulate the survival and sprouting of endothelial cells.33-37 It is thus not surprising that Ang1 could promote sprouting or survival of the lymphatic endothelium. Furthermore, Ang1 is also able to rescue the lymphatic phenotype in Ang2 knockout mice, suggesting that either ligand acts as a receptor agonist in LECs.8 Such activity is consistent with our present findings. In addition, up-regulation of VEGFR-3 in response to the Ang1 stimulation of Tie2 in the LECs could account for at least part of the observed increase in the rate of LEC proliferation, as VEGFR-3 signals have been shown to be sufficient for lymphatic vessel growth in vivo.38
Ang1 induces proliferation of lymphatic endothelial cells. (A-C) Double staining of VEGFR-3 (red) and the proliferation marker phosphohistone H3 (green) 14 days after adenovirus transduction. Double-positive cells (arrowheads) are identified from confocal stacks by X and Y projections (white arrows). (D-G) PCNA/LYVE-1 double staining of ears from mice treated with the indicated adenoviral vectors. PCNA-positive LEC nuclei have been marked with arrowheads in panels D and E. (H) Quantification of the proportion of PCNA plus LYVE-1 double-positive cells relative to AdLacZ-negative control samples. Bars represent mean values ± SEM (n ≤ 3). Scale bars: 100 μm.
Ang1 induces proliferation of lymphatic endothelial cells. (A-C) Double staining of VEGFR-3 (red) and the proliferation marker phosphohistone H3 (green) 14 days after adenovirus transduction. Double-positive cells (arrowheads) are identified from confocal stacks by X and Y projections (white arrows). (D-G) PCNA/LYVE-1 double staining of ears from mice treated with the indicated adenoviral vectors. PCNA-positive LEC nuclei have been marked with arrowheads in panels D and E. (H) Quantification of the proportion of PCNA plus LYVE-1 double-positive cells relative to AdLacZ-negative control samples. Bars represent mean values ± SEM (n ≤ 3). Scale bars: 100 μm.
Our previous data have indicated that embryonic and tumor lymphangiogenesis can be inhibited by intravenous application of an adenovirus encoding the soluble VEGFR-3-Ig, demonstrating that this molecule is a potent inhibitor of VEGFR-3 signaling.39,40 Indeed, circulating soluble VEGFR-3-Ig inhibited the sprouting observed in response to VEGF-C or Ang1. It is possible that by up-regulating VEGFR-3, Ang1 renders lymphatic vessels more susceptible to VEGF-C and VEGF-D signals emanating from the surrounding microenvironment, including vascular smooth muscle cells, which have been shown to express both VEGF-C and VEGF-D.24,41 Even though baseline expression of VEGF-C was similar in the Ang1 and control samples, lymphatic vessels sprouted toward these cells only in the AdAng1-treated ears, suggesting that Ang1 is able to sensitize LECs for the VEGF-C sprouting signals.
Adenoviral Ang1 up-regulates the expression of VEGFR-3 in vivo and in vitro. (A) Quantification of VEGFR-3-stained immunofluorescent area intensities, normalized to LYVE-1 staining intensities, in ears transduced with either AdAng1 or AdLacZ. The 2.5-fold increase in VEGFR-3 levels on the lymphatic endothelium 4 days after gene transduction was statistically significant (*P < .001). The horizontal broken line indicates the baseline of VEGFR-3 expression in the control samples. Images and surface plots above panel B show changes in VEGFR-3 staining intensity after AdAng1 transduction compared with control. Bars represent mean values ± SEM (n ≤ 5). (B) Northern blot of total RNA extracted from LECs and BECs transduced with either AdAng1 or AdLacZ. (C-E) Double immunofluorescent staining of VEGFR-3 (red) and VEGF-C (green) in the ears 2 weeks after transduction with AdAng1 (C), AdVEGF-C (D), or AdLacZ (E). VEGF-C-positive cells are indicated with arrowheads. Scale bars: 100 μm.
Adenoviral Ang1 up-regulates the expression of VEGFR-3 in vivo and in vitro. (A) Quantification of VEGFR-3-stained immunofluorescent area intensities, normalized to LYVE-1 staining intensities, in ears transduced with either AdAng1 or AdLacZ. The 2.5-fold increase in VEGFR-3 levels on the lymphatic endothelium 4 days after gene transduction was statistically significant (*P < .001). The horizontal broken line indicates the baseline of VEGFR-3 expression in the control samples. Images and surface plots above panel B show changes in VEGFR-3 staining intensity after AdAng1 transduction compared with control. Bars represent mean values ± SEM (n ≤ 5). (B) Northern blot of total RNA extracted from LECs and BECs transduced with either AdAng1 or AdLacZ. (C-E) Double immunofluorescent staining of VEGFR-3 (red) and VEGF-C (green) in the ears 2 weeks after transduction with AdAng1 (C), AdVEGF-C (D), or AdLacZ (E). VEGF-C-positive cells are indicated with arrowheads. Scale bars: 100 μm.
However, direct evidence for the cooperation of the Tie2 and VEGFR-3 signaling systems in lymphangiogenesis is lacking. Interestingly, Ang1 has been reported to bind to integrin β1, even in the absence of Tie2, and to stimulate cell adhesion to fibronectin via a β1 integrin.42,43 On the other hand, VEGFR-3 has been shown to form a ligand-independent signaling complex with integrin β1.44 It is thus possible that Ang1 modulates signaling via the integrin β1-VEGFR-3 complex in addition to activation of Tie2.
Ang1 is an important regulator of vascular permeability. It is capable of preventing plasma leakage even after treatment of blood vessels with highly potent permeabilizing agents, such as VEGF.3,10 Our findings show that Ang1 can also be implicated as a lymphangiogenic factor. Ang1 could then act as a more global antagonist of plasma leakage and tissue edema by decreasing blood vascular permeability and by promoting growth of lymphatic vessels and thereby facilitating removal of excess fluid and other plasma components from the interstitium. These findings reinforce the idea that Ang1 might have therapeutic value in certain conditions involving tissue edema due to increased blood vascular permeability.
Prepublished online as Blood First Edition Paper, March 3, 2005; DOI 10.1182/blood-2004-08-3327.
Supported by the Human Frontier Science Program, European Union grants (QLK3-CT-2002-02059 and LSHG-CT-2004-503573), the Finnish Academy, the Finnish Cancer Organizations, the Farmos Research Foundation, the Ida Montin Foundation, and the Finnish Medical Foundation.
G.T. has declared a financial interest in Regeneron Pharmaceuticals Inc, whose potential product, angiopoietin-1, was studied in the present work.
A.S. and M.L. contributed equally to this study.
Presented as a poster at the Gordon Conference on Angiogenesis and Microcirculation, Newport, RI, August 10-15, 2003; at the Gordon Conference on Molecular Mechanisms of Lymphatic Function and Disease, Ventura, CA, March 7-12, 2004; and at the European School of Hematology Euroconference on Angiogenesis, Helsinki, Finland, May 21-24, 2004.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Peter Baluk and Dr Donald McDonald for their valuable help with our work. We also thank Dr Yulong He, for help with the experiments, and Sanna Lampi, Mari Helanterä, Johannes Lyytikkä, Paula Hyvärinen, and Tapio Tainola for excellent technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal