Abstract
Little is known about the ontogeny of naturally occurring CD4+CD25+ regulatory/suppressor T cells that play a major role in maintaining self-tolerance in mice and humans. In rodents, thymectomy on day 3 of life leads to multiple organ-specific autoimmune diseases that can be prevented by adoptive transfer of regulatory T cells, suggesting their neonatal development. We investigated regulatory T-cell ontogeny in 11 human fetuses. Together with the first mature T cells, thymic CD4+CD25+ cells were detected as early as 13 weeks of gestation. Thymic CD25+ cells appeared to be positively selected at the CD4+CD8+CD3hi differentiation stage, as assessed by CD1a and CD69 expression. The proportion of thymic CD4+CD25+ cells appeared quite stable with age, around 6% to 7%, similar to the proportion observed in infant thymi. Extrathymic CD4+CD25+ T cells could hardly be detected at 13 weeks of gestation but were present from week 14 onwards. As adult regulatory T cells, purified CD4+CD25+ fetal cells were anergic and suppressed T-cell proliferative responses; they expressed intracellular cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and Foxp3 mRNA. Altogether, our results indicate that the generation of regulatory/suppressor T cells is consubstantial to the generation of a functional and self-tolerant immune system. (Blood. 2005;105:4715-4721)
Introduction
T cells capable of suppressing immune responses have been hypothesized for a long time.1 Functional assays have supported their existence2,3 but the inability to phenotypically identify them has hampered their further study. However, there is now clear evidence that CD4+ cells expressing the interleukin-2 receptor α chain (CD25) contain a population of naturally and ubiquitously present cells endowed with suppressive activities.4-7 The lack of a known specific surface marker makes it hard to distinguish CD4+CD25+ regulatory T cells (Treg's) from CD4+ activated effector T cells, which may transitorily express CD25.8 As of today, Treg's are best identified by the expression of (1) high levels of CD25 (CD25hi),8-10 (2) the forkhead/winged helix transcription factor Foxp3,11 (3) high levels of intracytoplasmic cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4),12,13 and (4) the glucocorticoid-induced tumor necrosis factor receptor (GITR) surface marker.14 Since these individual markers are neither specific nor always simultaneously expressed, the authentication of bona fide Treg's relies on the demonstration that (1) they are anergic in vitro in the absence of exogenous IL-2 and (2) they indeed suppress effector T-cell responses.
It is now well established that Treg's play a major role in maintenance of self-tolerance and control of autoimmune diseases.7,15 They are also involved in regulation of T-cell homeostasis16-18 and modulation of immune responses to alloantigens, tumor cells, and various pathogens.19-26 In the periphery, at steady-state, Treg's represent a stable proportion of the CD4+ T cells, typically 5% to 12% in mice and humans. Although Treg's are anergic in vitro, in vivo studies have shown that some Treg's are quiescent with a long lifespan whereas others divide extensively due to continuous activation by tissue self-antigens.27 T-helper type 3 (Th3) and T-regulatory type 1 (Tr1)28,29 are other types of regulatory cells whose relation to Treg's is poorly understood. Th3 and Tr1 cells are generated in the periphery in particular cytokine environments, whereas Treg's are naturally occurring and thought to have a thymic origin.30
The thymic production of Treg's has been suggested to be developmentally programmed, with their egress beginning on day 3 after birth.31 Indeed, thymectomy on day 3 of life induces depletion of Treg's and development of a severe autoimmune syndrome that can be prevented by adoptive transfer of Treg's. In agreement with this observation, murine Treg's cannot be detected at the periphery before day 3 of life,31 and it was shown that neonatal infection with the mouse T-lymphotropic virus, which selectively depletes CD4+ T cells in the thymus and periphery, causes various organ-specific autoimmune diseases in otherwise normal mice.32 Importantly, thymic selection of Treg's appears to favor the emergence of autoreactive Treg's.30,33-35
In humans, little is known about Treg ontogeny although the development of the thymus and T cells has been extensively studied. The human thymus has a mixed origin, developing from both the third pharyngeal pouch endoderm36 and neural crest-derived mesenchyme. This stroma is later colonized by hematopoietic cells.37 The epithelial primordium of the thymus begins its development at the end of the fourth week of gestation (WG) then migrates to the rostral level of the sternum up until the seventh WG. Colonization of the thymic primordium by T-lymphocyte progenitors appears to be a very early event in humans, taking place during the eighth WG. However, mature T cells are not detected in the thymus before 12 to 13 WGs38,39 ; they start egressing the thymus and progressively colonize the periphery from about the end of the 13th/begining of the 14th WG,40,41 with numbers and percentages of CD3+ cells increasing exponentially during gestation.42
Different subsets of cells with high suppressive activities have already been described in human cord blood43 : (1) directly suppressive CD8+CD4- cells,44 (2) CD4+CD8- cells able to induce secondary suppressor cells,45,46 (3) and CD3+CD4-CD8- CD45R+ cells able to inhibit IL-2 production.47 More recently CD8+CD25+ cells48 and CD4+CD25+49-51 regulatory T cells have been identified in human cord blood or thymus. We report here for the first time the ontogeny of CD4+CD25+ regulatory T cells in different fetal organs. Our results show that these cells originate from the thymus and possess all the phenotypic and functional characteristics of Treg's found in adults.
Patients, materials, and methods
Origin of fetuses
The use of human fetal and postnatal tissues was approved by the National Advisory Ethical Committee for Life Sciences and Health. Informed consent was given by the mother according to the committee guidelines. Thymus, spleen, liver, and bones were dissected from human fetuses obtained after legal or spontaneous abortion. Fetuses macerated or presenting chromosomal abnormalities were discarded. The fetal age was carefully established according to the date of the last menstrual period and to early echographic biometry. The dissection of the fetus and its viscera follows the standard protocols established for such a process by the Fetopathology Department of the Hospital. A sample of a thymus, the spleen, and the liver were carefully removed from each fetus. These samples were maintained in cold medium solution (RPMI 1640 [Gibco-Invitrogen, Carlsbad, CA] complemented with 10% fetal calf serum [PAA Laboratories, Pasching, Austria]) before processing for their analysis. Bone marrow (when available) was obtained by removing the upper moiety of the femur. Eleven fetuses were included in the study (6 males and 5 females). Their age varied from 13 WGs to 29 WGs. The causes for abortion were as follows: chorioamnionitis (4), placental infarcts due to maternal vascular disease (4), maternal dysembryoplastic tumor lying in the right temporal lobe (1), spina bifida (1), and severe maternal nephritic syndrome (1). Two infant thymi from 8-week-old and 24-month-old cardiac surgery patients were analyzed too.
Cell purification
For mixed lymphocyte reactions, CD4+ thymocytes and splenocytes were purified using a CD4+ T-cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to manufacturer's protocol. Briefly, after a mechanical dissociation, thymus and spleen were first incubated in phosphate-buffered saline (PBS) supplemented with 3% fetal calf serum and saturating amounts of an antibody cocktail (CD8, CD11b, CD16, CD19, CD36, and CD56). Cells were then incubated with microbeads (Miltenyi Biotec) and CD4+ cells were purified using magnetic separation columns (Miltenyi Biotec). The negatively selected cells (∼95% CD4+) were incubated with CD25 microbeads (Miltenyi Biotec), and CD25+ and CD25- cells were purified from CD4+ fraction using magnetic cell separation columns (Miltenyi Biotec). To increase cell purity, the positive fraction was separated in another column leading to a greater than 95% purity of the CD8-CD4+CD25+ cell population.
For reverse transcriptase-polymerase chain reaction (RT-PCR) analysis, thymocytes were labeled with fluorescein isothiocyanate (FITC)-conjugated anti-CD3 (clone UCHT; Immunotech, Marseille, France), phycoerythrin (PE)-conjugated anti-CD25 (clone M-A251; BD Biosciences, San Jose, CA), and allophycocyanin (APC)-conjugated anti-CD4 (clone RPAT4; BD Biosciences). The CD3hiCD4+CD25+ and CD3hiCD4+CD25- cells were sorted on a FACSvantageSE (Becton Dickinson, Mountain View, CA), giving a purity of 98.5% to 99.5%.
Antibodies and flow cytometric analysis
After a mechanical dissociation, cells from thymus, spleen, bone marrow, and liver (liver lymphocytes were purified by ficoll using Histopaque-1077; Sigma, St Quentin Fallavier, France) were counted and stained in PBS 3% fetal calf serum with saturating amounts of combinations of the following monoclonal antibodies (mAbs) or their isotype-matched irrelevant mAbs as controls: FITC-conjugated anti-CD3 (clone UCHT; Immunotech); anti-CD4 (clone SCFI12T4D11; Beckman Coulter, Hialeah, FL); anti-CD8 (clone B9.11; Immunotech); anti-CD25 (clone M-A251), anti-CD45RO (clone UCHL1), anti-CD62L (clone Dreg 56), anti-CD134 (OX40; clone ACT35), anti-CD154 (CD40L; clone TRAP1) purchased from BD Biosciences; anti-GITR (clone 110416; R&D Systems, Minneapolis, MN); PE-conjugated anti-CD8 (clone RPAT8), anti-CD25 (clone M-A251), anti-HLA A, B, C (clone G46-2.6) from BD Biosciences; anti-HLA DR (clone L243; Becton Dickinson); anti-CD62L (clone SFCI28T17G6; Beckman Coulter); Cy-chrome (CyC)-conjugated anti-CD1a (clone H149), anti-CD4 (clone RPAT4), anti-CD45 RA (clone HI100), anti-CD152 (CTLA-4; clone BNI3) all purchased from BD Biosciences; and APC-labeled anti-CD3 (UCHT1; Immunotech); anti-CD4 (clone RPAT4; BD Biosciences); anti-CD8 (clone RPAT8; BD Biosciences); anti-CD25 (clone M-A251; BD Biosciences); and anti-CD45 (clone J.33; Immunotech). For intracellular staining, cells were first surface labeled with the relevant antibody before being permeabilized using a BD CytoFix/CytoPerm kit. They were then incubated with the experimental antibodies or the isotype-matched irrelevant mAbs as controls. Lymphocytes were gated according to their forward and side scatter characteristics and 4-color acquisitions were performed on a FACScalibur (Becton Dickinson). Data were analyzed using FlowJo (Tree Star, San Carlos, CA).
Proliferation and suppression assays
For mixed lymphocyte cultures, 5 × 104 CD4+, CD4+CD25-, or CD4+CD25+ fresh thymocytes or splenocytes were stimulated by 5 × 104 fetal irradiated allogeneic splenocytes in complete RPMI 1640 medium supplemented with 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer, 2 mM l-glutamine, penicillin (100 μg/mL), streptomycin (100 U/mL; Gibco-Invitrogen), 10% fetal calf serum (PAA Laboratories), and 50 μM 2-mercaptoethanol (Sigma). For assessing suppressive activity, different numbers of purified CD8-CD4+CD25+ or CD8-CD4+CD25- cells were added to the culture of 5 × 104 fresh CD8-CD4+CD25- autologous fetal thymocytes and stimulated by 20-Gy irradiated 5 × 104 allogeneic splenocytes. Cells were cultured in round-bottom 96-well plates for 4 days and then pulsed with methyl-[3H]thymidine (1.5 μCi [0.0555 MBq]/well) for the last 18 hours before measuring incorporated radioactivity using a liquid scintillation counter. The background proliferation of cells in the absence of allogeneic APC was composed of between 10 and 200 counts per minute (cpm).
RT-PCR analysis
Expression of Foxp3 mRNA was analyzed by nested RT-PCR as follows. Samples of 100 sorted cells in 10 μL ice-cold reaction buffer were denatured to 95°C for 5 minutes. Reverse transcription and 2 PCRs were carried out in the same tube. The reaction conditions were 50 minutes at 42°C for reverse transcription with Superscript II (Invitrogen, Carlsbad, CA), followed by a first PCR using a Perkin-Elmer (Shelton, CT) DNA thermocycler 9600 by 35 cycles of 30 seconds at 94°C, 30 seconds at 60°C, 40 seconds at 72°C, and a final extension of 2 minutes at 72°C. The following primers spanning the entire Foxp3 mRNA coding sequence were used for the Foxp3 PCR: upper primer 5′-CGTGACAGTTTCCCACAAGC-3′ and lower primer 5′-CCTGTTCGTCCATCCTCCTT-3′ for the first PCR. A secondary PCR of 35 cycles was then performed in the same conditions using the following pairs of nested primers: upper primer 5′-ATGCCCAACCCCAGGCCTGG-3′ and lower primer 5′-TCAGGGGCCAGGTGTAGGGT-3′. β-Actin PCR was performed using the following primers: upper primer 5′-TCGACAACGGCTCCGGCA-3′, lower primer 5′-CGTACATGGCTGGGGTGT-3′. The predicted sizes (in base pairs) of the amplicons were as follows: Foxp3, 1296 base pair (bp); and β-actin, 370 bp. PCR products of Foxp3 and β-actin were visualized by electrophoresis in a 1.5% agarose gel containing ethidium bromide.
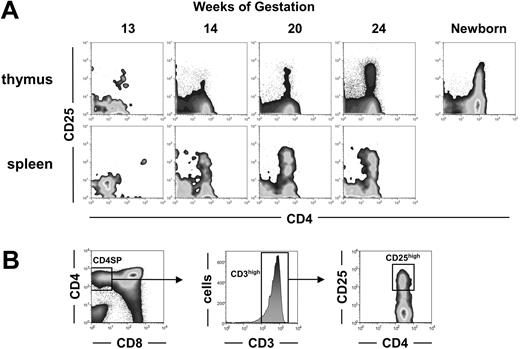
Development of CD4+CD25+ cells during human fetal ontogeny. (A) Thymic and splenic T cells from fetuses of the indicated ages and from one 8-week-old newborn were analyzed by flow cytometry for CD4 and CD25 expression. Density plot windows shown are gated on CD3+ lymphocytes. (B) Successive gates used to determine the proportion of CD25hi cells among CD3hiCD4+CD8-SP cells reported in Table 1 are shown.
Development of CD4+CD25+ cells during human fetal ontogeny. (A) Thymic and splenic T cells from fetuses of the indicated ages and from one 8-week-old newborn were analyzed by flow cytometry for CD4 and CD25 expression. Density plot windows shown are gated on CD3+ lymphocytes. (B) Successive gates used to determine the proportion of CD25hi cells among CD3hiCD4+CD8-SP cells reported in Table 1 are shown.
Results
Fetal development of CD4+CD25+ thymocytes and peripheral T cells
As of today, human Treg's are best identified by CD4 and high levels of CD25 coexpression.9,10,52 We thus first looked for CD4+CD25+ cells within the whole population of CD3+ fetal thymocytes and peripheral T cells (Figure 1A). In the youngest fetus analyzed, at 13 WGs, CD4+CD25+ T cells could already be detected and represented a large proportion of the very few thymocytes. In older fetuses, the CD4+CD25+ T cells, including CD4+CD25hi cells, became readily detectable as the overall CD3+ thymocyte population increased. We then quantified the percentage of CD25hi cells within CD8-CD4+CD3hi mature T cells as shown in Figure 1B. From 14 WGs onwards, the percentage of CD25hi cells within mature CD3hiCD4+CD8- cells appeared almost constant, around 6% to 7% (Table 1). In contrast, CD4+CD25+ cells could hardly be detected in the spleen of the 13-week-old fetus analyzed (Figure 1A). They were however present and represented 10% to 27% of the CD3+ T cells in the spleen from 14 WGs onwards. These observations suggest that, as for other T cells, the seeding of CD4+CD25+ cells to the periphery occurs rapidly after their generation. Significant numbers of CD4+CD25hi could not be detected in the liver until 14.5 WGs and in the bone marrow until 16 WGs (Table 1). In peripheral organs, the frequency of CD4+CD25hi cells was around 20% (Table 1). Importantly, we never observed the presence of CD4+CD25- or CD8+ lymphocytes without the simultaneous presence of CD4+CD25+ T cells in any of the organs analyzed. Altogether, the generation of CD4+CD25+ T cells appears concomitant to the generation of other mature T cells.
Frequencies of CD25hi Treg's among CD3hiCD4+CD8- T cells in various organs from human fetuses
Age . | Thymus . | Spleen . | Liver . | Bone marrow . |
|---|---|---|---|---|
| Fetus, wk | ||||
| 13 | 26 | ND | ND | ND |
| 14 | 5 | 10 | ND | ND |
| 14.5 | 5 | 18.5 | 32 | ND |
| 16 | 8 | 26 | ND | 29 |
| 19.5 | 8 | 20 | 10 | 16 |
| 20.5 | 7 | 27 | 29 | NA |
| 21.5 | 6 | 16 | 24 | 17 |
| 22 | 6 | NA | NA | NA |
| 24 | 6 | 20 | 18 | 16 |
| 29 | 7 | 14.5 | 19 | NA |
| 29 | 7 | 16.5 | 20 | NA |
| Mean | 8.3 | 19.8 | 21.7 | 19.5 |
| SD | 6.0 | 6.3 | 7.4 | 6.4 |
| Child, mo | ||||
| 2 | 7 | NA | NA | NA |
| 24 | 7 | NA | NA | NA |
Age . | Thymus . | Spleen . | Liver . | Bone marrow . |
|---|---|---|---|---|
| Fetus, wk | ||||
| 13 | 26 | ND | ND | ND |
| 14 | 5 | 10 | ND | ND |
| 14.5 | 5 | 18.5 | 32 | ND |
| 16 | 8 | 26 | ND | 29 |
| 19.5 | 8 | 20 | 10 | 16 |
| 20.5 | 7 | 27 | 29 | NA |
| 21.5 | 6 | 16 | 24 | 17 |
| 22 | 6 | NA | NA | NA |
| 24 | 6 | 20 | 18 | 16 |
| 29 | 7 | 14.5 | 19 | NA |
| 29 | 7 | 16.5 | 20 | NA |
| Mean | 8.3 | 19.8 | 21.7 | 19.5 |
| SD | 6.0 | 6.3 | 7.4 | 6.4 |
| Child, mo | ||||
| 2 | 7 | NA | NA | NA |
| 24 | 7 | NA | NA | NA |
Expression of CD25 during maturation of fetal thymocytes
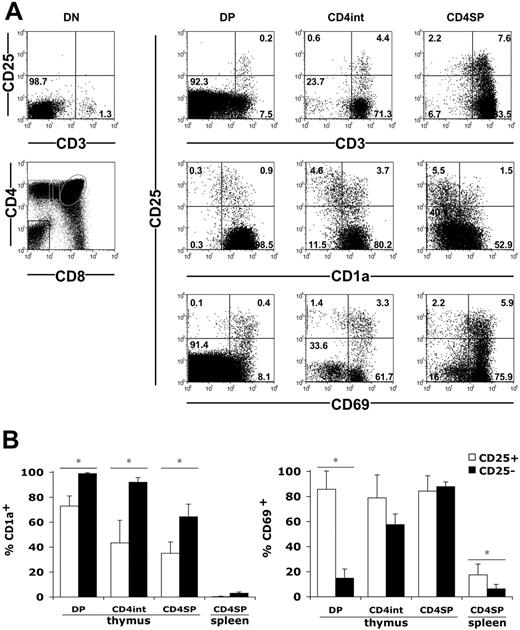
We then examined the expression of CD25 during the maturation of fetal and infant thymocytes. Schematically, the thymocyte maturation according to CD4 and CD8 expression starts with immature CD4-CD8- double-negative (DN) cells that mature into CD4+CD8+ double-positive (DP) cells; these cells then proceed to a CD4+CD8lo or CD4loCD8+ intermediate stage to become terminally differentiated CD4+CD8- or CD4-CD8+ single-positive (SP) cells. We first analyzed the development of CD25+ cells according to CD3, which belongs to the T-cell receptor complex. We could not detect CD25hi cells among DN cells, including their small CD3+ fraction (Figure 2A). Low percentages of CD25hi cells could be detected in total DP cells. Strikingly, they all belong to the CD3hiDP population and represented approximately 3% of these cells. Increasing proportions of CD25+ cells were observed among the CD4+CD8lo cells and the CD4SP cells, also within the CD3hi cells (Figure 2A). Noteworthy, a few CD25lo cells could also be detected among the CD3hiCD8+CD4lo and CD8SP cells (not shown). We then analyzed CD25 expression according to CD1a, a marker of functional maturation of human thymocytes that is expressed at the DP stage and is lost in mature SP cells.53 Very few DN thymocytes expressed CD1a (not shown), whereas most DP thymocytes were CD1a+ (Figure 2). The progressive decrease in CD1a expression observed from DP to CD4SP stages was more pronounced in CD25+ than in CD25- cells (Figure 2A). Indeed, while all CD25-DP cells were CD1a+, 30% of the CD25+ cells had already lost CD1a at the DP stage (Figure 2B). The downregulation of the CD1a molecule proceeded further in CD4+CD8lo and CD4SP cells and was always more pronounced in CD25+ cells (Figure 2B). We also analyzed CD25 expression according to CD69, which is a marker of positive selection in the thymus54 (Figure 2A). In CD25- cells, CD69 expression was progressively acquired during thymic maturation from DP to CD4SP cells. In contrast, a vast majority of CD25+ cells already expressed CD69 at the DP stage and maintained this high level of expression until the CD4SP stage (Figure 2B). In the spleen, both CD25+ and CD25- cells had lost CD1a and CD69 expression (Figure 2B). Altogether, CD25+ cells were predominantly detected among positively selected CD4+CD3hiCD1a-CD69+ cells. Therefore, during the differentiation of DP cells to CD4SP cells in fetal thymi, the CD25hi cells tended to loose CD1a expression and to acquire CD69 sooner than the CD25- cells. Similar results were obtained from the 2 infant thymi analyzed (not shown).
Positive selection of human fetal CD25hi thymocytes. (A) CD3, CD1a, CD69, and CD25 expression in CD4-CD8- (DN), CD4+CD8+ (DP), CD4+CD8lo (CD4int), and CD4+CD8- (CD4SP) thymocytes gated as shown on the bottom left panel. Numbers indicate cell percentages in each quadrant. Data from 20-WG fetuses are shown. (B) Mean percentages ± SD of CD1a+ and CD69+ cells in CD25+ and CD25- lymphocytes within subsets defined as in panel A. Cells were obtained from 8 fetuses (13 to 29 WGs) for CD69 analyses and 6 fetuses (14 to 24 WGs) for CD1a analyses. *P < .05 by Student t test.
Positive selection of human fetal CD25hi thymocytes. (A) CD3, CD1a, CD69, and CD25 expression in CD4-CD8- (DN), CD4+CD8+ (DP), CD4+CD8lo (CD4int), and CD4+CD8- (CD4SP) thymocytes gated as shown on the bottom left panel. Numbers indicate cell percentages in each quadrant. Data from 20-WG fetuses are shown. (B) Mean percentages ± SD of CD1a+ and CD69+ cells in CD25+ and CD25- lymphocytes within subsets defined as in panel A. Cells were obtained from 8 fetuses (13 to 29 WGs) for CD69 analyses and 6 fetuses (14 to 24 WGs) for CD1a analyses. *P < .05 by Student t test.
In vitro proliferation and suppressive activity of fetal CD4+CD25+ T cells
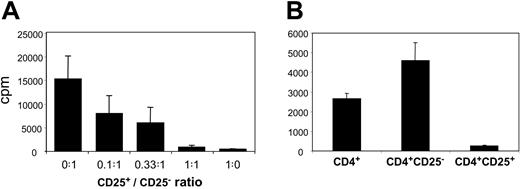
To assess whether CD4+CD25+ thymocytes and splenocytes were bona fide Treg's, we tested their proliferative and suppressive properties in vitro (Figure 3). We observed that CD4+CD8-CD25+ fetal thymocytes were anergic in response to allogeneic stimulation (Figure 3A). Moreover, they were able to inhibit CD4+CD8-CD25- T-cell proliferation in a dose-dependent manner, with almost complete suppression at a 1:1 ratio (Figure 3A). When we substituted CD4+CD25- cells for CD4+CD25+ cells we observed a dose-dependent increase in proliferation indicating that the inhibition was not due to culture conditions (not shown). Due to the paucity of the fetal splenocytes available, we could not test the suppressive activity of purified splenic fetal Treg's in a dose-dependent assay. However, we could assay the proliferative capacity of highly purified CD4+, CD4+CD25+, and CD4+CD25- fetal splenocytes stimulated with irradiated allogeneic splenocytes. While unfractionated CD4+ T splenocytes proliferated, CD4+CD25+ ones did not (Figure 3B). Furthermore, the depletion of CD4+CD25+ cells, which represent 20% of the whole CD4+ population, consistently resulted in almost 100% increased proliferation of the remaining CD4+CD25- cells (Figure 3B). This suggested that CD4+CD25+ splenocytes suppressed lymphocyte proliferation.
Proliferation and suppressive activity of human fetal CD4+CD25+ T cells. Results from 19.5-WG fetus are given as mean counts per minute (cpm) ± SD results of triplicate cultures and are representative of 4 independent experiments performed on 4 fetuses aged 16, 19.5, 29, and 29 WGs. (A) CD4+CD8-CD25- (50 000) and increasing numbers of CD4+CD8-CD25+ thymocytes were cocultured at the indicated ratios of CD4+CD8-CD25+/CD4+CD8-CD25- cells and stimulated by irradiated allogeneic fetal splenocytes (20.5 WGs). (B) Proliferation of 40 000 purified CD4+, CD4+CD25-, or CD4+CD25+ splenocytes stimulated by irradiated allogeneic splenocytes. All the differences between CD4+, CD4+CD25+, and CD4+CD25- splenocyte proliferations are statistically significant (P < .05 by Student t test).
Proliferation and suppressive activity of human fetal CD4+CD25+ T cells. Results from 19.5-WG fetus are given as mean counts per minute (cpm) ± SD results of triplicate cultures and are representative of 4 independent experiments performed on 4 fetuses aged 16, 19.5, 29, and 29 WGs. (A) CD4+CD8-CD25- (50 000) and increasing numbers of CD4+CD8-CD25+ thymocytes were cocultured at the indicated ratios of CD4+CD8-CD25+/CD4+CD8-CD25- cells and stimulated by irradiated allogeneic fetal splenocytes (20.5 WGs). (B) Proliferation of 40 000 purified CD4+, CD4+CD25-, or CD4+CD25+ splenocytes stimulated by irradiated allogeneic splenocytes. All the differences between CD4+, CD4+CD25+, and CD4+CD25- splenocyte proliferations are statistically significant (P < .05 by Student t test).
Altogether, CD4+CD25+ splenocytes and thymocytes are anergic and have a suppressive activity like their adult counterpart; they can thus be considered as fetal Treg's.
Fetal Treg phenotype
We then analyzed the phenotypic profile of CD4+CD25hi cells in various fetal organs (Figure 4). Most if not all of the fetal thymic or splenic Treg's expressed CD3, CD45, HLA class I, and αβ-T-cell receptor (αβ-TCR) molecules, whereas only a minority expressed a γδ-TCR (data not shown). HLA class II molecules were expressed on a higher proportion of Treg's than of CD4+CD25- cells (30.3 ± 13.1 vs 17.5 ± 5.8 and 42.7 ± 13 vs 30.7 ± 10.3 in the thymus and spleen, respectively), suggesting that CD4+CD25hi cells were enriched for activated T cells. A fraction of fetal Treg's in the thymus expressed OX40 (CD134) whereas most expressed CD62L and CD45RO (Figure 4A). In contrast, they did not express CD40L that is otherwise expressed by activated effector T cells in adults. In the spleen, a similar pattern of OX40, CD40L, and CD62L expression was found among Treg's, but CD45RO+ cells were drastically reduced (Figure 4B). Despite this, a higher proportion of CD45RO+ cells was observed in Treg's than in CD4+CD25- cells. Based on HLA class II, CD45RO (Figure 4B), and CD69 (Figure 2) expression in peripheral organs, we conclude that Treg's were enriched for activated T cells compared with their CD4+CD25- counterparts.
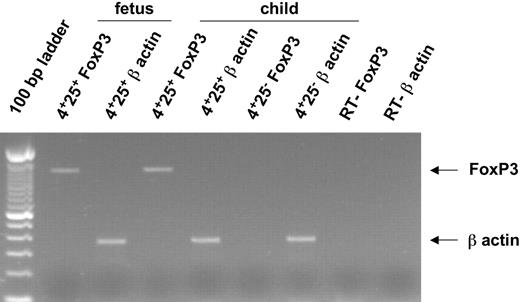
CTLA-4 (CD152) and GITR expression, which are closely associated with the regulatory function of Treg's, were also evaluated in fetal T cells. Most CD4+CD25hi Treg's express intracytoplasmic CTLA-4 and approximately 40% express cell surface GITR (Figure 4) whereas none expressed cell surface CTLA-4 (not shown). This phenotypic profile was similar in all fetuses examined irrespective of ages and was also found in the liver and the bone marrow (Figure 4B). Finally, we analyzed the expression of Foxp3 mRNA (Figure 5), which is considered the most specific marker of adult Treg's of mice and humans. As expected, RT-PCR performed on highly purified cells showed that Foxp3 was expressed by 14-WG fetal as well as newborn CD3hiCD4+CD25+ thymocytes but not CD3hiCD4+CD25- fetal and newborn thymocytes (Figure 5; not shown). Similar results were obtained with 16-, 20.5-, and 29-WG fetal cells (data not shown).
Phenotypic analysis of human fetal Treg. (A) CD25 high (top row) or negative (bottom row) T cells from fetal thymus were analyzed for cell surface expression of the indicated markers. Percentages of positive cells among CD4+CD3+ cells are indicated. Gray histograms represent the background staining with isotype-matched irrelevant mAbs. (B) Mean frequencies ± SD of positive cells for each indicated marker among CD3+CD4+CD25hi Treg's and CD3+CD4+CD25- cells from thymus, spleen, liver, and bone marrow cells. Each marker was tested in 3 to 11 fetuses (13 to 29 WGs), except for GITR in liver that was tested once in a 19.5-WG fetus. *P < .05 by Student t test.
Phenotypic analysis of human fetal Treg. (A) CD25 high (top row) or negative (bottom row) T cells from fetal thymus were analyzed for cell surface expression of the indicated markers. Percentages of positive cells among CD4+CD3+ cells are indicated. Gray histograms represent the background staining with isotype-matched irrelevant mAbs. (B) Mean frequencies ± SD of positive cells for each indicated marker among CD3+CD4+CD25hi Treg's and CD3+CD4+CD25- cells from thymus, spleen, liver, and bone marrow cells. Each marker was tested in 3 to 11 fetuses (13 to 29 WGs), except for GITR in liver that was tested once in a 19.5-WG fetus. *P < .05 by Student t test.
Discussion
We have identified a population of T cells from human fetal organs that clearly appeared to be the equivalent of Treg's found in adults49 and recently described in cord blood.51 These T cells (1) shared the same CD4+CD25hi phenotype, (2) were enriched in GITR+OX40+ cells,52,55,56 and (3) expressed the intracellular form of CTLA-4, a molecule that seems essential for Treg function12,13 and that is otherwise only expressed, constitutively, by Treg's in adults.56 Additionally, they expressed the transcription factor Foxp3, which is the most specific Treg marker so far. Indeed, its forced expression in effector T cells transform them into Treg's11,57 and genetic mutations of Foxp3 result in lethal autoimmune diseases, the scurfy and IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome) of mice and humans, respectively.58-60 Furthermore, they were anergic in vitro and suppressed lymphocyte proliferation in a dose-dependent fashion as described for their adult counterparts.48,56 Thus, as in adult peripheral blood and cord blood, CD4+CD25hi fetal T cells contain regulatory/suppressor Treg's. We also observed CD8SP CD25lo cells in our fetal thymi, which could correspond to the recently described CD8+CD25+ thymic regulatory cells found in newborns and children.48
Expression of Foxp3 and β-actin mRNAs in human fetal thymocytes.Foxp3 mRNA and β-actin mRNA were amplified by RT-PCR from sorted CD8-CD4+CD25+ and CD8-CD4+CD25- thymocytes from a 24-month-old child and CD8-CD4+CD25+ thymocytes from a 14-WG fetus. Arrows indicate the specific PCR band corresponding to Foxp3 and β-actin mRNA.
Expression of Foxp3 and β-actin mRNAs in human fetal thymocytes.Foxp3 mRNA and β-actin mRNA were amplified by RT-PCR from sorted CD8-CD4+CD25+ and CD8-CD4+CD25- thymocytes from a 24-month-old child and CD8-CD4+CD25+ thymocytes from a 14-WG fetus. Arrows indicate the specific PCR band corresponding to Foxp3 and β-actin mRNA.
It has been previously reported that the first mature thymocytes are detected between 12 to 14 WGs.38,39 We show here that Treg's are detected in the thymus by 13 WGs and they are therefore most likely generated concomitantly with other T cells. Interestingly, at that time, they could not be detected in peripheral organs. Thus, while a circulation to the thymus of Treg's generated at the periphery could have been possible, our data strongly support a thymic rather than a peripheral origin for human Treg's.61 Treg's can be detected in the spleen as early as at 14 WGs, the first time at which mature functional T splenocytes have been detected previously.40,41 In liver and bone marrow, Treg's and other CD4+ or CD8+ T cells were absent in the 14-WG fetus analyzed and were first detected between 14.5 and 16 WGs. This indicates that they reach these organs around the 15th WG. Altogether, the appearance of T cells in the thymus, as well as in the periphery, was always accompanied by the appearance of Treg's.
The generation of Treg's during fetal life in humans differs from that in mice where many lines of evidence indicate that they appear only 3 days after birth.31,32 We believe that this reflects differences in pregnancy duration. Full maturation of thymic T-cell progenitors, a process that requires more than 2 weeks in adult mice,62-64 can hardly be achieved in an organ generated at day 12 of a 3-week pregnancy. Likewise, peripheral effector T cells are not detected before day 2 after birth in mice whereas their numbers are fairly high at birth in humans. Our results may explain why neonatal thymectomy is associated with the development of autoimmune disease in mice31 but not in humans thymectomized at birth during cardiac surgery.65,66
Except for the youngest fetus analyzed, which had a significantly higher proportion of CD25hi cells among its very few CD3hiCD4+ thymocytes, the frequency of thymic Treg's appeared quite stable in fetuses of increasing ages and comparable to that of newborns. This contrasts with the variation in the proportion of other thymic populations such as DN, DP, CD8SP, and CD4SP (data not shown) and suggests a linked homeostasis of Treg's with other mature T cells. During thymocyte differentiation, CD25hi expression was detected at the late DP stage (ie, CD3hiDP cells). In addition, at this stage, almost all CD25+ cells were CD69+, suggesting that the commitment to the regulatory lineage occurs during positive selection. The fact that most Treg's expressed CD69 and lost CD1a more rapidly than other thymocytes further suggests that these cells might receive more vigorous activation signals during selection. We speculate that the self-antigen-specific TCR of the Treg's would be more efficiently engaged by the presence of self-peptides than would the non-self-specific TCR of other T cells, which are selected through “degenerated recognition” in the absence of specific peptide(s). That potentially stronger activation could be the committing signal to the regulatory/suppressive pathway. This hypothesis is supported by the finding that thymic epithelium cells engaged in positive selection are necessary for the Treg generation67-69 and that murine Treg's seem to be positively selected to recognize their ligands with very high affinity.30,33,70 In the periphery, activation markers are also expressed in a larger proportion of Treg's than of other T cells. This could be explained by the presence of recent thymic immigrants and/or by the existence in each organ of a pool of self-specific Treg's activated by the presence of their cognate antigens. This latter hypothesis is supported by the observation that mouse self-specific Treg's have a high turnover and express activation markers.27,71-73
The fetal origin of Treg's raises the question of the role(s) that they could play in the fetus. It has been previously shown that fetal suppressor cells were able to inhibit in vitro the activation/proliferation of maternal lymphocytes,43 suggesting that they protect fetal tissues from the mother's immune responses. We suggest that fetal Treg's could rather play the same or similar role as their equivalent found in adults (ie, protecting tissues from autoimmune attacks). Altogether, the concomitant generation of Treg's together with other T cells in human fetuses demonstrates that the development of Treg's is consubstantial to the development of a self-tolerant immune system.
Prepublished online as Blood First Edition Paper, February 24, 2005; DOI 10.1182/blood-2004-10-4051.
Supported by the Assistance Publique des Hôpitaux de Paris (AP-HP), the UPMC, the CNRS, the Association Française contre les Myopathies (AFM), and the Fondation pour la Recherche Médicale (FRM). G.D.-J. is supported by a predoctoral fellowship from the Ministère de l'Education Nationale et de la Recherche and G.M. is supported by postdoctoral fellowships from the FRM and the AFM.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Béatrice Levacher for performing the RT-PCR experiments; Véronique Thomas-Vaslin, José Cohen, and Charlotte Frisén for critical reading of the manuscript; Helen Forher, Orly Azogui, and Ahmed Hamaï for their kind help and advice; Alain-Jacques Valleron for advice concerning statistical analyses; and all the lab members for stimulating discussions.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal