Abstract
Nonhomologous end-joining (NHEJ) DNA factors maintain genomic stability through their DNA double-strand break (DSB) repair and telomere-associated activities. Unrepaired or misrepaired DSBs can lead to apoptotic death or chromosomal damage. The B cells of some B-chronic lymphocytic leukemia (B-CLL) patients are resistant to radiation-induced apoptosis in vitro. We show here that the novel DNA-dependent protein kinase (DNA-PK) inhibitor, NU7026 (2-(morpholin-4-yl)-benzo[h]chomen-4-one), and the phosphatidylinositol 3 (PI-3) kinase inhibitor, wortmannin, restored sensitivity to DNA damage-induced apoptosis of otherwise resistant cells. These resistant malignant B cells also escaped DSB-induced apoptosis following exposure to etoposide or neocarzinostatin. We found that at 15 minutes after irradiation, the levels of NHEJ (as measured by an in vitro DSB end-ligation assay) and DNA-PK catalytic subunit (DNA-PKcs) activity were, respectively, 2-fold and 4-fold higher in radio-resistant than in radio-sensitive B-CLL cells or Epstein-Barr virus (EBV)-transformed B cells. Ku70/Ku80 heterodimer DNA end-binding activity was also 2- to 3-fold higher in the resistant B-CLL cell subset compared with the sensitive B-CLL cell subset. Our results provide the first evidence that overactivating the NHEJ DNA repair pathway impairs DNA damage-induced apoptosis in malignant B cells and that this may contribute to their resistance to current chemotherapy. (Blood. 2005;105:4776-4783)
Introduction
Mammalian cells respond to a variety of genotoxic stresses, including exposure to ionizing radiation and topoisomerase inhibitors, and endogenous reactive oxygen species, via 2 mechanisms for maintaining genome integrity: DNA repair and apoptosis. Inaccurate repair or a lack of repair of double-strand breaks (DSBs) can lead to apoptosis1 or to mutations or large-scale genomic instability through the generation of nonlethal chromosomal aberrations.2
DSBs are repaired by homologous recombination (HR) and by nonhomologous end-joining (NHEJ). NHEJ is the predominant mechanism in higher eukaryotes, whereas single-celled organisms (such as yeast) rely more heavily on HR. DNA-dependent protein kinase (DNA-PK) is a key component of the NHEJ pathway.3 DNA-PK is a nuclear serine/threonine protein kinase, comprising a 460-kDa catalytic subunit, DNA-PKcs, and a DNA-binding subunit, the Ku autoantigen (a Ku70 and Ku80 protein dimer). The Ku70/Ku80 heterodimer binds the free DNA ends generated by DSBs and recruits the catalytic subunit of the complex.4-6 In vitro, this active DNA-PK complex can phosphorylate many DNA-bound proteins including protein 53 (p53), Ku, X-ray cross-complementing group 4 (XRCC4), and DNA-PKcs3,7,8 and can bind to XRCC4 and ligase IV, which join together the ends of the broken DNA strands.8-10
Little is known about the role of the NHEJ repair pathway in apoptosis. Studies suggesting that DNA-PK plays a role in apoptosis remain controversial and principally relate to the interaction of this protein with p53.11 The results from some studies indicate that DNA-PK activates and phosphorylates p5312,13 and murine double mutant 2 (MDM2)14 after DNA damage. However, other groups reported that DNA-PKcs-defective mice and DNA-PKcs-defective cell lines display normal p53-mediated apoptosis responses.15,16 DNA-PK activity has been implicated in resistance to nitrogen mustard therapy in leukemia cells,17,18 but further studies are necessary to understand how these cells escape apoptosis after this genotoxic stress.
We investigated this phenomenon using lymphocytes from patients with B-chronic lymphocytic leukemia (B-CLL), a disease characterized by the accumulation of mature-looking malignant B cells in the peripheral blood, bone marrow, and lymph nodes.19 B-CLL cells also display both chromosomal instability and defects in apoptotic cell death. We investigated whether the NHEJ DNA repair system prevents apoptosis in response to DNA damage in these cells. We reported previously that some B-CLL patients' B cells are completely resistant to radiation-induced apoptosis.20,21 DNA damage is repaired more rapidly in these resistant cells than in sensitive B-CLL cells, but more chromosomal aberrations accumulate in the resistant cells after the first cell division.22
We show here that B-CLL cells resistant to γ-radiation-induced apoptosis are also completely resistant to apoptosis induced by neocarzinostatin (NCS) and etoposide VP-16 (4′-demethyl-epipodophyllotoxin-9-[4,6-O-ethylidene-β-D-glucopyranoside]), compounds that specifically cause DNA DSBs. Treatment of these resistant cells with the DNA-PKcs-specific inhibitor, NU7026 (2-(morpholin-4-yl)-benzo[h]chomen-4-one),23-25 or with the phosphatidylinositol 3 (PI-3) kinase inhibitor, wortmannin,26,27 led to them undergo apoptosis after DNA damage. In vitro NHEJ assay for DSB end-ligation revealed that end-ligation efficiency was 2-fold higher in resistant B-CLL cells than in sensitive B-CLL cells or in Epstein-Barr virus (EBV)-transformed B lymphocytes at 15 minutes after irradiation (10 Gy). This alteration in NHEJ activity after DNA damage correlated with our findings that DNA-PKcs activity was 4-fold higher 15 minutes after irradiation and that Ku70/80 heterodimer DNA end-binding (DEB) activity was constitutively 2- to 3-fold higher in the resistant cells. This is the first time that a deregulated DNA repair system has been shown to allow human malignant cells to escape apoptosis after DNA damage. These results should improve current understanding of cancer cell resistance to genotoxic chemotherapy.
Patients, materials, and methods
Patients, isolation of B-CLL lymphocytes, and cell culture
The patients enrolled in the study were diagnosed with B-CLL following cytologic and immunologic analyses and followed-up at the Pitié-SalpêtrièreHospital (Paris, France) between 1998 and 2004. Approval for these studies was obtained from the institutional review boards of the Commissariat à l'Energie Atomique and the Hospital Pitié-Salpêtrière in Paris. All patients gave informed consent. B lymphocytes were purified and maintained in culture as previously described.20,21 The MO59J, MO59K,28 CHO-K1, and xrs6 cell lines29 were grown in Dulbecco modified Eagle medium (DMEM; Invitrogen, Cergy Pointoise, France) supplemented with 10% fetal calf serum (D. Dutscher, France) and an antibiotic-antimycotic cocktail (Invitrogen). The EBV-transformed B-lymphocyte cell line was grown in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum.
Irradiation and cell treatments
Purified B-CLL lymphocytes were irradiated as previously described.30 Drugs were then added directly to the cell culture medium at the concentrations indicated. Neocarzinostatin was prepared and titrated as described.31 Working solutions were prepared immediately before use in cold 0.01 M sodium phosphate buffer (pH 6.6). VP-16, okadaic acid, NU7026 (Calbiochem, Fontenay-Sous-Bois, France), and wortmannin (Sigma, Saint Quentan Fallavier, France) were prepared in dry dimethyl sulfoxide and stored at -20°C. Equal volumes of the solvents or buffers used were added to untreated cell cultures as controls. To determine the effect of NU7026 and wortmannin on irradiation-induced apoptosis, cells were pretreated with either 10 μM NU7026 for one hour or 1 μM wortmannin for 30 minutes, washed twice in phosphate-buffered saline (PBS), and irradiated at 10 Gy. Cells were then cultured in medium containing either 10 μM NU7026 or 1 μM wortmannin, and the number of apoptotic cells was then counted as described in the next paragraph.
Apoptotic cell counts: B-CLL cell classification according to sensitivity to γ-radiation-induced apoptosis
After 24 hours of cell culture with or without the various drug treatments, the number of cells with chromatin-associated fluorescence and characteristic apoptotic morphology (ie, chromatin condensation and nuclear fragmentation) was counted as previously described.30 B-CLL cells were then classified into 2 groups depending on their sensitivity to γ-irradiation-induced apoptosis. B lymphocytes with postirradiation apoptosis scores identical to those of untreated cells (spontaneous apoptosis generally occurs in < 20% of untreated cells) were classified as the resistant subset (R), and B lymphocytes with an apoptosis score of more than 40% were classified as the sensitive subset (S). Approximately 15% of B-CLL patients produce B cells that are completely resistant to radiation-induced apoptosis, and so far B cells from 225 B-CLL patients have been tested for their sensitivity to radiation-induced apoptosis in vitro.
Preparation of in vitro cell-free extracts and DSB end-ligation assay (“NHEJ activity assay”)
In vitro cell-free extracts and DSB end-ligation assays were prepared and carried out as described previously.32
Cell-free extracts
B-CLL extracts (0.15-0.2 mL) with a protein concentration of between 5 and 10 mg/mL were prepared from approximately 1 × 109 B-CLL lymphocytes. MO59J, MO59K, and EBV-transformed B-cell extracts (0.3-0.8 mL) were prepared from 5 × 108 cells and had protein concentrations of between 7 and 10 mg/mL. Extracts were stored as 20-μL aliquots in liquid nitrogen and remained active for 6 to 12 months. Before being measured for NHEJ activity, extracts were dialyzed against freshly prepared M-buffer (50 mM 3-(N-morpholino)-2-hydroxy-propane sulfonic acid [MOPSO]-NaOH [pH 7.5], 40 mM KCl, 10 mM MgCl2, 5 mM 2-mercaptoethanol) for 30 minutes at 4°C using microdialysis filters (0.025-μm pore diameter; Millipore, Molsheim, France).
DNA substrates
The 2 substrates for 5′-cohesive end-ligation were derived from pSP65 and pUC18 (2.69 kb; Sigma) after linearization of the 2 vectors by digestion with BamHI and EcoRI (New England Biolabs, Beverly, MA), respectively. Similar results were obtained with both DNA substrates (data not shown). Substrates were recovered from gels using a gel extraction kit (Qiagen, Courtabeuf, France).
NHEJ assay and analysis of the products
Preliminary experiments revealed that ligation and NHEJ were complete for both the cell line and B-CLL lymphocyte extracts after incubation for 3 hours at 25°C. For standard reactions, 10 ng linearized plasmid (1 μL) was incubated in a total volume of 10 μL reaction medium containing 40 μg protein extract (8 μL) in M-buffer (pH 7.5) supplemented with 1 mM adenosine triphosphate (ATP), 200 μM deoxynucleoside triphosphate (dNTPs) (50 μM of each nucleotide), and 50 ng/μL bovine serum albumin. Where indicated, protein samples were pretreated with wortmannin (1 μM) or NU7026 (10 μM) for 20 minutes on ice before being assayed for end-joining activity. Reactions were stopped by adding a solution containing 20 mM Tris (tris(hydroxymethyl)aminomethane)-HCL (pH 7.5), 10 mM EDTA (ethylenediaminetetraacetic acid), 1% sodium dodecyl sulfate (SDS) and incubating at 65°C for 5 minutes. Samples were then digested with 2 mg/mL proteinase K for 30 minutes at 37°C. The equivalent of 2 ng DNA substrate was subjected to electrophoresis in 1% agarose gels containing 1 μg/mL ethidium bromide followed by Southern blot analysis using a pSP65- or PUC18-specific probe labeled with [α-32P] deoxycytidine triphosphate (dCTP) by random priming (random labeling kit; Roche Diagnostics, Meylan, France). Reaction products were quantified by phosphor imaging (Storm 860 and ImageQuant software; Amersham-Pharmacia Biotech, Orsay, France). End-ligation activity was assessed by measuring the conversion of monomeric plasmids to ligated products. Ligation efficiency was calculated by dividing the densitometry readings for the sum of all converted plasmid products by those for the sum of all products. The changes in NHEJ activity after treatment of B cells with 10 Gy γ-irradiation were calculated by dividing the ligation efficiency of B-cell extracts exposed to irradiation for 15 minutes by the ligation efficiency of untreated control B cells.
DNA-PKcs activity
DNA-PK “pull-down” kinase assays were performed as previously described.33 Nuclear extracts were prepared as follows: at the indicated time points after irradiation, 1 × 107 B-CLL lymphocytes were washed twice in PBS and lysed by incubation in 200 μL ice-cold hypotonic buffer (1.5 mM MgCl2, 5 mM KCl, 10 mM HEPES [pH 7.5], 5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride [PMSF], Mini-Complete protease inhibitor cocktail [Roche Diagnostics], and 0.5% nonidet P-40). After centrifugation (5000g, 10 minutes, 4°C), the pellet containing cell nuclei was recovered and suspended in 100 μL modified buffer (50 mM NaF, 20 mM Hepes [pH 7.5], 450 mM NaCl, 25% glycerol, 0.2 mM EDTA, 0.5 mM 1,4-dithiothreitol [DTT], 0.5 mM PMSF, and Mini-Complete protease inhibitor cocktail) as described previously for whole-cell extracts.33 The nuclear suspension was then frozen at -70°C and thawed at 30°C 3 times. After centrifugation (10 000g, 10 minutes, 4°C), supernatants were recovered and stored at -80°C until use. Protein concentration was determined by the Bradford method. Where indicated, protein samples were pretreated with wortmannin (1 μM) or NU7026 (10 μM) before being used in DNA-PKcs assays. Samples were assayed in the presence either of a native (EPPLSQEAFADLWKK; Promega, Sarl, France) or of a mutant (EPPLSEQAFADLWKK; negative control) peptide derived from p53. Radiolabeled, phosphorylated substrate was subjected to tricine SDS-polyacrylamide gel electrophoresis (PAGE, 18% gels). [γ-32P] ATP incorporation into the substrate peptide was quantified using the Storm 860 and ImageQuant software (Amersham-Pharmacia Biotech). The MO59J and MO59K cell lines were used as controls.
Ku heterodimer DEB activity
Whole-cell extracts were prepared from B-CLL lymphocytes using the modified buffer used to make the nuclear extracts for the DNA-PK pull-down assays. The double-stranded 18-mer nucleotide (5′GATCTAGCTGCACCGGAC3′,5′GTCCGGTGCAGCTAGATC3′) was used to examine Ku DNA-end binding. Electrophoretic mobility shift assays (EMSAs) were performed as previously described,18 with the exception that electrophoresis conditions were modified to enable us to detect supershifts (6% acrylamide gels and 0.25X Tris-borate-EDTA [TBE] running buffer). Extracts from the CHO-K1, xrs6, MO59K, and MO59J cell lines were used as controls. Supershifts were performed using a polyclonal Ku70 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at a 1:10 dilution. Immunodepletion studies were performed by incubating 5 μg B-CLL cell extract with 125 ng or 250 ng monoclonal Ku70 antibody (Ab4; Neomarkers, Fremont, CA) for one hour at 4°C on a rotary wheel. Extracts were then incubated for a further 2 hours at 4°C with protein A-Sepharose (Amersham-Pharmacia Biotech). After centrifugation (5 minutes at 4°C, 13 000 rpm), the supernatant was assayed for Ku heterodimer DEB activity and subjected to Western blot analysis.
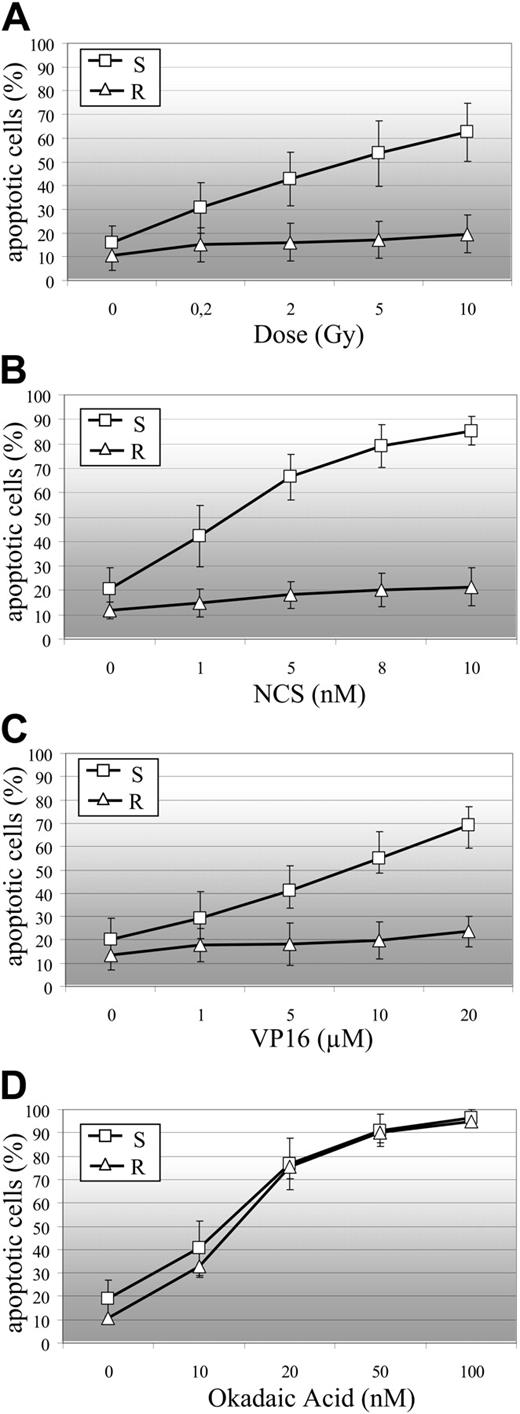
B-CLL cell sensitivity to DSB-induced apoptosis. R indicates resistant B-CLL cells; and S, sensitive B-CLL cells. (A) Sensitivity of B-CLL cells to γ-irradiation-induced apoptosis (R: n = 13 samples; S: n = 79 samples). Sensitivity of R (n = 5 samples) and S (n = 15 samples) B-CLL cells to NCS-induced (B), VP16-induced (C), and okadaic acid-induced (D) apoptosis. Percentages of apoptotic cells are the means (± SEM) of duplicate experiments on B-CLL samples.
B-CLL cell sensitivity to DSB-induced apoptosis. R indicates resistant B-CLL cells; and S, sensitive B-CLL cells. (A) Sensitivity of B-CLL cells to γ-irradiation-induced apoptosis (R: n = 13 samples; S: n = 79 samples). Sensitivity of R (n = 5 samples) and S (n = 15 samples) B-CLL cells to NCS-induced (B), VP16-induced (C), and okadaic acid-induced (D) apoptosis. Percentages of apoptotic cells are the means (± SEM) of duplicate experiments on B-CLL samples.
Western blot
Protein extracts were analyzed by Western blotting as previously described.34 Proteins were detected using Ku70 (Ab-4, clone N3H10), Ku80 (Ab-2, clone 111), Ku80 variant form (Ab-7, clone S10B1; Neomarkers), and DNA-PKcs (ab230; Abcam, Cambridge, United Kingdom) antibodies at dilutions of 1:10 000, 1:5000, 1:200, and 1:5000, respectively. The XRCC4 antibody (ab145; Abcam) was used at a dilution of 1:2000 and the ligase IV antibody was used at a dilution of 1:500 (a gift from Tomas Lindahl, Imperial Cancer Research Fund [ICRF], United Kingdom).
Results
A B-CLL cell subset is specifically resistant to DSB-induced apoptosis
B cells from most B-CLL patients underwent apoptosis within 24 hours of γ-irradiation, whereas B cells from some patients did not (Figure 1A). Of 92 independent B-CLL cell samples, 13 were totally resistant to apoptosis induced by exposure to 10 Gy irradiation (mean ± SEM percentage of cells in this subset undergoing spontaneous apoptosis: 10.7% ± 6.2%, whereas those undergoing 10 Gy γ-ray-induced apoptosis: 19.5% ± 8%). More than 40% of the cells in the remaining 79 samples underwent apoptosis after exposure to only 2 Gy γ-irradiation (15.8% ± 7.3% underwent spontaneous apoptosis, 42.7% ± 11.3% underwent apoptosis after exposure to 2 Gy, and 62.5% ± 12.3% underwent apoptosis after exposure to 10 Gy). We investigated whether the difference in sensitivity to irradiation-induced apoptosis between these 2 B-CLL cell subsets was associated with the level of DSB injury. We used the radiomimetic compound NCS. NCS cleaves DNA during the course of a reaction that leads to complete degradation of the drug after a few minutes.35 As found previously for γ-ray dose,36 the incidence of DSB increased linearly with NCS concentration; 1 nM NCS lead to the same amount of DSBs as 1.21 Gy γ-rays.37 We studied the sensitivity of 5 representative resistant (in which 9.7% ± 2% of cells underwent spontaneous apoptosis and 14.7% ± 2.8% underwent apoptosis after exposure to 10 Gy γ-rays; data not shown) and 15 representative sensitive (in which 19.7% ± 8.8% of cells underwent spontaneous apoptosis and 74.8% ± 14.1% of cells underwent apoptosis after exposure to 10 Gy of γ-rays; data not shown) B-CLL samples to increasing concentrations of NCS (Figure 1B). Radio-resistant B-CLL samples were also resistant to NCS-induced apoptosis (11.9% ± 4.0% of cells underwent spontaneous apoptosis and 20.2% ± 8.0% underwent 8 nM NCS-induced apoptosis). In the sensitive subset, 1.65 nM NCS (yielding the same number of DSBs as 2 Gy γ-rays) induced apoptosis in 45% plus or minus 17% of cells (Figure 1B) and 2 Gy γ-rays induced apoptosis in 42.7% plus or minus 11.3% of cells (Figure 1A). We then studied the sensitivity of the same 5 resistant and 15 sensitive B-CLL samples to VP-16 (Figure 1C). VP-16 causes DSBs by inhibiting topoisomerase II and is used in cancer chemotherapy.38 As with NCS, radio-resistant B-CLL cells were also resistant to VP-16-induced apoptosis (13.6% ± 6.6% of cells underwent spontaneous apoptosis and 23.6% ± 9.9% of cells underwent apoptosis induced by 20 μM VP16). Radio-sensitive B-CLL cells were also sensitive to VP-16-induced apoptosis (20.0% ± 9.2% of cells underwent spontaneous apoptosis and 69.3% ± 7.7% underwent apoptosis induced by 20 μM VP16). The protein phosphatase inhibitor, okadaic acid (OA), has been shown previously to induce apoptosis in B-CLL cells.39 This inhibitor induced apoptosis in both the radio-resistant and radiosensitive B-CLL samples. The percentage apoptosis did not differ significantly between the 2 cell types at any of the concentrations tested (0-100 nM OA). These results indicate that the OA-induced cell death pathway is fully functional in both types of B-CLL cells (Figure 1D).
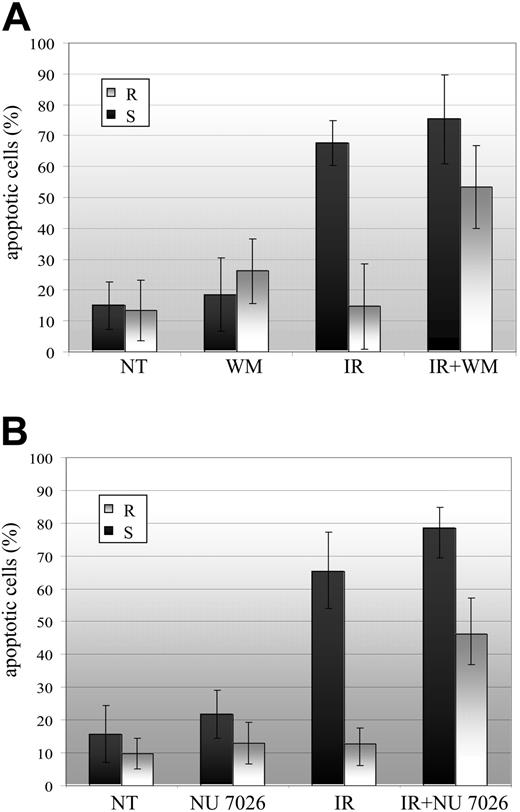
The PI-3 kinase inhibitor wortmannin and the DNA-PKcs inhibitor NU7026 sensitize resistant B-CLL cells to γ-irradiation-induced apoptosis. R indicates resistant B-CLL (n = 5 samples); and S, sensitive B-CLL (n = 7 samples). (A) B-CLL cells were either left untreated (NT) or treated with 1 μM wortmannin alone (WM), with 10 Gy irradiation alone (IR) or with 1 μM wortmannin and 10 Gy irradiation (IR+WM). (B) B-CLL cells were either left untreated (NT) or treated with 10 μM NU7026 alone (NU7026), with 10 Gy irradiation alone (IR), or with 10 μM NU7026 and 10 Gy irradiation (IR+NU7026). Percentages of apoptotic cells are means (± SEM) of duplicate experiments on n B-CLL samples.
The PI-3 kinase inhibitor wortmannin and the DNA-PKcs inhibitor NU7026 sensitize resistant B-CLL cells to γ-irradiation-induced apoptosis. R indicates resistant B-CLL (n = 5 samples); and S, sensitive B-CLL (n = 7 samples). (A) B-CLL cells were either left untreated (NT) or treated with 1 μM wortmannin alone (WM), with 10 Gy irradiation alone (IR) or with 1 μM wortmannin and 10 Gy irradiation (IR+WM). (B) B-CLL cells were either left untreated (NT) or treated with 10 μM NU7026 alone (NU7026), with 10 Gy irradiation alone (IR), or with 10 μM NU7026 and 10 Gy irradiation (IR+NU7026). Percentages of apoptotic cells are means (± SEM) of duplicate experiments on n B-CLL samples.
Inhibition of DNA-PKcs by NU7026 and wortmannin restores the sensitivity of resistant B-CLL cells to irradiation-induced apoptosis
We hypothesized that the NHEJ pathway was responsible for the resistance of some B-CLL cells to DSB-induced apoptosis. To test this hypothesis, we used 2 DNA-PK inhibitors: NU7026, which at low concentrations inhibits DNA-PKcs (median inhibitory concentration [IC50] = 0.23 μM) but not PI-3 kinase, ATM, and ATR (IC50 = 13 μM, IC50 > 100 μM, and IC50 > 100 μM, respectively),24 and wortmannin, which inhibits all PI-3 kinases and PI-3-like kinases, including DNA-PKcs.27 The resistance of some B-CLL cells to irradiation-induced apoptosis was nearly abolished by inhibiting DNA-PK (Figure 2). The number of apoptotic cells was higher for γ-irradiated (10 Gy) resistant B-CLL cells treated with 1 μM wortmannin (53.3 ± 14.3%) than for cells treated with irradiation alone (14.7 ± 8.1%) (Figure 2A). NU7026 also restored the sensitivity of resistant B-CLL cells to radiation-induced apoptosis (Figure 2B): the number of apoptotic cells was significantly higher for γ-irradiated (10 Gy) resistant B cells treated with 10 μM NU7026 (45.7 ± 9.1%) than for resistant cells treated with irradiation alone (13.7 ± 6.4%). NU7026 and wortmannin had no significant effect on percentage apoptosis in γ-irradiated sensitive B-CLL cells (Figure 2A-B). In addition, wortmannin and NU7026 inhibited NHEJ-mediated DNA repair and DNA-PKcs phosphorylation in both the sensitive and resistant B-CLL cells (Figures 3C, 4D).
NHEJ is overactivated after DNA damage in resistant B-CLL cells
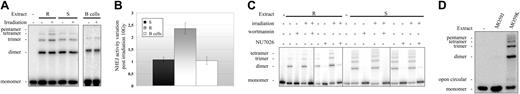
We performed the DSB end-ligation assay to measure NHEJ activity in 3 sensitive and 3 resistant B-CLL cell extracts. These samples were representative of the 2 B-CLL cell subsets, and, with the exception of one resistant sample displaying p53 dysfunction and carrying a mutation at position 220 (Y→A; data not shown), all the samples used produced wild-type p53. The ligation efficiencies of resistant and sensitive B-CLL cell extracts were compared with those of an EBV-transformed B-cell line (B cells). Measurements were taken before and 15 minutes after exposure to 10 Gy γ-irradiation. All experiments were performed at 25°C with 40 μg protein extract, and reactions were allowed to continue for 3 hours (linear phase of the end-ligation reaction under conditions where all monomers are not converted to ligation products; data not shown). We found that NU7026 and wortmannin inhibited DNA repair in both untreated and irradiated extracts from resistant and sensitive B-CLL cells (Figure 3C), indicating that DSB end-ligation activity was completely dependent on DNA-PK activity. Moreover, DSB endligation activity was detected in extracts from MO59K (DNA-PKcs proficient) cells but not in extracts from MO59J (DNA-PKcs deficient) cells, demonstrating that the DNA repair is a DNA-PKcs-dependent NHEJ process (Figure 3D). We found that the efficiency of NHEJ repair increased by 2-fold (2.36 ± 0.23) in the resistant B-CLL cells after exposure to 10 Gy irradiation, whereas no increase after irradiation was detected in sensitive B-CLL cells or EBV-transformed B lymphocytes (1.07 ± 0.1 and 1.03 ± 0.15 respectively, Figure 3A and 3B). Experiments were done in duplicate with 3 different resistant and 3 different sensitive B-CLL samples; smallest and highest values calculated with resistant samples were, respectively, 2.01 and 2.64, whereas smallest and highest values calculated with sensitive B-CLL samples were, respectively, 0.94 and 1.24, indicating that only small variations appeared between patient samples of each group. However, as there is a relatively small number of samples, the variation shown in Figure 3 (2-fold increase in resistant cells) may not reflect that seen in a larger population. Western blot analysis of extracts from resistant and sensitive B-CLL cells revealed no significant differences in DNA-PKcs, Ku80, and Ku70 levels, before or after irradiation (Figure 5E). Although we found that the levels of both XRCC4 and ligase IV may be slightly higher in 3 resistant than in 3 sensitive B-cell samples, both before and after irradiation (Figure 5E), experiments done on a larger series of samples (6 resistant and 7 sensitive) revealed that XRCC4 and ligase IV expression varied between samples and that, overall, resistant B-CLL cell samples did not produce higher amounts of these proteins than sensitive samples (data not shown). The Ku70 and Ku80 levels were similar in all the cell samples tested (data not shown).
NHEJ DNA repair pathway is overactivated in resistant B-CLL cells after DNA damage. (A) NHEJ activity in untreated cells (Irradiation -) and cells exposed to 10 Gy irradiation (Irradiation +). Assays were carried out using a resistant B-CLL lymphocyte extract (R), a sensitive B-CLL cell extract (S), and an EBV-transformed B-lymphocyte extract (B cells). The results shown are representative of those obtained with all the cell extracts tested (n = 3 for resistant B-CLL samples, n = 3 for sensitive B-CLL samples, and n = 3 for EBV-transformed B-cell samples). (B) Changes in NHEJ activity after exposure to 10 Gy irradiation were calculated from the mean (± SEM) activities recorded in duplicate experiments on 3 different resistant B-CLL cell extracts (R), 3 different sensitive B-CLL cell extracts (S), and 3 different EBV-transformed B-lymphocyte extracts (B cells). (C) NHEJ activity in cells that were either left untreated (-) or treated (+) with 10 Gy irradiation, 1 μM wortmannin, or 10 μM NU7026. Assays were performed on resistant B-CLL cell extracts (R) and sensitive B-CLL cell extracts (S). A representative experiment is shown. (D) NHEJ activity assay with DNA-PKcs-deficient cell line extract (MO59J) and wild-type cell line extract (MO59K). A representative experiment is shown. See Supplemental Figure S1 for more information.
NHEJ DNA repair pathway is overactivated in resistant B-CLL cells after DNA damage. (A) NHEJ activity in untreated cells (Irradiation -) and cells exposed to 10 Gy irradiation (Irradiation +). Assays were carried out using a resistant B-CLL lymphocyte extract (R), a sensitive B-CLL cell extract (S), and an EBV-transformed B-lymphocyte extract (B cells). The results shown are representative of those obtained with all the cell extracts tested (n = 3 for resistant B-CLL samples, n = 3 for sensitive B-CLL samples, and n = 3 for EBV-transformed B-cell samples). (B) Changes in NHEJ activity after exposure to 10 Gy irradiation were calculated from the mean (± SEM) activities recorded in duplicate experiments on 3 different resistant B-CLL cell extracts (R), 3 different sensitive B-CLL cell extracts (S), and 3 different EBV-transformed B-lymphocyte extracts (B cells). (C) NHEJ activity in cells that were either left untreated (-) or treated (+) with 10 Gy irradiation, 1 μM wortmannin, or 10 μM NU7026. Assays were performed on resistant B-CLL cell extracts (R) and sensitive B-CLL cell extracts (S). A representative experiment is shown. (D) NHEJ activity assay with DNA-PKcs-deficient cell line extract (MO59J) and wild-type cell line extract (MO59K). A representative experiment is shown. See Supplemental Figure S1 for more information.
Early activation of DNA-PKcs in resistant but not sensitive B-CLL cell samples in response to γ-irradiation
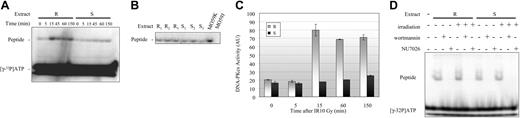
We investigated whether the increase in NHEJ DNA repair efficiency in resistant cells was related to an alteration in DNA-PKcs activity. A “pull-down” assay33 was performed before and at different times after irradiation in 3 resistant and 3 sensitive B-CLL cell extracts. DNA-PKcs kinase activity was determined by measuring phosphorylation of a peptide substrate derived from wild-type p53. The radioactivity associated with the mutated peptide did not exceed 6% of that associated with the substrate peptide (data not shown). Furthermore, no phosphorylation of the wild-type p53-derived substrate was detected in extracts from the DNA-PKcs-deficient MO59J cell line, indicating that phosphorylation of the wild-type substrate is a result of DNA-PKcs activity, rather than of other protein kinases (Figure 4B). We found that DNA-PKcs kinase activity was slightly higher in nuclear protein extracts from resistant than from sensitive B-CLL samples before irradiation treatment (Figure 4A-C). As expected, wortmannin and NU7026 completely inhibited DNA-PKcs activity in both the sensitive and resistant B-CLL cells (Figure 4D). At 15 minutes after irradiation, kinase activity was 4-fold higher in the resistant B-CLL cells than in the sensitive B-CLL cells (Figure 4A,C). The kinase activity in the resistant cells remained 3- to 3.5-fold higher than in sensitive cells at 60 and 150 minutes after irradiation (Figure 4C). This increase in kinase activity was completely abolished by treatment of the resistant cells with NU7026 and wortmannin (Figure 4D).
Early increases in DNA-PKcs activity in resistant B-CLL cell extracts after γ-irradiation. (A) DNA-PKcs activity in resistant B-CLL (R) nuclear protein extracts (25 μg/lane) and sensitive B-CLL (S) nuclear protein extracts (25 μg/lane) at the indicated times after treatment with 10 Gy γ-irradiation. The results shown are representative of those obtained in all extracts tested (n = 3 for resistant samples and n = 3 for sensitive samples). (B) DNA-PKcs activity in untreated nuclear protein extracts from 3 resistant B-CLL samples (R1,R2,R3) and from 3 sensitive B-CLL samples (S1,S2,S3). (C) Mean (± SEM) DNA-PKcs activity in 3 resistant B-CLL samples (R) and 3 sensitive B-CLL samples (S) at the indicated times after irradiation. Values are given in arbitrary units (AU). (D) DNA-PKcs activity in resistant (R) and sensitive (S) B-CLL nuclear protein extracts (25 μg/lane) from cells that were either left untreated (-) or treated with 10 Gy irradiation (+, measurements were carried out 15 minutes after irradiation), 1 μM wortmannin, or 10 μM NU7026. A representative experiment is shown. See Supplemental Figure S2 for more information.
Early increases in DNA-PKcs activity in resistant B-CLL cell extracts after γ-irradiation. (A) DNA-PKcs activity in resistant B-CLL (R) nuclear protein extracts (25 μg/lane) and sensitive B-CLL (S) nuclear protein extracts (25 μg/lane) at the indicated times after treatment with 10 Gy γ-irradiation. The results shown are representative of those obtained in all extracts tested (n = 3 for resistant samples and n = 3 for sensitive samples). (B) DNA-PKcs activity in untreated nuclear protein extracts from 3 resistant B-CLL samples (R1,R2,R3) and from 3 sensitive B-CLL samples (S1,S2,S3). (C) Mean (± SEM) DNA-PKcs activity in 3 resistant B-CLL samples (R) and 3 sensitive B-CLL samples (S) at the indicated times after irradiation. Values are given in arbitrary units (AU). (D) DNA-PKcs activity in resistant (R) and sensitive (S) B-CLL nuclear protein extracts (25 μg/lane) from cells that were either left untreated (-) or treated with 10 Gy irradiation (+, measurements were carried out 15 minutes after irradiation), 1 μM wortmannin, or 10 μM NU7026. A representative experiment is shown. See Supplemental Figure S2 for more information.
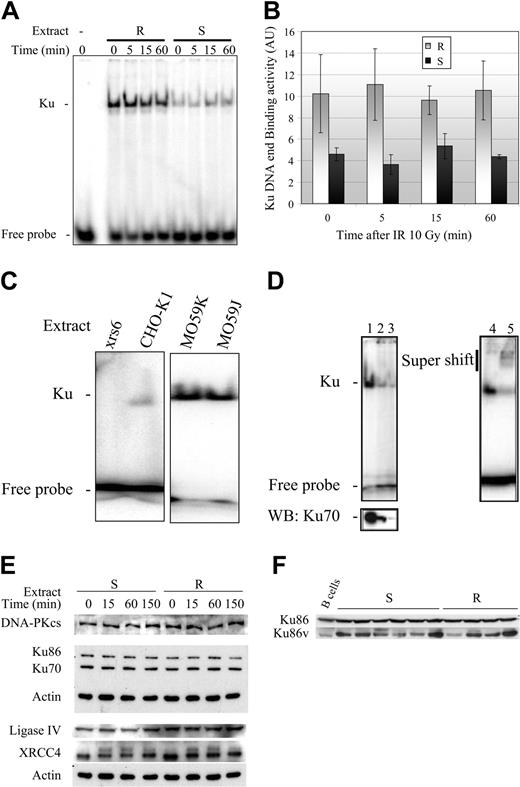
Regulation of DNA-PK activity: increased Ku70/80 heterodimer DNA-binding activity in resistant B-CLL cells
The DNA end-binding activity of the Ku70/80 heterodimer is the first limiting step in the formation of an active DNA-PK complex. Thus, the increase in DNA-PK activity in the resistant B-CLL samples may be associated with an increase in Ku heterodimer DEB activity. We therefore tested this possibility using the 3 resistant and 3 sensitive B-CLL samples described earlier. EMSA was performed to assess binding of the Ku heterodimer complex to radiolabeled double-stranded DNA.18 Assays were carried out in the presence of a circular plasmid (competitive substrate), and shifts in mobility were detected by autoradiography (Figure 5A). The following lines of evidence indicate that the band-shifts observed corresponded to binding of the Ku70/Ku80 heterodimer DNA complex to the double-stranded DNA: (1) the band-shift was not observed following immunodepletion of Ku70 (Figure 5D lanes 1-3), (2) a supershift was observed after the addition of anti-Ku70 antibodies (Figure 5D lanes 4-5), and (3) the complex was not detected in extracts from xrs6 cells, expressing a mutated form of Ku80, but was detected in extracts from CHO-K1 control cells (Figure 5C). We also noticed that both MO59J and MO59K cells had functional Ku heterodimer DNA end-binding activity (Figure 5C). The Ku DEB activity was quantified in 3 resistant and 3 sensitive B-CLL samples, before and at various times following irradiation. Unlike DNA-PKcs activity, Ku DEB activity was significantly higher (2- to 3-fold) in resistant B-CLL samples than in sensitive B-CLL samples, both before and after γ-irradiation (Figure 5B). Differences in Ku DEB activity between the resistant and sensitive B-CLL samples were not caused by differences in the level of expression of the Ku70 and Ku80 proteins (Figure 5E). Previous studies have reported that a variant form of Ku80 (estimated molecular weight [MW] = 69 kDa) may be expressed in cells with low DNA-PK activity.18 Although the Ku70/Ku80 variant heterodimer binds to DNA ends, it has a decreased ability to recruit the catalytic subunit, DNA-PKcs.18,40 Western blot analysis revealed that the variant Ku80 protein was produced in similar amounts in both resistant (n = 4) and sensitive (n = 6) B-CLL samples and in normal B lymphocytes (Figure 5F).
Constitutive increase in Ku70/80 heterodimer DNA-end binding activity. (A) Ku DNA-end binding activity in whole protein extracts from resistant (R) and sensitive B-CLL (S) cells (1 μg/lane) at the indicated times after treatment with 10 Gy γ-irradiation. The results shown are representative of those obtained in all extracts tested (n = 3 for resistant samples and n = 3 for sensitive samples). (B) Ku DNA-end binding activity (mean ± SEM in arbitrary units, AU) in 3 resistant B-CLL samples (R) and 3 sensitive B-CLL samples (S) at the indicated times after irradiation. (C) Untreated whole cell protein extracts (1 μg/lane) from the Ku80-deficient xrs6 cell line and the CHO-K1 line (expressing both Ku70 and Ku80 proteins). MO59J (DNA-PKcs deficient) and MO59K (normal levels of DNA-PKcs activity) were used as controls. (D) Untreated B-CLL lymphocyte protein extracts (5 μg, lane 1) were immunodepleted using 125 ng (lane 2) and 250 ng (lane 3) monoclonal Ku70 antibody, assayed for Ku DNA-end binding activity (1 μg protein extract/lane) (upper panel) and then subjected to Western blot (WB) analysis (lower panel). For supershift assays (lanes 4-5), samples were treated with 5 μg untreated B-CLL lymphocyte protein extract (lane 5) or with 250 ng polyclonal Ku70 antibody (lane 4), and then assayed for Ku DNA-end binding (1 μg of protein extract/lane). (E) Whole-cell extracts (5 μg) were obtained from representative sensitive and resistant B-CLL cell samples at the indicated times after irradiation. Extracts were subjected to electrophoresis on a 10% SDS polyacrylamide gel (DNA-PKcs analysis was carried out using a 6% SDS polyacrylamide gel). Proteins were then blotted onto polyvinylidenefluoride (PVDF) membranes and were detected using the appropriate antibodies. (F) Whole-cell extracts (5 μg) from 6 sensitive (S) and 4 resistant (R) untreated B-CLL cell samples, and 4 untreated normal B-cell samples from healthy donors (B cells) were subjected to Western blotting analysis as described in panel E. See Supplemental Figure S3 for more information.
Constitutive increase in Ku70/80 heterodimer DNA-end binding activity. (A) Ku DNA-end binding activity in whole protein extracts from resistant (R) and sensitive B-CLL (S) cells (1 μg/lane) at the indicated times after treatment with 10 Gy γ-irradiation. The results shown are representative of those obtained in all extracts tested (n = 3 for resistant samples and n = 3 for sensitive samples). (B) Ku DNA-end binding activity (mean ± SEM in arbitrary units, AU) in 3 resistant B-CLL samples (R) and 3 sensitive B-CLL samples (S) at the indicated times after irradiation. (C) Untreated whole cell protein extracts (1 μg/lane) from the Ku80-deficient xrs6 cell line and the CHO-K1 line (expressing both Ku70 and Ku80 proteins). MO59J (DNA-PKcs deficient) and MO59K (normal levels of DNA-PKcs activity) were used as controls. (D) Untreated B-CLL lymphocyte protein extracts (5 μg, lane 1) were immunodepleted using 125 ng (lane 2) and 250 ng (lane 3) monoclonal Ku70 antibody, assayed for Ku DNA-end binding activity (1 μg protein extract/lane) (upper panel) and then subjected to Western blot (WB) analysis (lower panel). For supershift assays (lanes 4-5), samples were treated with 5 μg untreated B-CLL lymphocyte protein extract (lane 5) or with 250 ng polyclonal Ku70 antibody (lane 4), and then assayed for Ku DNA-end binding (1 μg of protein extract/lane). (E) Whole-cell extracts (5 μg) were obtained from representative sensitive and resistant B-CLL cell samples at the indicated times after irradiation. Extracts were subjected to electrophoresis on a 10% SDS polyacrylamide gel (DNA-PKcs analysis was carried out using a 6% SDS polyacrylamide gel). Proteins were then blotted onto polyvinylidenefluoride (PVDF) membranes and were detected using the appropriate antibodies. (F) Whole-cell extracts (5 μg) from 6 sensitive (S) and 4 resistant (R) untreated B-CLL cell samples, and 4 untreated normal B-cell samples from healthy donors (B cells) were subjected to Western blotting analysis as described in panel E. See Supplemental Figure S3 for more information.
Discussion
The primary response of cells with excessive DNA damage is to repair the lesions. Maintenance of the switching mechanisms that shift the cell from DNA repair to apoptosis is of central importance for avoiding progression to malignancy.
Previous investigations of the in vitro apoptotic response of B cells from B-CLL patients to irradiation have revealed resistant and sensitive subsets.20,21 Approximately 15% of B-CLL patients present B cells resistant to irradiation-induced apoptosis. We propose that human B-CLL cells can escape DNA damage-induced apoptosis by up-regulating the NHEJ DNA repair system. In a previous study, we showed that increased DNA damage repair was associated with the accumulation of an unusually high number of chromosomal aberrations in the resistant cell subset.22 Irradiation has been reported to induce apoptosis via DNA damage-independent processes such as generation of ceramide by the activated acid sphingomyelinase.41 We showed here that the sensitivity of the 2 B-CLL cell subsets to irradiation-induced apoptosis was dependent on the response to DSBs. We found that B-CLL subsets that were resistant or sensitive to irradiation-induced apoptosis were also resistant or sensitive to apoptosis induced by NCS and the topoisomerase II inhibitor VP-16. NHEJ is the predominant pathway for the repair of both NCS- and topoisomerase II-mediated DNA damage: wild-type and repair-deficient yeasts and LIG4-/- and Ku70-/- cells, which are defective in NHEJ, are extremely sensitive to NCS42,43 and to both VP-16 and ICRF-193.44 We found that okadaic acid, an inhibitor of protein phosphatases 1 and 2A, induced apoptosis in both resistant and sensitive B-CLL samples, indicating the existence of a functional executive cell death pathway in resistant cells. Okadaic acid induces apoptosis in many cell types including B-CLL cells.39,45,46
Approximately 10% of B-CLL patients present p53 mutations and consecutive drug resistance.47 To test the possibility that resistant B-CLL cells display p53 dysfunction and/or mutations, 22 B-CLL samples (10 sensitive and 12 resistant samples) were assessed for p53 status using a functional assay in yeasts48 and sequence-mutation analysis. Only 4 resistant B-CLL samples presented both a nonfunctional p53 protein and a mutation in the p53 gene (Blaise et al49 and J.D., unpublished data, July 2004). ATM is one of the signal transducers thought to be essential for the general DNA damage response.50 The ATM gene is mutated in 18% of B-CLL patients, and loss of heterozygosity (LOH) at 11q22-23 is observed in 14% of cases.51,52 P53 activity has been reported to involve ATM.53-55 However, none of the 10 B-CLL samples displaying radio-resistant apoptosis and p53 wild type (p53wt) examined presented ATM LOH.49 Thus, our data from previous studies suggest that neither p53 nor ATM deficiencies can fully explain the resistance of some B-CLL cells to DNA damage-induced apoptosis.
We previously found that DNA damage repair was enhanced in the resistant B-CLL subset, however, we also found that this rapid DNA repair was highly prone to errors.22 Considering that G0 yeast cells with unrepressed NHEJ capacity have an increased frequency of small-scale mutations, and thus large-scale chromosomal instability,56 and the fact that more than 95% of neoplastic B-CLL cells are in a G0 quiescent state57 (supplemental data, available on the Blood website; see the Supplemental Figures link at the top of the online article), we sought whether this DNA repair system could also define the cell susceptibility to undergo or not DNA damage-initiated apoptosis. This hypothesis is also consistent with studies reported elsewhere in the literature suggesting that NHEJ plays a role in the control of the apoptotic response following DNA damage.58,59 We found here that wortmannin, a known inhibitor of DNA-PKcs,27 restored sensitivity to irradiation-induced apoptosis to cells that were otherwise resistant to this cell death pathway. Wortmannin enhances B-CLL cell cytotoxicity to chlorambucil by inhibiting DNA-PK activity.26 Our results showed that 1 μM wortmannin increased the incidence of apoptosis in irradiation-treated resistant B-CLL cells by 4- to 5-fold. However, at the concentrations used, wortmannin inhibits ATM and PI-3 K in addition to DNA-PKcs.24 We also investigated the effect of NU7026, a DNA-PKcs-specific inhibitor, at concentrations of 10 μM on irradiation-induced apoptosis in the resistant cells. NU7026 enhances topoisomerase II inhibitor-induced cytotoxicity.23 Our results were consistent with this model: we found that 10 μM NU7026 restored irradiation-induced apoptosis sensitivity to otherwise resistant cells as efficiently as wortmannin.
A striking finding of this study is that overall NHEJ and DNA-PKcs activity increased 2- and 4-fold, respectively, in resistant B-CLL cells 15 minutes after exposure to irradiation. In contrast, only small increases of NHEJ and DNA-PKcs activity were detected in the sensitive cells. We also found that both NU7026 and wortmannin suppressed NHEJ and DNA-PKcs activity in the B-CLL extracts. These results show that the increases in DSB DNA repair and DNA-PKcs activity detected in resistant B-CLL cells after exposure to irradiation cannot be attributed to DNA-PK-independent repair or mechanisms involving other PI-3 kinases. We did not detect increases in NHEJ activity in irradiation-treated EBV-transformed B cells. Although we did not investigate DNA-PK activity in the normal B cells in detail, these results are consistent with those obtained in our previous study showing that the sensitivity of the normal B cells to irradiation treatment was similar to that of the sensitive B-CLL cell subset.22 Likewise, both NHEJ and DNA-PK activity have been reported to be higher in samples from patients with myeloid leukemias associated with concomitant DNA misrepair and in samples of B-CLL cells resistant to nitrogen mustard treatment than in normal B lymphocytes from healthy donors.18,60 Interestingly, Ku80-/- p53-/- mice succumb to disseminated pro-B-cell lymphoma before 3 months of age. Tumors result from a specific set of chromosomal translocations and gene amplifications involving immunoglobulin heavy chain (IgH) and c-Myc, reminiscent of Burkitt lymphoma.61 DNA-PKcs-deficient mice also display accelerated aging and increased lymphoma.62 Moreover, mice with XRCC4 or ligase IV deficiencies die during late embryonic development. They also display extremely high levels of apoptosis in newly generated neurons throughout their developing nervous system.63-65 Although p53 deficiency allowed postnatal survival of XRCC4-deficient mice, they routinely succumbed to pro-B-cell lymphomas that had chromosomal translocations, supporting a crucial role for the NHEJ pathway as a caretaker of the mammalian genome.58 Altogether, these results show that NHEJ deficiencies58,61,62 or NHEJ up-regulation22,60 could potentially lead to genomic instability. Investigation of XRCC4 and ligase IV protein levels in B-CLL samples revealed that the levels of these 2 proteins varied considerably between B-CLL cell samples, irrespective of their sensitivity to apoptosis. Our data suggest that the initial recognition of DSBs by the DNA-PK complex is the most crucial event for efficient NHEJ DNA repair and escape of apoptosis.
We propose that constitutive high levels of DNA end-binding by the Ku70/Ku80 heterodimer up-regulate DNA-PKcs and NHEJ activity and allow the resistant B-CLL subset to escape apoptosis despite irradiation-induced DNA damage. We found that Ku70/Ku80 heterodimer DNA-binding activity was higher in the resistant B-CLL cells than in sensitive cells, both before and after irradiation treatment. These data support the idea that regulation of DNA-PK activity occurs primarily through Ku.3 Other studies have reported that DNA-PK activity in B-CLL cells is regulated by the Ku heterodimer.18,66 The authors proposed that DNA-PK activity is down-regulated by a Ku80 variant.17,40,66 However, we found that the production of this variant form of Ku80 varied considerably between samples for both the resistant and sensitive B-CLL cells, as well as for normal B lymphocytes. These results show that production of the truncated Ku80 protein cannot explain the differences in DNA-PKcs and NHEJ activity between the 2 B-CLL subsets. Studies of the proteins interacting with Ku, the levels of Ku phosphorylation, and further biochemical studies will help us to determine how the differences in Ku heterodimer DEB activity in the sensitive and resistant B-CLL cell subsets lead to resistance to DNA damage-induced apoptosis. Our findings demonstrate that overactive NHEJ DSB repair allows human B-CLL cells to escape apoptosis in the presence of irradiation-induced DNA damage. The deregulation of a potentially mutagenic DNA repair system, such as NHEJ, may be one of the first steps toward carcinogenesis, as mutations in p53 and drug resistance occur as a consequence of DNA-damaging chemotherapy in B-CLL patients.47
Prepublished online as Blood First Edition Paper, February 17, 2005; DOI 10.1182/blood-2004-07-2888.
Supported by grants from Association pour la Recherche sur le Cancer, Ligue Nationale Contre Cancer, Société Française du Cancer, Société Française d'Hématologie, Commissariat à l'Energie Atomique, Fondation de France, Electricité de France, and Contract of European Community (CEC) RADINSTAB FIGH-1999-00003.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to the volunteer blood donors and to P. A. Jeggo for providing us with the pull-down DNA-PKcs protocol. We also thank O. Delattre for the pSP65 substrate and T. Lindahl for the DNA ligase IV antibody.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal