Abstract
Fibromodulin is an extracellular matrix protein normally produced by collagen-rich tissues; the fibromodulin gene has been found to be the most overexpressed gene in B-cell chronic lymphocytic leukemia. In this study, fibromodulin was expressed at the gene level (reverse transcription-polymerase chain reaction [RT-PCR]) in all patients with B-CLL (n = 75) and in most (5 of 7) patients with mantle cell lymphoma (MCL). No mutations in the fibromodulin gene were detected. Fibromodulin was also detected at the protein level in the cytoplasm of the B-CLL cells and in the supernatant after in vitro cultivation, but not at the cell surface. Fibromodulin was not found in patients with T-cell chronic lymphocytic leukemia (T-CLL), B-cell prolymphocytic leukemia (B-PLL), T-cell prolymphocytic leukemia (T-PLL), hairy cell leukemia, follicular lymphoma, lymphoplasmacytic lymphoma, multiple myeloma, acute lymphoblastic leukemia (ALL), acute myelogenous leukemia (AML), or chronic myelogenous leukemia (CML) or in 36 hematologic cell lines. Normal blood mononuclear cells (T and B lymphocytes, monocytes), tonsil B cells, and granulocytes did not express fibromodulin. Activation (phorbol 12-myristate 13-acetate [PMA]/ionomycin) of normal T and B lymphocytes induced weak fibromodulin gene expression, but not to the extent seen in freshly isolated B-CLL cells. The reason for the exclusive ectopic expression of fibromodulin in B-CLL and MCL is unknown. However, its unique protein expression makes it likely that fibromodulin is involved in the pathobiology of B-CLL and MCL. (Blood. 2005;105:4828-4835)
Introduction
Fibromodulin is an extracellular matrix protein normally produced by collagen-rich tissues. Through study of the global gene expression profile of B-cell chronic lymphocytic leukemia (B-CLL) cells, fibromodulin was found to be the most overexpressed (more than 300-2200 times) gene.1,2
B-CLL cells originate from the CD5+ B-lymphocyte subpopulation and display distinct immunophenotypic features and various genetic defects and chromosomal aberrations.3,4 Microenvironment nontumoral cells (follicular dendritic cells, CD4+ T lymphocytes, bone marrow-derived stromal cells, and endothelial cells) appear to have an indispensable role in the onset and progression of B-CLL.5 Stromal cells have a role in extending leukemic cell survival. Direct physical contact between leukemic cells and stromal cells is essential for the inhibition of apoptosis.6 Similarly, endothelial cells have the capacity to protect B-CLL cells from apoptosis by means of physical interaction.7 The viability of B-CLL cells cultured on fibronectin is consistently higher than the viability of control cultures.8 It has been suggested that direct cell-cell contact and matrix or membrane-bound cytokines rather than soluble factors may be important for the in vivo survival of B-CLL cells.9 Bidirectional malignant lymphocyte-microenvironment interactions may lead to the amplification of a microenvironment able to inhibit the apoptosis of B-CLL cells.
The overexpression of numerous genes, including a marked increase of fibromodulin (more than 300-2200 times), has been shown through a microarray technique.1,2 Fibromodulin (42-80 kDa) is a member of the extracellular class 2 leucin-rich proteoglycan family involved in the regulation of the assembly of collagen in connective tissues.10,11 It is a cytosolic protein with a secretory sequence but no transmembrane or extracellular domain. Although fibromodulin has thus far been ignored as a molecule involved in the pathogenesis of cancer, it has become evident that this and other members of the proteoglycan family are not only involved in collagen fibrillogenesis and cell adhesion, they contribute to the modulation of cytokine activity, the suppression of tumor growth, and the prevention of apoptosis.12-15 Sulfation of tyrosine residues at the N-terminal part of fibromodulin indicates that fibromodulin might be involved in chemokine receptor signaling.16
In the present study, we extended our preliminary finding17 and could now demonstrate that fibromodulin is a unique, nonmutated molecule selectively expressed at the gene and protein level in B-CLL and mantle cell lymphoma (MCL) cells and in the supernatant of cultured B-CLL cells. The reason for the exclusive expression of fibromodulin in these B-cell malignancies is unknown. Understanding the biologic functions of fibromodulin in B-CLL/MCL is critical because this unique molecule may be used as a target for therapeutic intervention.
Patients, materials, and methods
Patients and controls
The World Health Organization (WHO) classifications of neoplasms of the hematopoietic and lymphoid tissues were applied.18 The diagnosis of CLL was based on immunophenotyping (CD5+/CD19+/CD23+/IgM+) and on the presence of more than 5.0 × 109/L lymphocytes in peripheral blood. Clinical characteristics of the B-CLL patients are shown in Table 1. In 3 patients the phenotype of T-cell leukemic cells was CD3+/CD4+/CD8-, and in 2 patients it was CD3+/CD4-/CD8+. Diagnoses of acute myelogenous leukemia (AML), acute lymphoblastic leukemia (ALL), and chronic myelogenous leukemia (CML) were based mainly on cytomorphology. Karyotyping was performed on 5 of the CML patients, and all were Philadelphia chromosome positive.
Clinical characteristics of the B-CLL patient material at testing time
Characteristic . | Patients, % . |
|---|---|
| Sex | |
| Male | 68 |
| Female | 32 |
| Age, y | |
| 50-59 | 15 |
| 60-69 | 46 |
| 70-79 | 36 |
| 80-89 | 3 |
| Rai stage | |
| 0 | 24 |
| I | 30 |
| II | 13 |
| III | 16 |
| IV | 17 |
| Treatment | |
| Previously untreated | 32 |
| Previously treated* | 68 |
Characteristic . | Patients, % . |
|---|---|
| Sex | |
| Male | 68 |
| Female | 32 |
| Age, y | |
| 50-59 | 15 |
| 60-69 | 46 |
| 70-79 | 36 |
| 80-89 | 3 |
| Rai stage | |
| 0 | 24 |
| I | 30 |
| II | 13 |
| III | 16 |
| IV | 17 |
| Treatment | |
| Previously untreated | 32 |
| Previously treated* | 68 |
n = 75 patients with B-CLL.
Most patients received chlorambucil or fludarabine therapy. At least 1 month elapsed since the last treatment.
Heparinized peripheral blood was collected from patients with B-CLL (n = 75) and from those with MCL (n = 7), T-cell chronic lymphocytic leukemia (T-CLL) (n = 5), hairy cell leukemia (HCL) (n = 10), B-cell prolymphocytic leukemia (B-PLL) (n = 1), T-cell prolymphocytic leukemia (T-PLL) (n = 1), CML (n = 15), AML (n = 5), and ALL (n = 14). Bone marrow tumor cells were obtained from patients with B-CLL (n = 2), multiple myeloma (n = 4), follicular lymphoma (n = 2), and lymphoplasmacytic lymphoma (n = 4).
Control blood was drawn from healthy donors (n = 70) whose mean age was 38 years (range, 19-75 years). Fresh human tonsils were obtained from 2 children after routine tonsillectomy. This study was approved by the ethics committee of each institution, and informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
Hematologic and fibroblast cell lines
Thirty-six cell lines derived from a variety of hematologic tumors were also included (Table 3). Characteristics of the Burkitt lymphoma cell lines have been described previously.19 One B-CLL cell line (EHEB)20 was obtained from the German collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany), and another B-CLL cell line (I83-E95)21 was a kind gift from Professor Kenneth Nilsson (Uppsala, Sweden). The natural killer lymphoma cell line (YT) was obtained from DSMZ. The remaining hematologic cell lines were provided by the National Cell Bank of Iran (NCBI; Pasteur Institute of Iran, Tehran). A human fetal foreskin fibroblast cell line (HFFF-PI 6) expressing fibromodulin was obtained from NCBI. All cell lines were adapted to grow in RPMI 1640 medium (no. 42401) (Gibco, Paisley, Scotland) supplemented with 10% fetal bovine serum (FBS) (Gibco), l-glutamine (2 mM), penicillin (100 U/mL), and streptomycin (100 μg/mL) (Gibco) (complete medium).
Fibromodulin expression in hematologic cell lines
Cell line . | No. . | Fibromodulin expression . |
|---|---|---|
| Burkitt lymphoma | 20 | Negative |
| T-cell leukemia | 3 | Negative |
| Multiple myeloma | 4 | Negative |
| ALL | 3 | Negative |
| Promyelocytic leukemia | 1 | Negative |
| Myelocytic leukemia | 1 | Negative |
| Histiocytic leukemia | 1 | Negative |
| NK cell lymphoma | 1 | Negative |
| B-CLL | 2 | Negative |
Cell line . | No. . | Fibromodulin expression . |
|---|---|---|
| Burkitt lymphoma | 20 | Negative |
| T-cell leukemia | 3 | Negative |
| Multiple myeloma | 4 | Negative |
| ALL | 3 | Negative |
| Promyelocytic leukemia | 1 | Negative |
| Myelocytic leukemia | 1 | Negative |
| Histiocytic leukemia | 1 | Negative |
| NK cell lymphoma | 1 | Negative |
| B-CLL | 2 | Negative |
Cell lines were kept under standard conditions in continuous culture in RPMI 1640 medium with penicillin, streptomycin, glutamine, and 10% FBS. Fibromodulin expression was determined using RT-PCR.
NK indicates natural killer.
Isolation of peripheral blood cells
Normal peripheral blood mononuclear cells (PBMCs) (lymphocytes and monocytes), blood tumor cells, and bone marrow tumor cells were isolated from blood or bone marrow, respectively, using Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) density-gradient centrifugation, as described.22 Granulocytes were recovered from the top of the erythrocyte layer after Ficoll-Hypaque density-gradient centrifugation. Erythrocytes were lysed by hypo-osmosis in cold water. More than 98% of the nucleated cells were granulocytes. Tonsil tissue was cut and passed through a metal grid, and suspension of tonsil mononuclear cells was prepared by Ficoll-Hypaque density-gradient centrifugation, as described.22
Isolation of B and T cells
PBMCs from healthy donors were isolated as described under “Isolation of peripheral blood cells.” T and B lymphocytes were purified by negative selection using MACS beads (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. The purity of the isolated populations was checked by direct immunofluorescence using conjugated monoclonal antibodies (mAbs) against CD3, CD19, and CD14 (BD Biosciences, San Jose, CA). Leukemic B cells and tonsil mononuclear cells were also enriched using nylon wool purification.22
Activation of normal B and T lymphocytes, tonsil B cells, and B-CLL cells
Stable CD40L-transfected mouse fibroblast cells (NIH-3T3) were irradiated (100 Gy) and transferred to 6-well plates (TPP, Trasadingen, Switzerland) (150 000 cells/well) in RPMI 1640 medium supplemented with 10% FBS, penicillin (100 U/mL), streptomycin (100 μg/mL), and l-glutamine (2 mM). After overnight incubation at 37°C in humidified air with 5% CO2, fibroblasts were washed in phosphate-buffered saline (PBS). Isolated normal B lymphocytes, tonsil B cells, and B-CLL cells were added (4 × 106 cells/well) and were cultured in a total volume of 2 mL Dulbecco modified Eagle medium (DMEM) (no. 41 965) (Gibco) supplemented with 10% FBS, penicillin, streptomycin, and l-glutamine at 37°C in humidified air with 5% CO2 for 48 hours. Cells cultured in medium alone were used as a control.
Isolated T lymphocytes were stimulated with anti-CD3 antibody (OKT3) (30 ng/mL) (Mabtech, Stockholm, Sweden) using the same culture conditions as described in the previous paragraph. In addition, normal B and T lymphocytes, tonsil B cells, and B-CLL cells were stimulated with 25 ng/mL PMA (phorbol 12-myristate 13-acetate) + 1 μg/mL ionomycin (Sigma, St Louis, MO).
After 48 hours of culture, cells were harvested. Activation was confirmed by an increased expression of CD25 and CD69 using mAbs (BD Biosciences) and flow cytometry. RNA was isolated, cDNA was prepared, and the relative increase in fibromodulin expression was compared with that of unstimulated cells (time, 0 hours) and was determined by real-time quantitative PCR (see “Real-time quantitative PCR”).
Activation of B-CLL cell lines
Under standard conditions, the B-CLL cell lines EHEB and I83-E95 were continuously cultured in RPMI 1640 (Gibco) supplemented with penicillin, streptomycin, l-glutamine, and 10% FBS. Cells in RPMI 1640 were stimulated by coculture with irradiated CD40L-transfected fibroblasts (see “Activation of normal B and T lymphocytes, tonsil B cells, and B-CLL cells”). EHEB cells were also stimulated with 25 ng/mL PMA + 1 μg/mL ionomycin.
Both B-CLL cell lines were also cultured in DMEM supplemented with penicillin (100 U/mL), streptomycin (100 μg/mL), l-glutamine (2 mM), and 10% FBS (unstimulated) and were stimulated with 20 ng/mL PMA + 1 μg/mL ionomycin for 48 hours. After 48 hours of culture, cells were harvested. Activation was tested by measuring the expression of CD25 and CD69 using mAbs (BD Biosciences) and flow cytometry. RNA was isolated, cDNA was prepared, and the relative increase in fibromodulin expression compared with unstimulated time 0 hours was determined by real-time quantitative PCR (see “Real-time quantitative PCR”).
Transformation of normal B cells with Epstein-Barr virus
Epstein-Barr virus (EBV) transformation was performed as previously described.23 Briefly, purified B lymphocytes (2 × 107 cells) from healthy controls (n = 16) were incubated with cell-free supernatant of B95-8 cells at 37°C for 1 hour. Cells were washed and incubated in RPMI 1640 supplemented with 10% FCS, l-glutamine (2 mM), penicillin (100 U/mL), and streptomycin (100 μg/mL).
RT-PCR amplification of fibromodulin
Total RNA was extracted from tumor cells and normal PBMCs using RNAzol B reagent (BioSite, Täby, Sweden) according to the manufacturer's instructions. First-strand cDNA was synthesized using 5 μg total RNA in 20 μL reaction mixture consisting of 4 μL 5 × reaction buffer, 1 μL 10 mM dNTP, 1.5 μL 100 μM dithiothreitol (DTT), 1 μL 10 pmol/mL random hexamer (N6), and 200 U M-MLV reverse transcriptase (Gibco). The mixture was incubated at 42°C for 45 minutes. PCR amplification was performed using fibromodulin-specific primers ACCGTCCCCGATAGCTACTT as sense and CATCCTGGACCTTCCAGCAAA as antisense (GenBank accession no. XM_001782). Briefly, 25 μL reaction mixture of PCR was prepared using 2.5 μL10 × buffer, 1 μL 25 mM MgCl2, 1.5 μL dNTP (10 mM), 5 pmol each primer, and 1 U Ampli-Taq Gold DNA polymerase (Perkin-Elmer/Applied Biosystems, Boston, MA). PCR was followed by 35 cycles at 92°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds leading to a 448-bp amplicon. PCR product was finally visualized by running agarose gel electrophoresis containing ethidium bromide. To ensure the specificity of primers, some of the PCR products were cloned into pGEM-T easy vector (Promega, Madison, WI) and were subjected to sequencing.
Real-time quantitative PCR
cDNA samples were used as template, and β-actin (endogenous housekeeping gene) was quantified as a positive control against which the different template values were normalized. For β-actin, the sense primer was 5′-CGACAGGATGCAGAAGGAGA-3′, the antisense primer was 5′-CGTCATACTCCTGCTTGCTG-3′, and the probe was 5′-FAM-TCCAACACCTTCAATTCCAGCAGGTAMRA-3′. Primers for fibromodulin were the same primers used for screening. Fibromodulin probe was 5′-FAM-TCCAACACCTTCAATTCCAGCAGCTAMRA-3′. Ampli-Taq Gold polymerase (Perkin-Elmer, Applied Biosystems, Brunchburg, NJ) was used to catalyze the amplification reaction. PCR conditions were optimized for primers, probes, and MgCI2. PCR amplification was carried out in 25-μL reaction volume in a 96-well optical PCR plate (N 801-0560; Perkin-Elmer). Each reaction volume contained 1 × TaqMan Buffer (Perkin-Elmer), 5 pmol sense primer (Cybergene AB, Stockholm, Sweden), 5 pmol antisense primer (Cybergene), 2.5 pmol probe (Cybergene), 0.5 U Ampli-Taq Gold, and 0.1 U uracil-N-glycosylase (UNG) (Perkin-Elmer). One microliter of the template cDNA was included in each reaction volume, except for the non-template control (NTC). Aliquots were then amplified by 1 cycle at 50°C (2 minutes) followed by 40 cycles of denaturation at 95°C (15 seconds) with annealing and extension at 60°C (1 minute). ABI Prism 7700 Sequence Detection System (Perkin-Elmer) was used for online quantification. For relative quantification of fibromodulin mRNA, the arithmetic formula [relative increase (fold increase) = 2-ΔΔCT] was used according to the 1997 Perkin-Elmer instruction manual, whereby ΔΔCT = (CT of target - CT of β-actin) at any time point (stimulated samples) - (CT of target - CT of β-actin) unstimulated samples (at time 0). CT is the point (cycle) at which the amplification plot crosses the threshold; mRNA gene expression is presented as the fold increase calculated in relation to unstimulated cells after normalization against β-actin.
Surface and cytoplasmic staining and flow cytometry
Blood leukemic cells of patients with B-CLL and PBMCs of healthy control donors were washed twice with cold PBS and were fixed with 1% paraformaldehyde in PBS for 10 minutes.
After they were washed with PBS, pelleted cells were treated with 0.5 mL FACS permeabilizing solution (BD Biosciences) for 10 minutes. Permeabilized cells were then washed with washing solution (PBS, 0.2% bovine serum albumin [BSA], 0.1% NaN3, 0.5 mM EDTA [ethylenediamine-tetraacetic acid], and 1% saponin) and were incubated for 60 minutes with an appropriate concentration (5 μg/mL) of affinity-purified rabbit antibody specific for a C-terminal peptide of human native fibromodulin.24 The antibody was a kind gift from Dr P. Roughley (Genetics Unit, Shriners Hospital for Crippled Children, Montreal, Canada). Non-immune polyclonal rabbit immunoglobulin G (IgG) (5 μg/mL) served as negative control. After 3 washings, F(ab')2-fragments of fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG (Biosource, Camarillo, CA) was added, and the cells were incubated for 45 minutes. Cells were finally washed twice with washing solution and once with PBS, fixed with 1% paraformaldehyde in PBS, and kept at 4°C until analysis by flow cytometry (FACScalibur; BD Biosciences). For direct surface staining, cells were incubated with FITC-conjugated mAb specific for CD19 or CD5 (BD Biosciences) for 30 minutes, washed with washing solution without saponin, and fixed as above. FITC-labeled irrelevant isotype-matched mAb served as control. Surface staining for membrane-bound fibromodulin was performed in indirect immunofluorescence using the same primary and secondary antibodies as stated earlier.
Western blot
PBMCs were lysed in buffer containing 2% Triton X-100, 10 mM Tris, pH 7.4, 100 mM NaCl, 1 mM EDTA, 1 mM NaF, 20 mM Na4P2O7, 1% glycerol, 0.1% sodium dodecyl sulfate (SDS), and 1% protease inhibitor cocktail. Cell lysate of 5 × 105 cells was run on 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) at 200 V for 1 hour under nonreducing conditions. After electrophoresis, resolved proteins were transferred onto Hybond enhanced chemiluminescence (ECL) nitrocellulose membranes (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom) in a mini-Transblot cell (Invitrogen, Carlsbad, CA). The membranes were blocked overnight with 5% nonfat milk in PBS plus 0.05% Tween 20 (PBS-T). All additional immunostaining steps, as well as washing steps, were performed in PBS-T supplemented with 5% nonfat milk (Semper, Stockholm, Sweden). Filters were incubated with a rabbit polyclonal antibody against native human fibromodulin (a kind gift from Prof D Heinegård, Department of Cell and Molecular Biology, Lund University, Sweden)25 overnight and a mouse anti-human leukocyte antigen (anti-HLA) class 1 mAb (Harlan Sera-Lab, Hillcrest, United Kingdom) for 2 hours as a control. After extensive washings, filters were incubated with the secondary antibody, a horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody or a goat anti-mouse immunoglobulin (DAKO, Glostrup, Denmark), respectively, for 1 hour. Filters were developed using the ECL system (Amersham Pharmacia) according to the manufacturer's instructions.
In vitro secretion of fibromodulin
PBMCs were isolated from the peripheral blood of CLL patients and healthy donors. T and B lymphocytes from healthy donors were also enriched by nylon wool purification (see “Isolation of B and T cells”). Isolated cells including the fibromodulin-positive fibroblast cell line (HFFF-PI 6) were cultured for 40 hours in 6-well plates (4 × 106 cells/well) (TPP, Trasadingen, Switzerland) in 2 mL AIM-V medium (Gibco) supplemented with penicillin (100 U/mL), streptomycin (100 μg/mL), and l-glutamine (2 mM) at 37°C in humidified air with 5% CO2. After culturing, supernatants were harvested and analyzed by Western blot using the same antibodies and conditions described under “Western blot.” Purified fibromodulin control protein was a kind gift from Prof D. Heinegård.
Mutation analysis of the fibromodulin gene
PBMCs from healthy controls and B-CLL patients were isolated and subjected to RNA and genomic DNA preparation, as previously described.26 Fibromodulin-specific primers were designed to amplify exon 2 and 3 coding regions from genomic DNA of healthy donors and full-length coding regions of cDNA and genomic DNA of CLL patients (sense, CACAGGCACGCACACTCTCA; antisense, GGTTCTCCAGGTTGGTGTTG (1082 bp DNA); sense, GAAAACATCTGCCCTCCATC; antisense, AGCCAAACCAAACCATCAAG (350 bp DNA); sense, CACAGGCACGCACACTCTCA; antisense, AGCCAAACCAAACCATCAAG (1328 bp cDNA). The PCR product was cloned into pGEM-T easy vector and was subjected to sequences using ABI310 genetic analyzer (Perkin-Elmer/Applied Biosystems, Foster City, CA).
Results
Fibromodulin gene expression in tumor cells from patients with hematologic malignancies and from hematologic cell lines
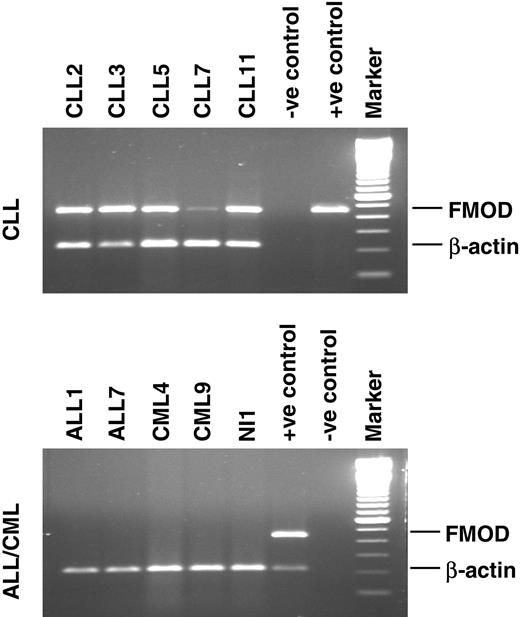
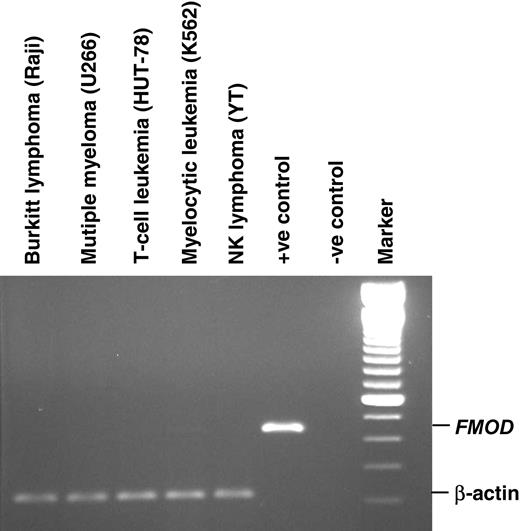
PBMCs of all B-CLL patients (n = 75) expressed fibromodulin at the mRNA level, as did the leukemic cells from 5 of 7 MCL patients. Bone marrow mononuclear cells from B-CLL patients (n = 2) also expressed fibromodulin (Table 2; Figure 1). Fibromodulin was not expressed in tumor cells of patients with T-CLL, B-PLL, T-PLL, hairy cell leukemia, follicular lymphoma, lymphoplasmacytic lymphoma, multiple myeloma, AML, ALL, or CML. Neither was fibromodulin expressed in any of 36 hematologic cell lines, including 2 B-CLL cell lines kept under standard cell culture conditions (RPMI 1640 medium) (Table 3; Figure 2). Fibromodulin was not expressed in fresh PBMCs (lymphocytes and monocytes; n = 70), isolated normal B cells (purity greater than 90%; n = 6), isolated T cells (purity greater than 70%; n = 4), or isolated blood granulocytes (purity greater than 98%; n = 10) of healthy donors, or in isolated tonsil B cells (purity greater than 90%; n = 2).
Fibromodulin gene expression in freshly isolated tumor cells of patients with hematologic malignancies and in nucleated blood cells of healthy control donors
Tumor or normal cells by tissue type . | No. positive/total no. cases . |
|---|---|
| Peripheral blood | |
| B-CLL | 75/75 |
| Mantle cell lymphoma | 5/7 |
| B-PLL | 0/1 |
| T-CLL | 0/5 |
| T-PLL | 0/1 |
| Hairy cell leukemia | 0/10 |
| CML | 0/15 |
| AML | 0/5 |
| ALL | 0/14 |
| Bone marrow | |
| B-CLL | 2/2 |
| Follicular lymphoma | 0/2 |
| Lymphoplasmacytic lymphoma | 0/4 |
| Multiple myeloma | 0/4 |
| Normal PBMCs, lymphocytes and monocytes | 0/70 |
| Normal blood B lymphocytes* | 0/6 |
| Normal tonsil B lymphocytes* | 0/2 |
| Normal blood granulocytes† | 0/10 |
| Normal blood T lymphocytes‡ | 0/4 |
Tumor or normal cells by tissue type . | No. positive/total no. cases . |
|---|---|
| Peripheral blood | |
| B-CLL | 75/75 |
| Mantle cell lymphoma | 5/7 |
| B-PLL | 0/1 |
| T-CLL | 0/5 |
| T-PLL | 0/1 |
| Hairy cell leukemia | 0/10 |
| CML | 0/15 |
| AML | 0/5 |
| ALL | 0/14 |
| Bone marrow | |
| B-CLL | 2/2 |
| Follicular lymphoma | 0/2 |
| Lymphoplasmacytic lymphoma | 0/4 |
| Multiple myeloma | 0/4 |
| Normal PBMCs, lymphocytes and monocytes | 0/70 |
| Normal blood B lymphocytes* | 0/6 |
| Normal tonsil B lymphocytes* | 0/2 |
| Normal blood granulocytes† | 0/10 |
| Normal blood T lymphocytes‡ | 0/4 |
Fibromodulin expression was determined using RT-PCR.
Purity >90%.
Purity >98%.
Purity >70%.
Fibromodulin protein expression in B-CLL cells
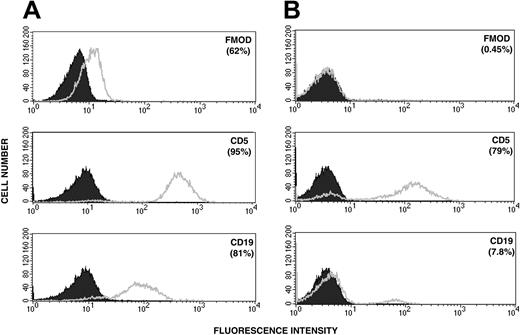
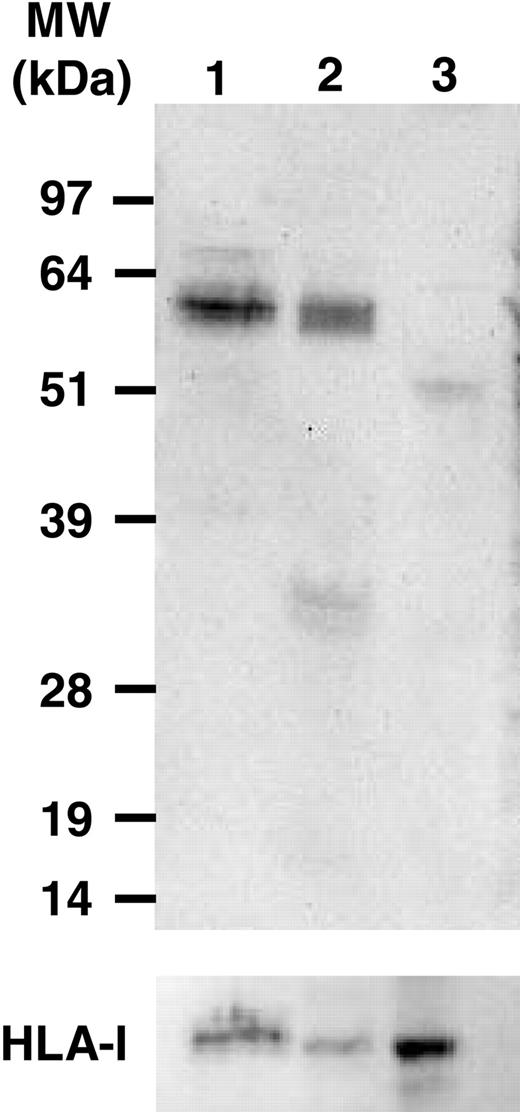
Cytoplasmic and surface staining for fibromodulin was performed using indirect immunofluorescence. Leukemic B cells from CLL patients (n = 2), but not B cells of control donors, were specifically stained in the cytoplasm. Results from a representative experiment are shown in Figure 3. No surface staining for fibromodulin could be detected (data not shown). Western blot analysis of cell lysates from 12 CLL patients demonstrated specific fibromodulin bands of approximately expected size in all of them. Representative immunoblots are depicted in Figure 4.
Expression profile of fibromodulin mRNA in leukemic cells. B-CLL (n = 5), ALL (n = 2), and CML (n = 2) patients and healthy control donor (NI 1). Positive (+ve) controls represent the PCR product cloned into pGEM-T easy vector (top panel). B-CLL pooled cDNA (bottom panel). Negative (-ve) control is the reaction mix without template. Marker is a 100-bp DNA ladder. FMOD indicates fibromodulin.
Expression profile of fibromodulin mRNA in leukemic cells. B-CLL (n = 5), ALL (n = 2), and CML (n = 2) patients and healthy control donor (NI 1). Positive (+ve) controls represent the PCR product cloned into pGEM-T easy vector (top panel). B-CLL pooled cDNA (bottom panel). Negative (-ve) control is the reaction mix without template. Marker is a 100-bp DNA ladder. FMOD indicates fibromodulin.
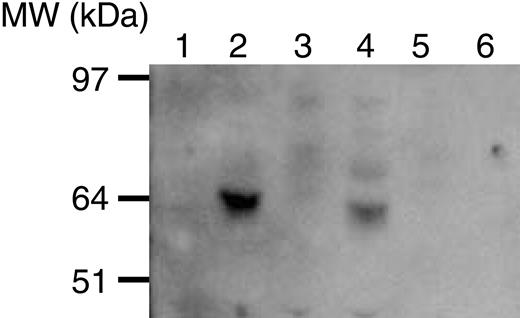
Supernatants of B-CLL cells cultured for 40 hours showed the presence of a 60-kDa protein reactive with a polyclonal anti-fibromodulin antibody. This was also seen in the culture medium from a fibromodulin-positive fibroblast cell line (HFFF-PI 6). No band could be seen in cultures of PBMCs or in enriched T and B cells of healthy donors (Figure 5).
Activation of normal B and T lymphocytes, tonsil B cells, CLL B cells, and B-CLL cell lines
In unstimulated normal B and T lymphocytes and tonsil B cells and in B-CLL cell lines cultured under standard conditions (RPMI 1640 medium), no fibromodulin expression at the gene level could be detected.
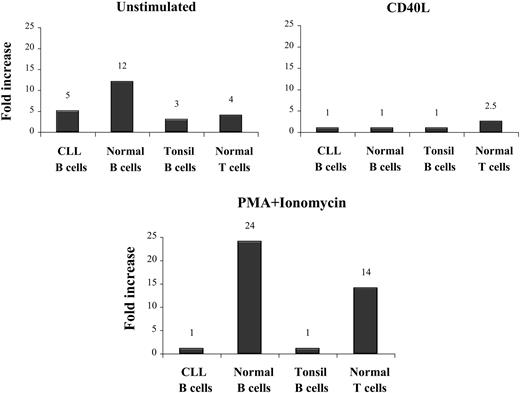
Culturing of normal B and T lymphocytes and of tonsil B cells in DMEM alone for 48 hours induced a weak expression of fibromodulin (12-, 4-, and 3-fold increase, respectively). A slight increase in fibromodulin was also noted in freshly isolated B-CLL cells (5-fold) (Table 4).
Fibromodulin expression in activated cells
Stimulator by cell type . | CD25+ cells, % . | CD69+ cells, % . | Fold increase of fibromodulin expression* . |
|---|---|---|---|
| Enriched normal B cells, purity >90% | |||
| Unstimulated | 24 | 6 | 1 |
| DMEM alone | 32 | 17 | 12 |
| DMEM + CD40L | 74 | 88 | 1 |
| DMEM + PMA/ionomycin | 95 | 62 | 24 |
| Enriched normal T cells, purity >70% | |||
| Unstimulated | 22 | 3 | 1 |
| DMEM alone | 8 | 8 | 4 |
| DMEM + anti-CD3 | 73 | 65 | 2.5 |
| DMEM + anti-CD3 | ND | ND | 14 |
| Enriched tonsil B cells, purity >90% | |||
| Unstimulated | 4 | 54 | 1 |
| DMEM alone | 36 | 51 | 3 |
| DMEM + CD40L | 75 | 65 | 1 |
| DMEM + PMA/ionomycin | 76 | 77 | 1 |
| B-CLL | |||
| Unstimulated | 48 | 21 | 1 |
| DMEM alone | 57 | 18 | 5 |
| DMEM + CD40L | 96 | 99 | 1 |
| DMEM + PMA/ionomycin | 100 | 30 | 1 |
| EHEB | |||
| RPMI medium alone† | 11 | 12 | 1 |
| RPMI medium alone | 17 | 14 | 1 |
| RPMI medium + CD40L | 21 | 15 | 1 |
| RPMI medium + PMA/ionomycin | 27 | 12 | 1 |
| DMEM alone | 13 | 26 | 12 |
| DMEM + PMA/ionomycin | ND | ND | 114 |
| I83-E95 | |||
| RPMI medium alone† | 3 | 5 | 1 |
| RPMI medium alone | 7 | 6 | 1 |
| RPMI medium + CD40L | 11 | 6 | 1 |
| RPMI medium + PMA/ionomycin | ND | ND | ND |
| DMEM alone | 3 | 3 | 17 |
| DMEM + PMA/ionomycin | 33 | 8 | 21 |
Stimulator by cell type . | CD25+ cells, % . | CD69+ cells, % . | Fold increase of fibromodulin expression* . |
|---|---|---|---|
| Enriched normal B cells, purity >90% | |||
| Unstimulated | 24 | 6 | 1 |
| DMEM alone | 32 | 17 | 12 |
| DMEM + CD40L | 74 | 88 | 1 |
| DMEM + PMA/ionomycin | 95 | 62 | 24 |
| Enriched normal T cells, purity >70% | |||
| Unstimulated | 22 | 3 | 1 |
| DMEM alone | 8 | 8 | 4 |
| DMEM + anti-CD3 | 73 | 65 | 2.5 |
| DMEM + anti-CD3 | ND | ND | 14 |
| Enriched tonsil B cells, purity >90% | |||
| Unstimulated | 4 | 54 | 1 |
| DMEM alone | 36 | 51 | 3 |
| DMEM + CD40L | 75 | 65 | 1 |
| DMEM + PMA/ionomycin | 76 | 77 | 1 |
| B-CLL | |||
| Unstimulated | 48 | 21 | 1 |
| DMEM alone | 57 | 18 | 5 |
| DMEM + CD40L | 96 | 99 | 1 |
| DMEM + PMA/ionomycin | 100 | 30 | 1 |
| EHEB | |||
| RPMI medium alone† | 11 | 12 | 1 |
| RPMI medium alone | 17 | 14 | 1 |
| RPMI medium + CD40L | 21 | 15 | 1 |
| RPMI medium + PMA/ionomycin | 27 | 12 | 1 |
| DMEM alone | 13 | 26 | 12 |
| DMEM + PMA/ionomycin | ND | ND | 114 |
| I83-E95 | |||
| RPMI medium alone† | 3 | 5 | 1 |
| RPMI medium alone | 7 | 6 | 1 |
| RPMI medium + CD40L | 11 | 6 | 1 |
| RPMI medium + PMA/ionomycin | ND | ND | ND |
| DMEM alone | 3 | 3 | 17 |
| DMEM + PMA/ionomycin | 33 | 8 | 21 |
Fibromodulin expression was determined using real-time quantitative PCR. Cells were stimulated for 48 hours unless otherwise marked.
ND indicates not determined.
Results are expressed as fold increase compared with unstimulated cells at time 0 h. Values are the mean of 2 experiments.
Cells were stimulated for 0 hours.
Coculturing of normal B lymphocytes, tonsil B cells, and B-CLL cells in DMEM with CD40L-transfected fibroblasts did not induce fibromodulin expression. Stimulation of T lymphocytes with anti-CD3 mAbs induced a weak fibromodulin expression (2.5-fold increase) (Table 4).
Expression profile of fibromodulin mRNA of hematologic cell lines. Positive (+ve) controls represent PCR product cloned into pGEM-T easy vector, and negative (-ve) control is the reaction mix without template. Marker is a 100-bp DNA ladder.
Expression profile of fibromodulin mRNA of hematologic cell lines. Positive (+ve) controls represent PCR product cloned into pGEM-T easy vector, and negative (-ve) control is the reaction mix without template. Marker is a 100-bp DNA ladder.
The polyclonal activator PMA/ionomycin induced stronger fibromodulin gene expression in normal B and T lymphocytes (24- and 14-fold increase, respectively). However, neither B-CLL cells nor tonsil B cells showed increased expression with this kind of stimulation (Table 4; Figure 6).
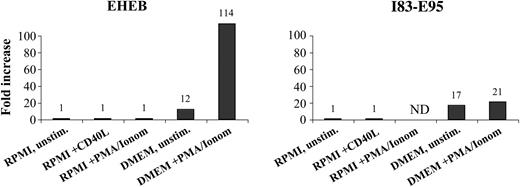
The CLL cell lines EHEB and I83-E95, cultured in RPMI 1640, did not express fibromodulin even after stimulation with CD40L or PMA/ionomycin. However, when cultured in DMEM, EHEB and I83-E95 cells showed induction of fibromodulin expression, which was further increased by PMA/ionomycin stimulation (Table 4; Figure 7). EBV-transformed normal B cells from healthy donors showed a weak fibromodulin gene expression in 2 of 16 tested donors (data not shown).
Intracytoplasmic staining for fibromodulin and surface-membrane staining for CD5 and CD19 of leukemic cells. (A) Patient with B-CLL and (B) PBMCs from a healthy control donor (1 representative experiment). The shaded curve represents background staining with a control antibody (see “Patients, materials, and methods”). Figures in brackets indicate percentages of positive cells.
Intracytoplasmic staining for fibromodulin and surface-membrane staining for CD5 and CD19 of leukemic cells. (A) Patient with B-CLL and (B) PBMCs from a healthy control donor (1 representative experiment). The shaded curve represents background staining with a control antibody (see “Patients, materials, and methods”). Figures in brackets indicate percentages of positive cells.
Mutation analysis of the fibromodulin gene in healthy controls and B-CLL patients
To determine mutations in the coding region of the fibromodulin gene giving rise to ectopic expression or truncation of fibromodulin, B-CLL cells (n = 10) and PBMCs of healthy controls (n = 10) were analyzed at the DNA and the cDNA levels. No mutations were found in the fibromodulin gene of B-CLL patients compared with the fibromodulin gene submitted to GenBank (accession no. XM_001782).
Representative immunoblots. Leukemic cell lysates of 2 B-CLL patients (lanes 1-2) and PBMCs of a normal healthy control donor (lane 3) incubated with anti-fibromodulin antibody (top) and anti-major histocompatibility complex (MHC) class 1 antibody (bottom) (see “Patients, materials, and methods”).
Representative immunoblots. Leukemic cell lysates of 2 B-CLL patients (lanes 1-2) and PBMCs of a normal healthy control donor (lane 3) incubated with anti-fibromodulin antibody (top) and anti-major histocompatibility complex (MHC) class 1 antibody (bottom) (see “Patients, materials, and methods”).
Discussion
Freshly isolated leukemic cells from all B-CLL patients and most patients with MCL expressed the extracellular matrix (ECM) protein fibromodulin at the gene and protein levels. No mutations in the fibromodulin gene were noted. The fibromodulin protein was detected in the cytoplasm but not on the cell surface. Short-term culture of CLL cells showed the presence of the fibromodulin protein in the cell culture medium. Expression of fibromodulin in hematologic malignancies seemed to be exclusive for B-CLL and MCL because fibromodulin was not detected in patients with T-CLL, B-PLL, T-PLL, hairy cell leukemia, follicular lymphoma, lymphoplasmacytic lymphoma, multiple myeloma, AML, CML, or ALL or in a panel of hematologic cell lines. Fibromodulin was not expressed in freshly isolated normal PBMCs (lymphocytes and monocytes), including enriched T and B lymphocytes, tonsil B cells, and purified granulocytes. However, 48-hour in vitro culture with a strong polyclonal activator (PMA/ionomycin) induced fibromodulin gene expression in normal B and T lymphocytes but did not induce it or only marginally increased it in B-CLL cells and tonsil B cells.
Immunoblotting (Western blot) of supernatants from cells cultured in AIM-V medium for 40 hours. Culture medium alone (lane 1), fibromodulin-positive (PCR) cell line (HFFF-PI 6) (lane 2), PBMCs of healthy donor (lane 3), B-CLL cells (lane 4), T cells of healthy donor (lane 5), and B cells of a healthy donor (lane 6). Molecular weight marker is to the left.
Immunoblotting (Western blot) of supernatants from cells cultured in AIM-V medium for 40 hours. Culture medium alone (lane 1), fibromodulin-positive (PCR) cell line (HFFF-PI 6) (lane 2), PBMCs of healthy donor (lane 3), B-CLL cells (lane 4), T cells of healthy donor (lane 5), and B cells of a healthy donor (lane 6). Molecular weight marker is to the left.
The reason for this unexpected exclusive and specific expression of fibromodulin in B-CLL and MCL is unknown. Gene expression profiling of B-CLL has shown an overexpression of many other genes, such as CD5 (a T-cell surface glycoprotein), ZAP70 (T-cell receptor [TCR]-associated signaling molecule), and fibromodulin, that are not B-cell specific. Abnormalities of chromosome 1, where the gene for fibromodulin is located (1q32), are rarely seen in patients with B-CLL,27 and no mutations in the gene were found. Recent published data indicate abnormalities of chromosome 1p34-36,28 where the gene for receptor tyrosine kinase Ror1 is located. Overexpression of this gene29 and other genes, including CD30, CD21, and interleukin-10, all located on chromosome 1, has been reported in patients with CLL.1,30,31 Our own unpublished data (E.M., A.H.D.M., A.Ö., H.M., and H.R., June 2004) also indicate that several other genes on chromosome 1 may be exclusively overexpressed in B-CLL cells. Such a gene expression signature involving several chromosomes raises the question of domain or cluster-specific regulation involved in tumorigenesis in B-CLL. This expression pattern, including T-cell markers, implies a possible disruption in regulatory element(s), possibly from a lymphoid progenitor cell (ie, before differentiation into B and T cells). The mechanism behind such a differential expression, with no major alteration in the genome, might be an alteration in DNA methylation covering, for example, the fibromodulin locus.
Fibromodulin expression in activated B and T lymphocytes, tonsil B cells, and B-CLL cells after 48 hours of culture. Increase was compared with time 0 hours measured by real-time quantitative PCR.
Fibromodulin expression in activated B and T lymphocytes, tonsil B cells, and B-CLL cells after 48 hours of culture. Increase was compared with time 0 hours measured by real-time quantitative PCR.
Fibromodulin expression in activated B-CLL cell lines after 48 hours of culture. Increase was compared with time 0 hours measured by real-time quantitative PCR.
Fibromodulin expression in activated B-CLL cell lines after 48 hours of culture. Increase was compared with time 0 hours measured by real-time quantitative PCR.
Fibromodulin is a cytosolic secreted protein with an expression pattern restricted mainly to cartilage, bone, connective tissue, and tissue rich in collagen.25 Fibromodulin is involved in fibrillogenesis, cell adhesion, and cytokine activity modulation. This suggests the involvement of fibromodulin in tumor suppression and prevention of apoptosis, as is true of other proteoglycans such as decorin.12-15 In normal tissues fibromodulin seems to be developmentally regulated, with higher levels of expression early in life and decreased expression during aging.32 Fibromodulin knock-out mice demonstrated collagen malformation and symptoms resembling osteoarthritis, but no life-threatening structural or physiologic defects were observed.33,34
Overexpression of ECM proteins has been observed in malignant cells,35-37 but the underlying mechanisms are poorly understood. ECM may promote angiogenesis.38 In addition, it may interact with cell surface-associated heparan sulfate proteoglycans and regulate proliferation and angiogenesis.39 Myeloma cells express the heparan sulfate proteoglycan syndecan-1, a receptor for the hepatocyte growth factor that may act as a growth factor for myeloma cells.40 Tumor stromal cells also produce and secrete increased amounts of ECM.41 This network of cells, ECM, and soluble growth factors may support the growth of tumor cells and angiogenesis and promote tumor progression and spread.
Tyrosine sulfation is a common posttranslational modification of proteins and seems to be of importance for protein-protein interactions.42 Tyrosine sulfation has been noted of ECM proteins, including tyrosine residues at the N-terminal of fibromodulin.16 Analyses of the functional consequences of tyrosine sulfation or phosphorylation of the N-terminal of fibromodulin in B-CLL might contribute to a better understanding of the function of this ectopically expressed protein in B-CLL. Sulfation may not exclude the phosphorylation of tyrosine residues within the same domain because serine phosphorylation of ECM sialoprotein has been reported.43
It is suggested that the microenvironment is important for the survival of tumor cells of low-grade B-cell neoplasms, including B-CLL.5 Cell-cell and cell-matrix interactions have been shown to be critical events in preventing the apoptosis of malignant lymphocytes. In B-CLL, direct contact between leukemic and bone marrow stromal cells has been found to be essential for tumor cell survival.6,44 Bone marrow stromal cells express various adhesion molecules involved in hematopoiesis. Leukemic cells exhibit several types of receptors that can bind to the adhesion molecules. This interaction may provide survival signals for the leukemic cells.9 Fibromodulin may play a role in the physical interaction and signaling between B-CLL cells and the microenvironment. Fresh B-CLL cells died rapidly in vitro when cultured in medium alone. However, when cultured on fibronectin, survival was prolonged.8
Resistance of B-CLL cells to proapoptotic and antiproliferative effects of transforming growth factor beta (TGF-β) has earlier been reported.45-47 TGF-β is secreted by B-CLL cells without a significant inhibitory effect on leukemic B cells, which is not the case for normal B cells in which autocrine-produced TGF-β inhibits differentiation and proliferation.45 These results may suggest an adaptation of the antiapoptotic mechanisms of TGF-β in B-CLL cells. It has been suggested that resistance to the inhibitory effects of TGF-β is associated with the loss of TGF-β receptors,46 but this has not been confirmed,45,48 indicating other mechanisms may be involved as well. Fibromodulin has been shown to modulate and suppress TGF-β functions in vivo and in vitro.12 Fibromodulin has binding sites for TGF-β, and binding of TGF-β to fibromodulin in B-CLL may inhibit the apoptotic activity of TGF-β.
Neither B-CLL cell line expressed fibromodulin when it was cultured in standard RPMI medium, and no effect of CD40L or PMA/ionomycin activation was noted. However, when cultured in DMEM, fibromodulin expression was induced and was further enhanced by PMA/ionomycin activation. DMEM has a higher glucose concentration than RPMI 1640 and contains Fe(NO3). Glucose and iron have been reported to induce TGF-β production.49,50 Iron is important for the expression of cell-cycle and apoptosis-related genes,51 and it may inhibit the proliferation of Burkitt lymphoma cells.52 Iron is also required for PMA-induced expression of certain genes53 and is necessary for protein kinase C activity.54 Fibromodulin and the closely related proteoglycan decorin bind TGF-β, inhibiting the function of TGF-β.12 In myocytes, iron overload increased the expression of decorin and decreased that of TGF-β. Reductions of TGF-β expression may result from binding to decorin.55 It may be that fibromodulin was induced secondarily to glucose/iron-induced TGF-β expression. Given that there was no genomic aberration of the fibromodulin locus, the expression might be considered the effect of epigenetic factors. Whether fibromodulin can be induced in the other hematologic cell lines using those culture conditions is unknown and requires further study.
In summary, we have shown that the ECM protein fibromodulin is exclusively expressed and secreted in patients with B-CLL and in most patients with MCL but not in patients with other hematologic malignancies. The gene showed no mutations, and its molecular weight was as expected. In normal B- and T-lymphocytes, a strong activation signal (PMA/ionomycin) induced a weak expression of fibromodulin. PMA induces cell differentiation signals through the protein kinase C pathways, but it also induces apoptosis.56 Fibromodulin may be a survival factor constitutively expressed in B-CLL cells and, on stimulation or stress, in normal B and T lymphocytes. Induction of fibromodulin was not seen in tonsil B cells, and the activation signal did not increase fibromodulin expression in B-CLL cells. Tonsil B cells similar to CLL cells do not have the propensity to undergo apoptosis.57 Tonsil B cells may exhibit other survival factors, making fibromodulin redundant. The functional role of fibromodulin in the pathogenesis of B-CLL and MCL remains to be established. The unique expression of fibromodulin in B-CLL also makes it an interesting structure for targeted therapy. Spontaneous expansion of CD8+ T cells that recognize fibromodulin peptides was recently shown in patients with B-CLL.58
Prepublished online as Blood First Edition Paper, March 1, 2005; DOI 10.1182/blood-2004-10-3941.
Supported by grants from the Swedish Research council/SIDA/SAREC, the Iranian Ministry of Health and Medical Education, the Swedish Cancer Society, the Torsten and Ragnar Söderberg Foundation, the Cancer Society in Stockholm, the King Gustaf Vth Jubilee Fund, the Cancer and Allergy Foundation, the Gunnar Nilsson Cancer Foundation, and the Karolinska Institute Foundations.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ms Ingrid Eriksson, Ms Barbro Näsman-Glaser, Dr R. Payam, K. Esmailzadeh, J. Ghasemi, and P. Ahmadi for their invaluable technical assistance. We thank Ms Gunilla Burén and Ms Gerd Ståhlberg for their excellent secretarial help.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal